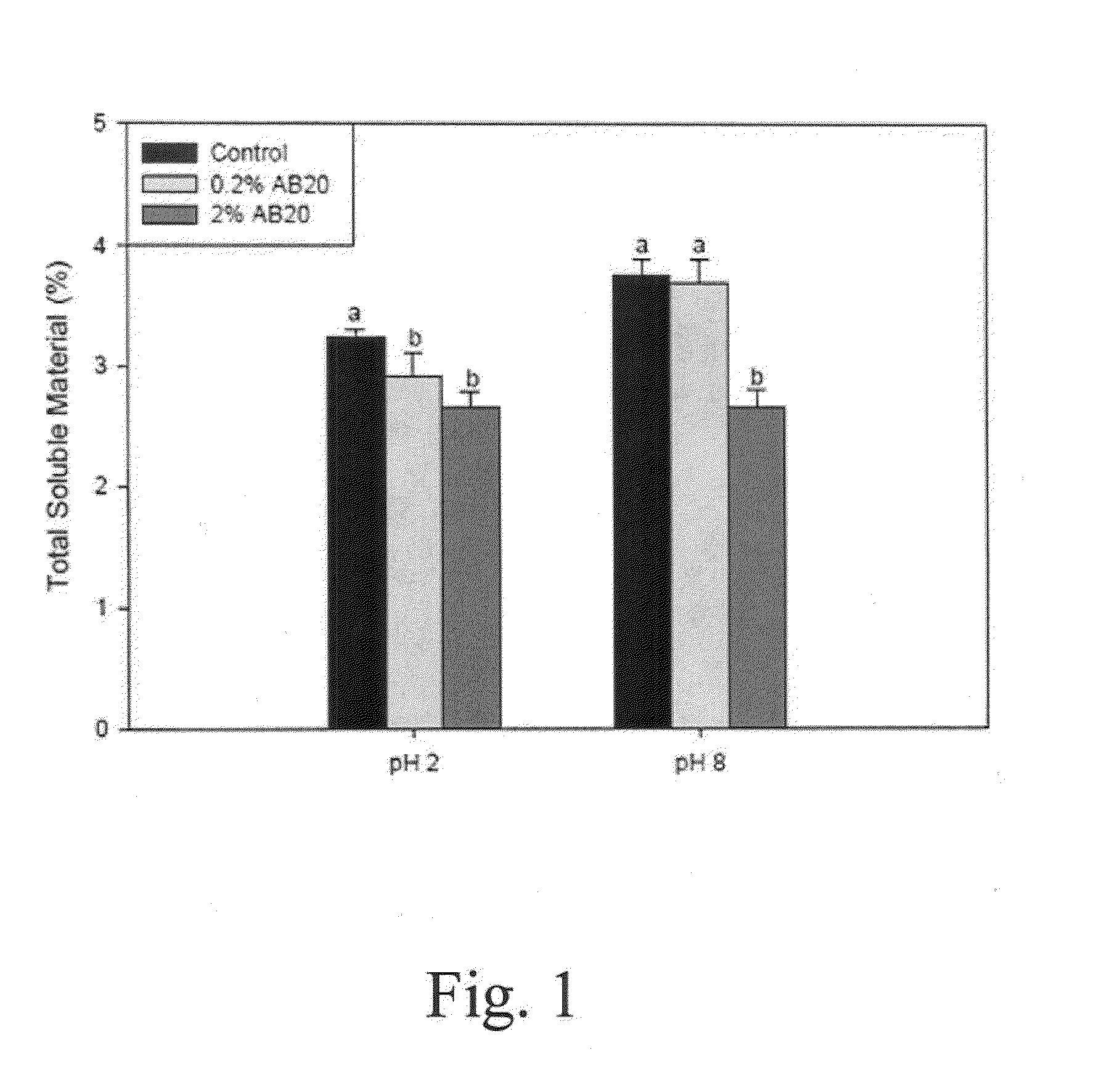
