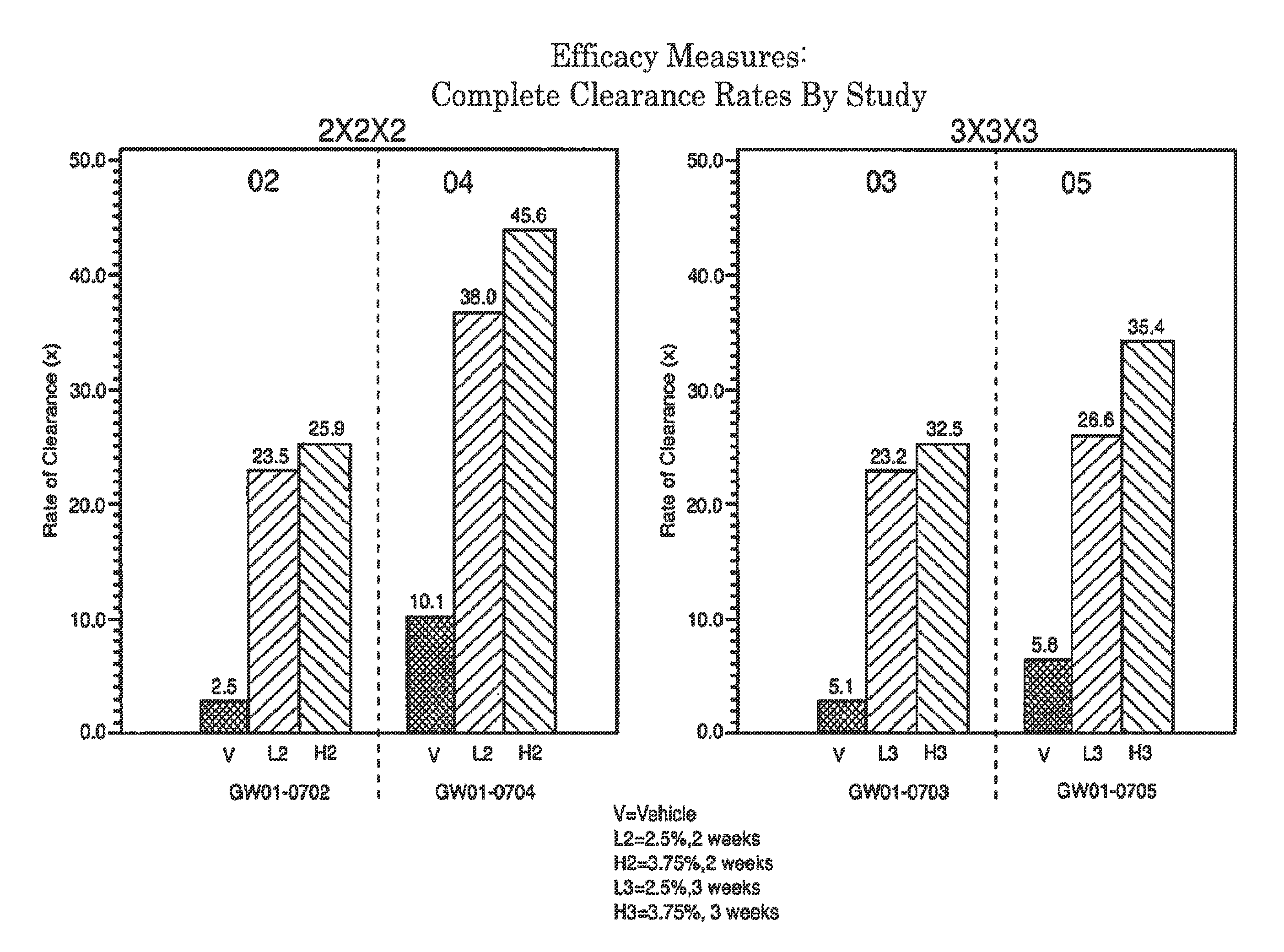
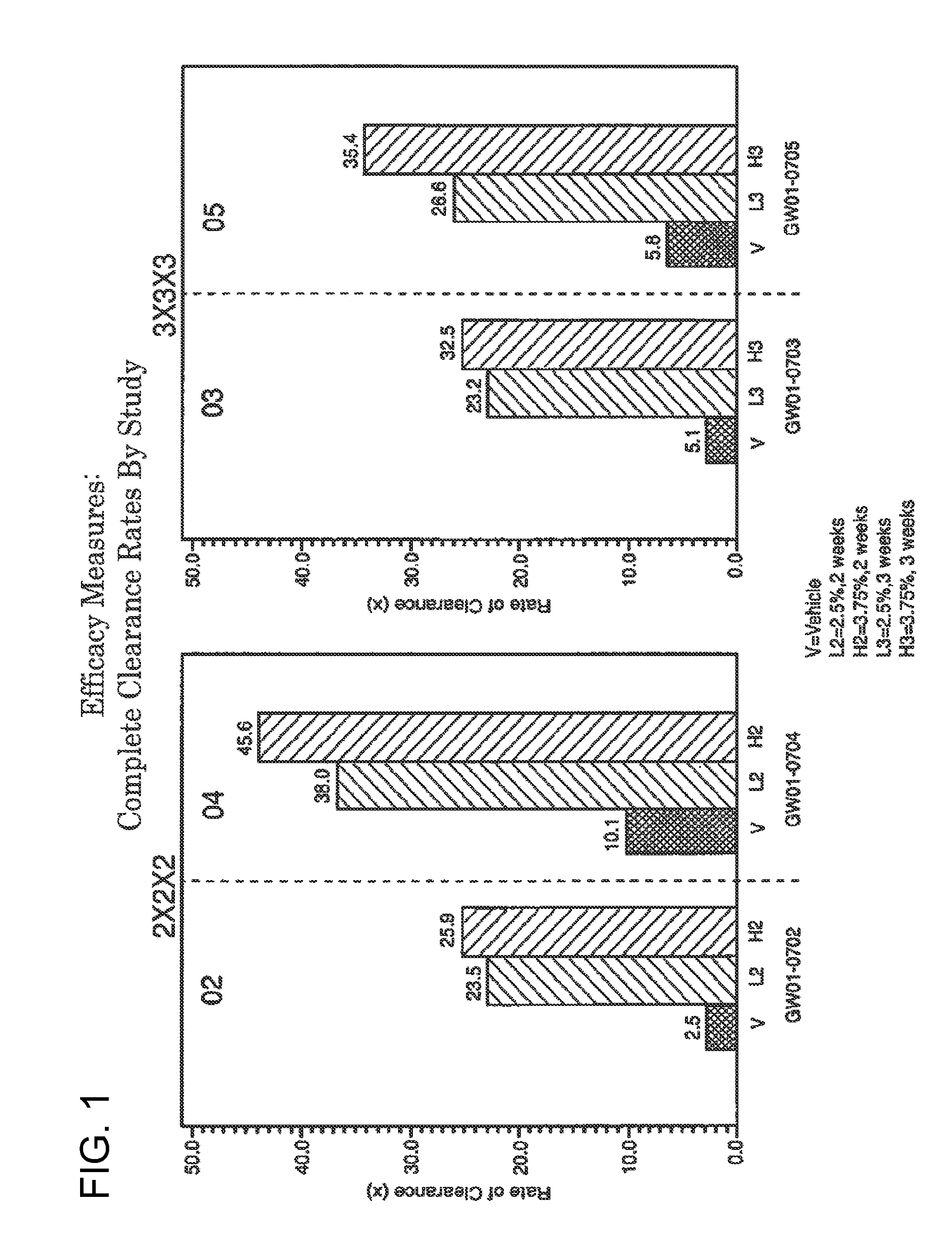
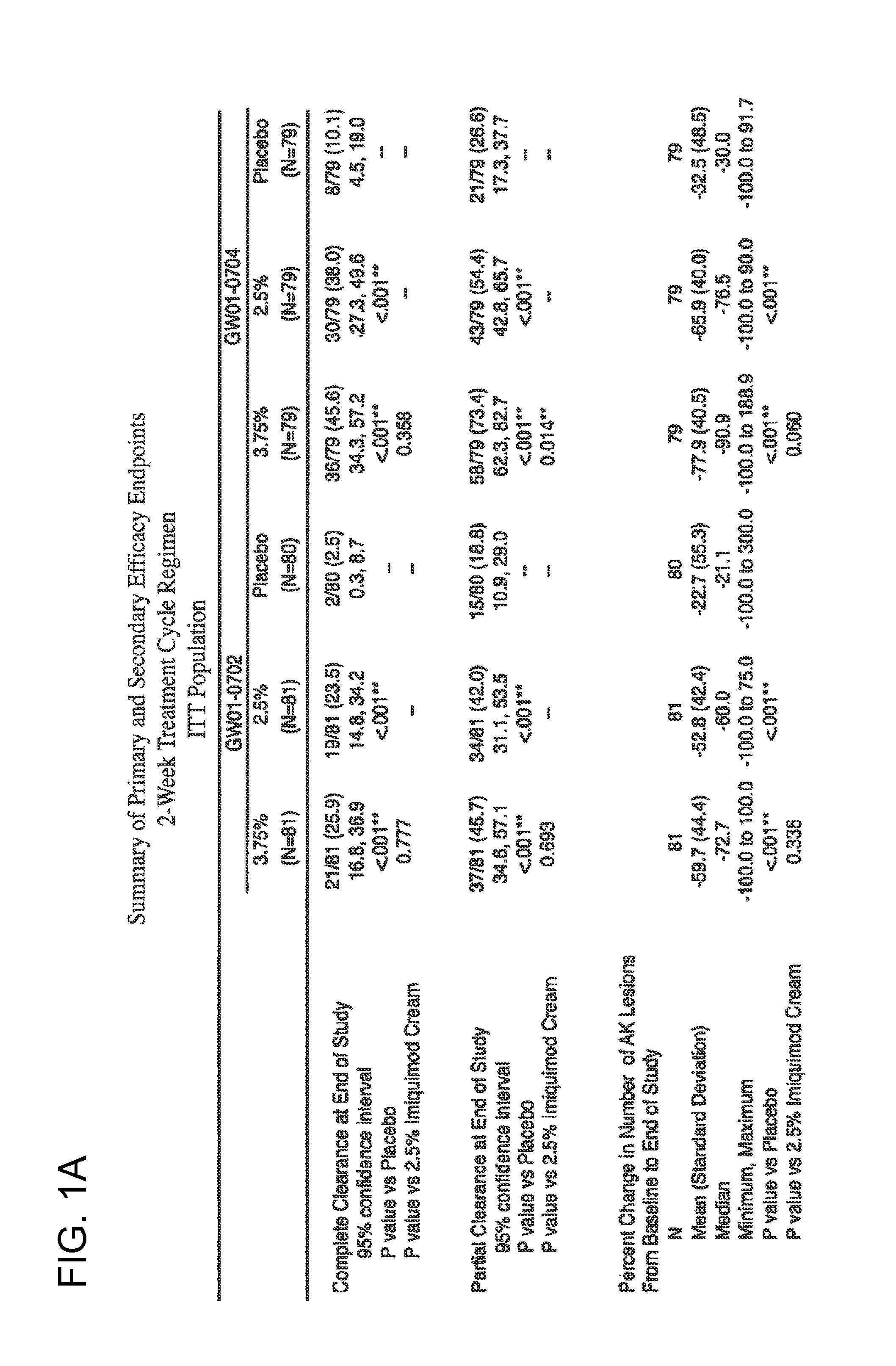
Lower dosage strength pharmaceutical compositions forumlated with 3.75% imiquimod
a technology of 3.75% and 3.75%, applied in the field of pharmaceutical formulations, can solve the problems of cellular damage even reaching the dermis, adult acne, and aging, and achieve the effect of simplified dosing regimens and low dosage strength imiquimod
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0302]A cream according to the present invention is prepared from the following ingredients:
% by WeightAmountOil Phase1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-1.040.0 gamineIsostearic acid10.0400.0 g Benzyl alcohol2.080.0 gCetyl alcohol2.288.0 gStearyl alcohol3.1124.0 g Polysorbate 602.55102.0 g Sorbitan monostearate0.4518.0 gAqueous PhaseGlycerin2.080.0 gMethylparaben0.2 8.0 gPropylparaben0.02 0.8 gPurified water76.483059.2 g