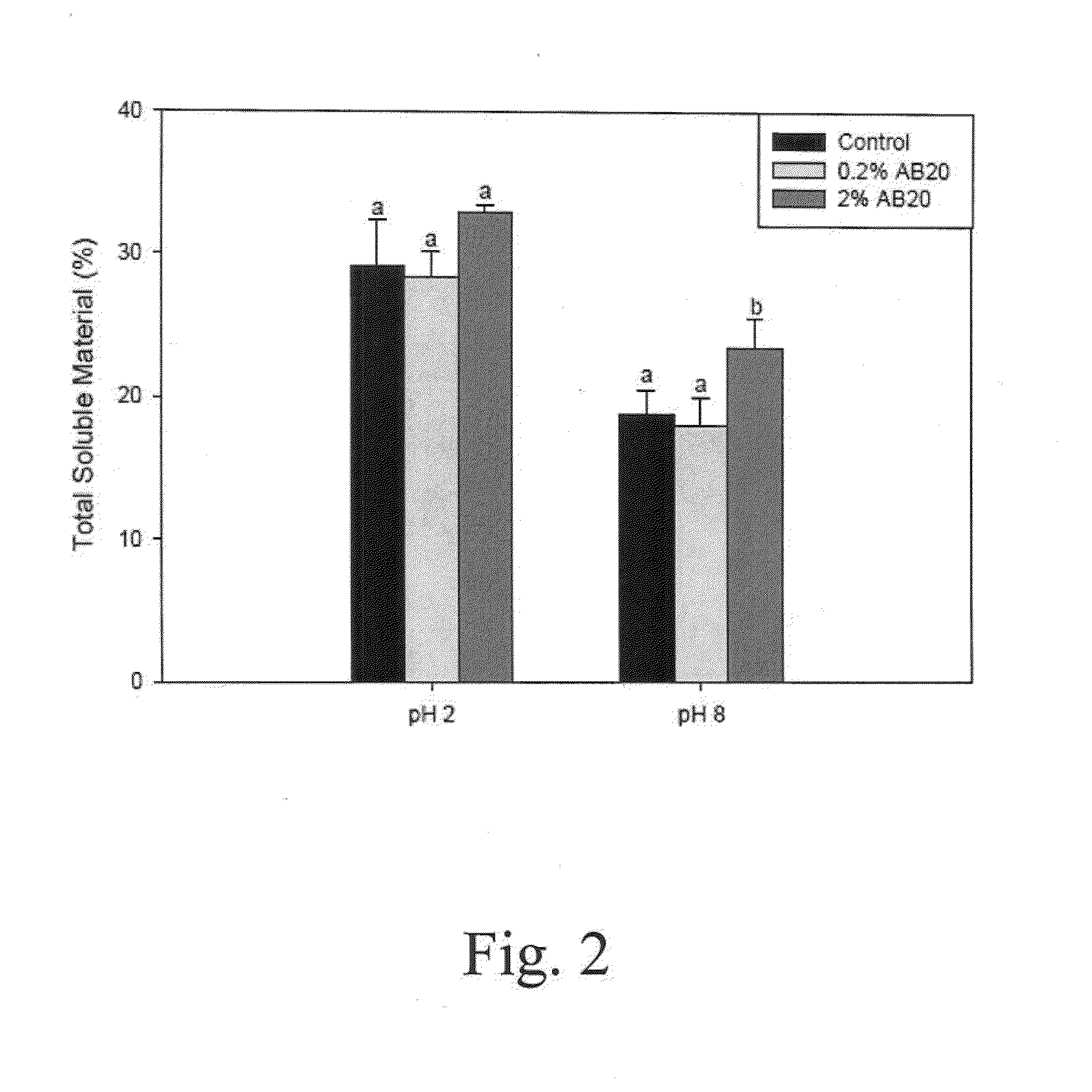
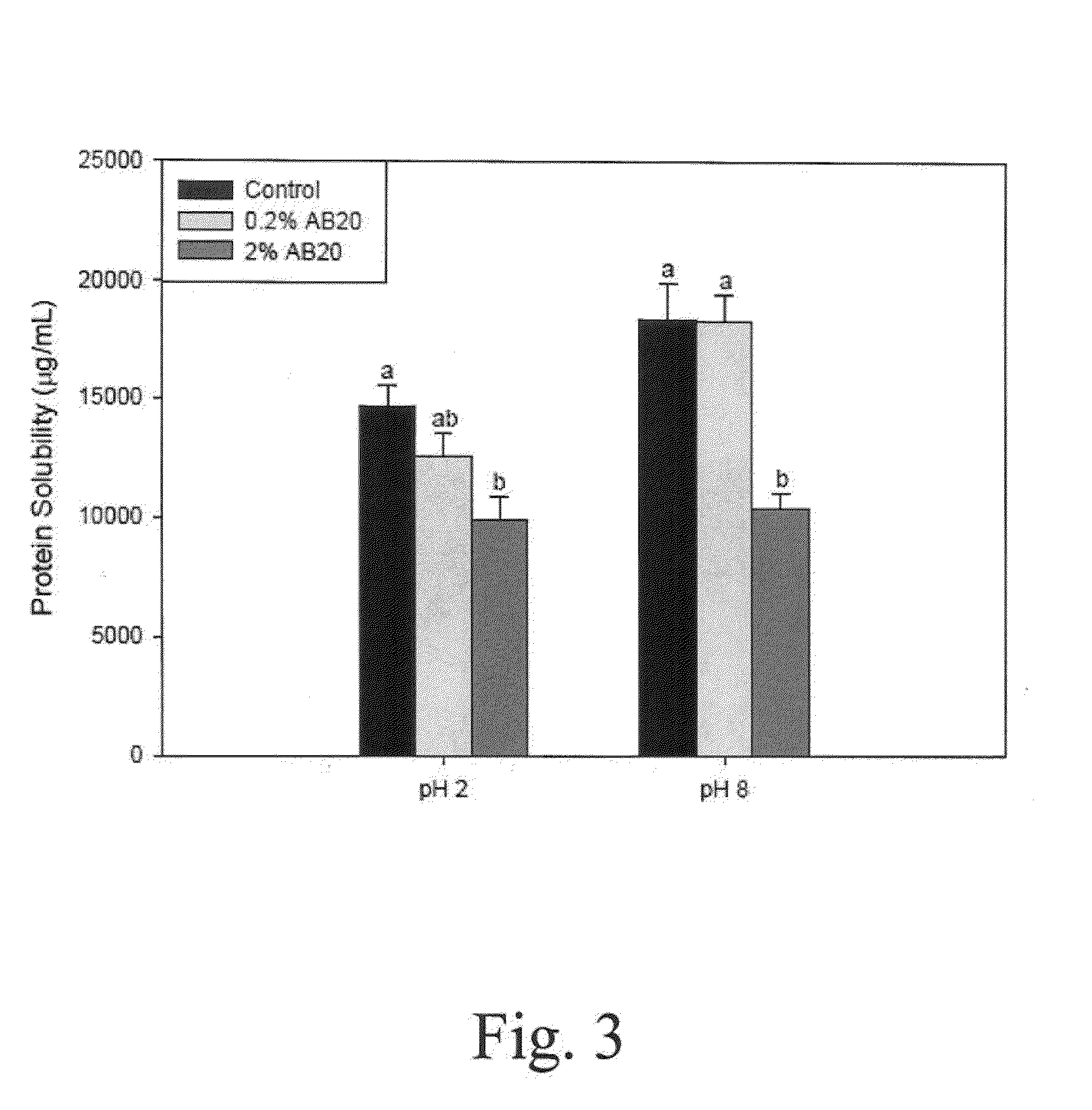
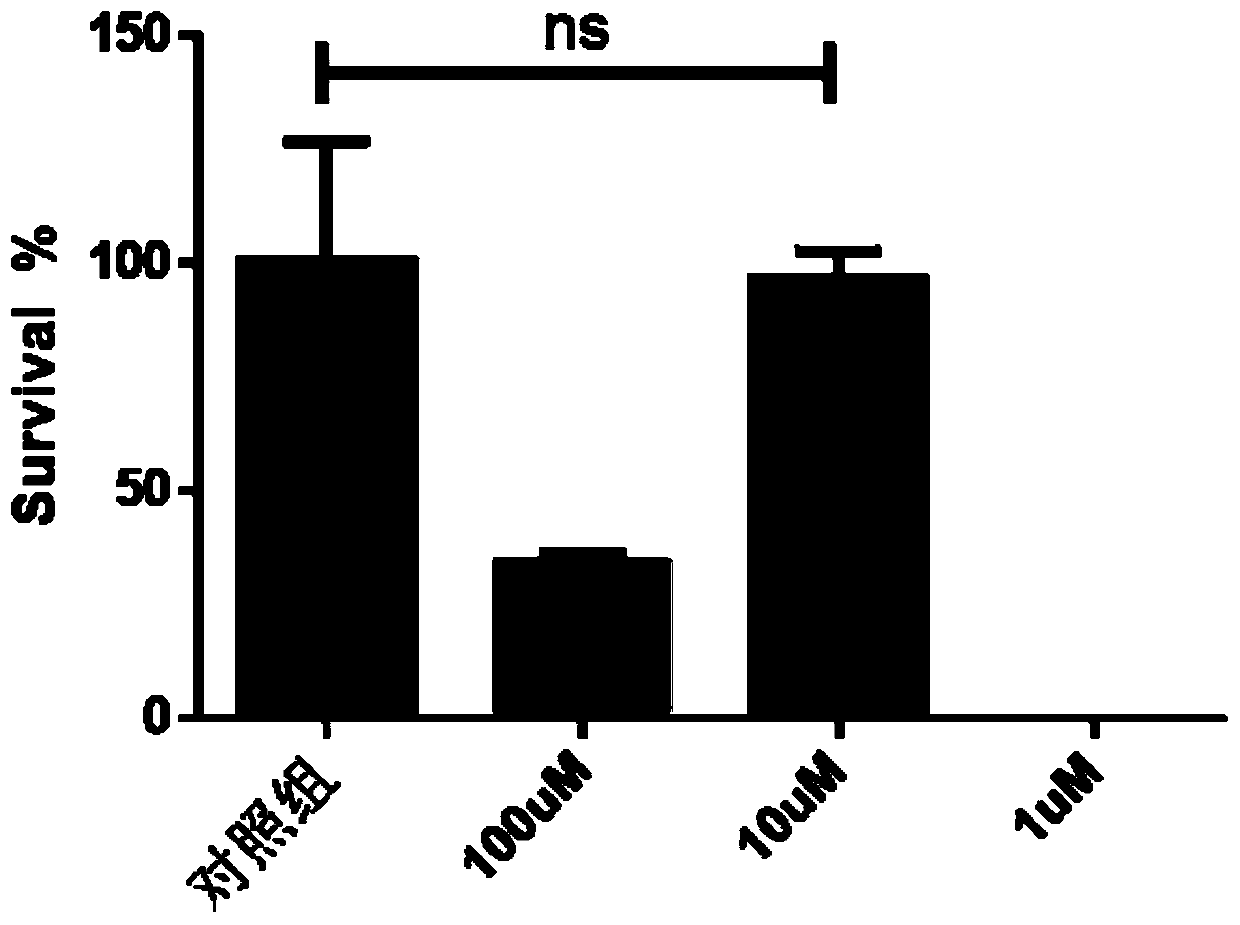