[0303]The materials listed above were combined according to the following procedure:
[0304]The glycerin, methylparaben, propylparaben and water were weighed into a 4 liter glass beaker then heated on a hot plate with stiffing until the parabens isostearic acid and 1-isobutyl-1H-imidazo[4,5-c]-quinolin-4-amine were weighed into an 8 liter stainless steel beaker and heated on a hot plate until the amine was in solution (the temperature reached 69° C.). The benzyl alcohol, cetyl alcohol, stearyl alcohol, polysorbate 60 and sorbitan monostearate were added to the is...
examples 2-9
[0305]Using the general method of Example 1, the cream formulations shown in Tables 1 and 2 are prepared.
TABLE 1% by WeightExample 2Example 3Example 4Example 5Oil Phase1-isobutyl-1H-imidazo-1.01.01.01.0[4,5-c]quinolin-4-amineIsostearic acid10.010.05.05.0Benzyl alcohol2.0Cetyl alcohol1.7Stearyl alcohol2.3Cetearyl alcohol6.06.06.0Polysorbate 602.552.552.552.55Sorbitan monostearate0.450.450.450.45Brij ™ 30a10.0Aqueous PhaseGlycerin2.02.02.02.0Methylparaben0.20.20.20.2Propylparaben0.020.020.020.02Purified water77.7877.7882.7872.78aBrij ™ 30 (polyoxyethylene(4) lauryl ether) is available from ICI Americas, Inc.
TABLE 2% by WeightExample 6Example 7Example 8Example 9Oil Phase1-isobutyl-1H-imidazo-1.01.01.01.0[4,5-c]quinolin-4-amineIsostearic acid10.025.010.06.0Benzyl alcohol2.02.0Cetyl alcohol2.21.7Stearyl alcohol3.12.3Cetearyl alcohol6.06.0Polysorbate 602.553.42.552.55Sorbitan monostearate0.450.60.450.45Brij ™ 30a10.0Aqueous PhaseGlycerin2.02.02.02.0Methylparaben0.20.20.20.2Propylparaben0....
example 10
[0306]A cream according to the present invention is prepared from the following ingredients in the following Table 3:
TABLE 3% by WeightAmountOil Phase1-isobutyl-1H-imidazo[4,5-1.03.00 gc]quinolin-4-amineIsostearic acid5.015.0 gWhite petrolatum15.045.0 gLight mineral oil12.838.4 gAluminum stearate8.024.0 gCetyl alcohol4.012.0 gWitconol ™ 14a3.09.00 gAcetylated lanolin1.0 3.0 gPropylparaben0.0630.19 gAqueous PhaseVeegum ™ Kb1.0 3.0 gMethylparaben0.120.36 gPurified water49.017147.05 g aWitconol ™ 14 (polyglyceryl4 oleate) is available from Witco Chemical Corp. Organics DivisionbVeegum ™ K (colloidal magnesium aluminum silicate) is available from R. T. Vanderbilt Company Inc.
[0307]The materials listed above were combined according to the following procedure:
[0308]The 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine and the isostearic acid were weighed into a glass jar and heated with occasional stirring until the amine was dissolved (the temperature reached 68° C.). To this solution was add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com