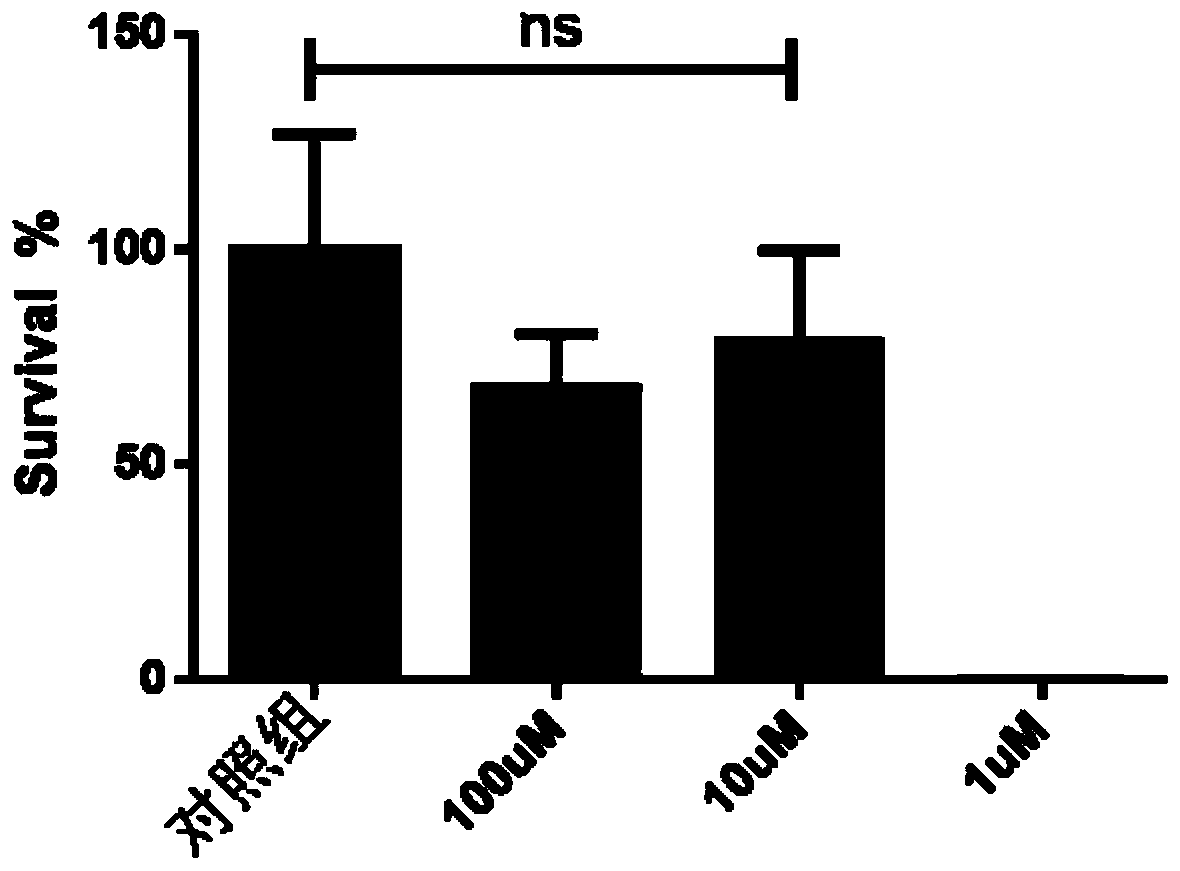
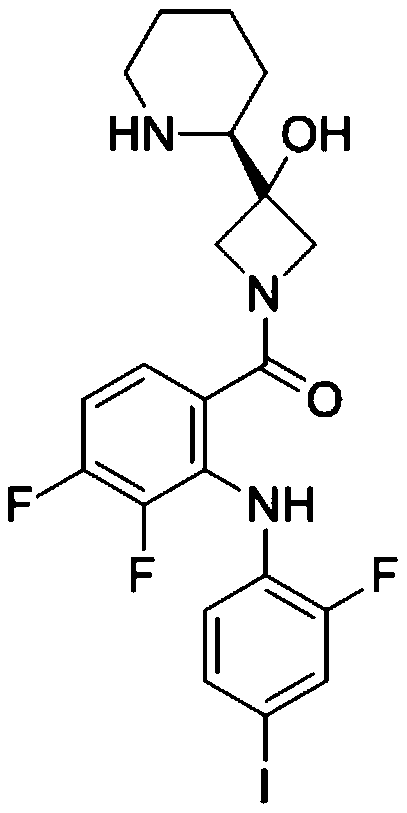
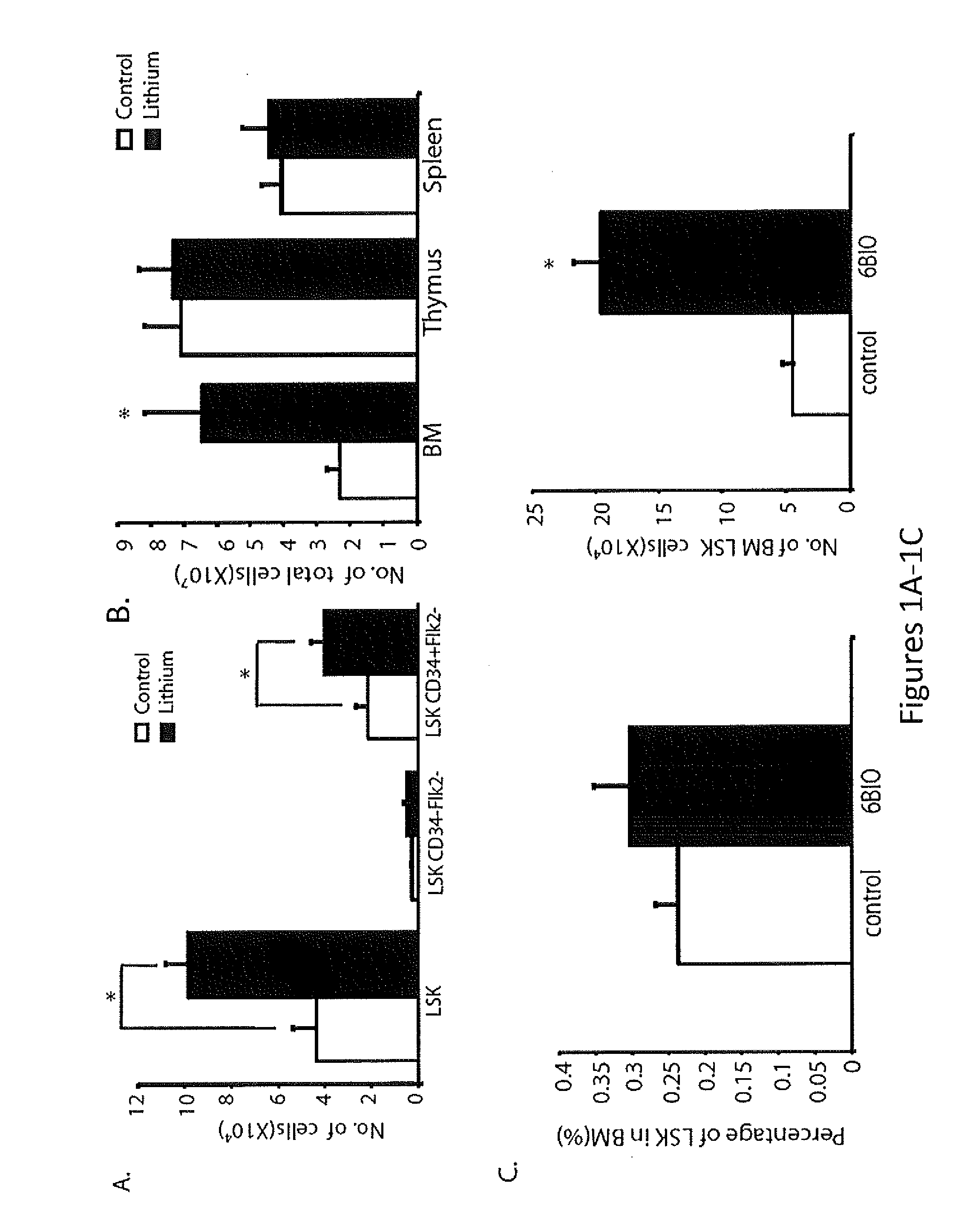
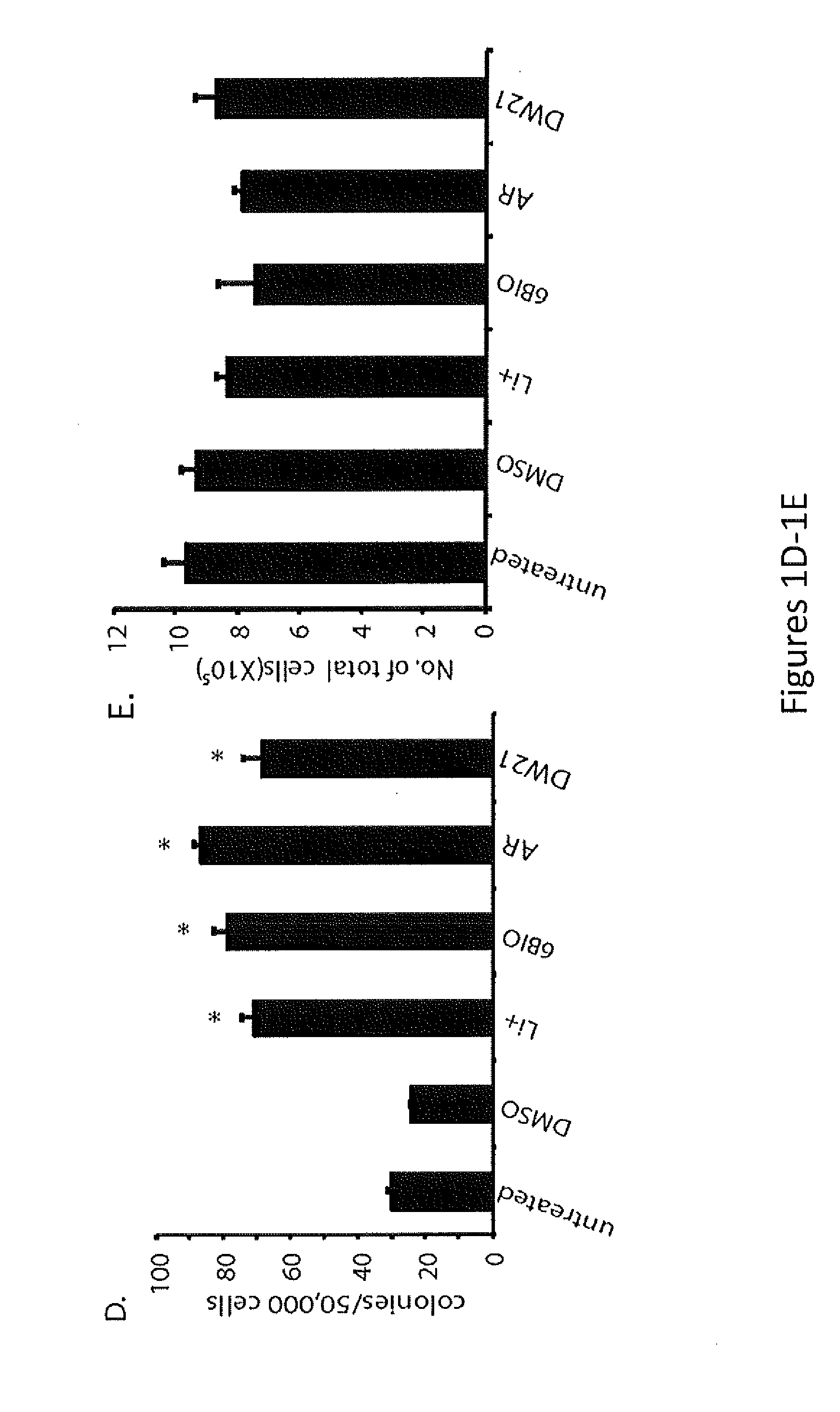
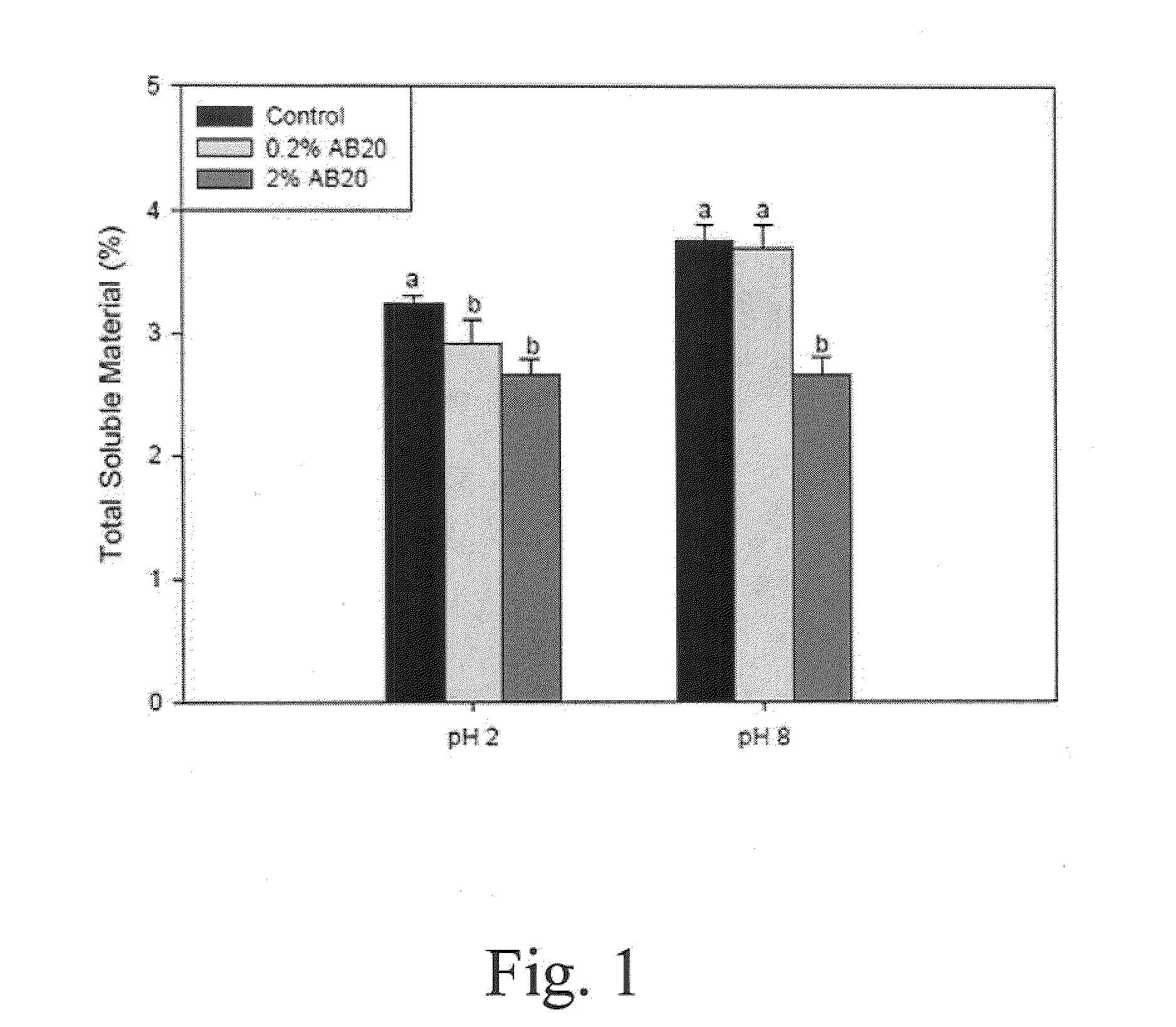
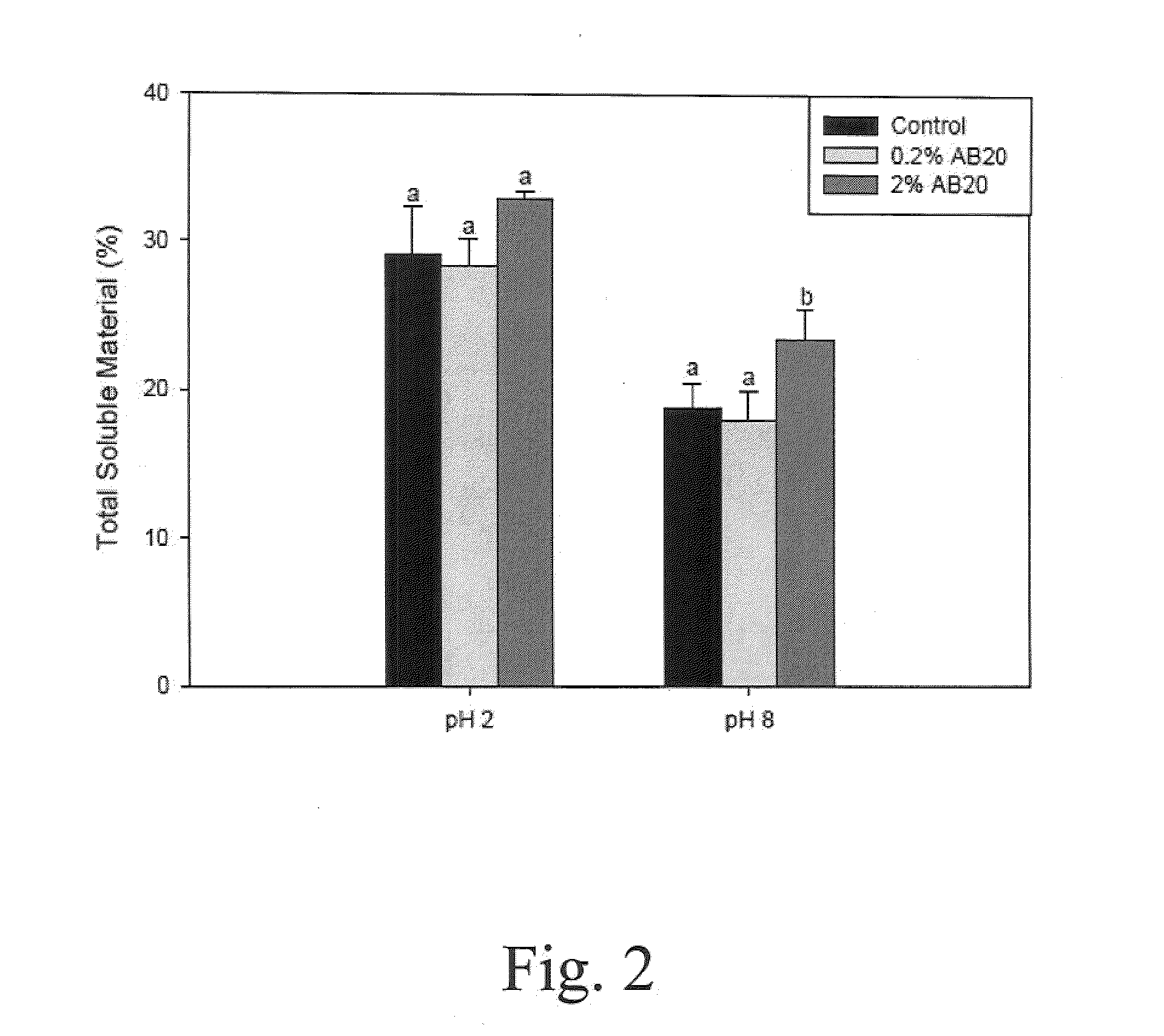
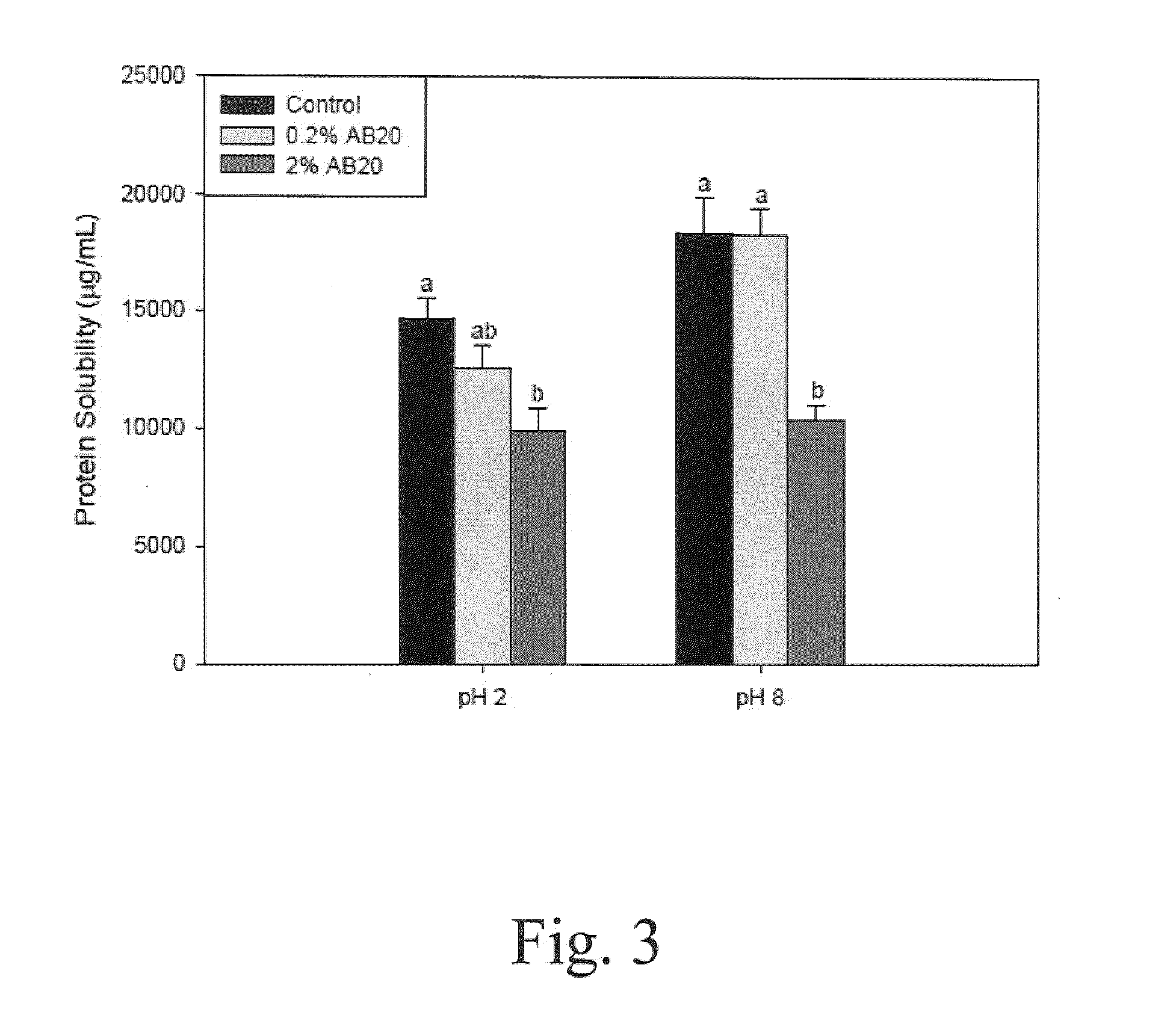
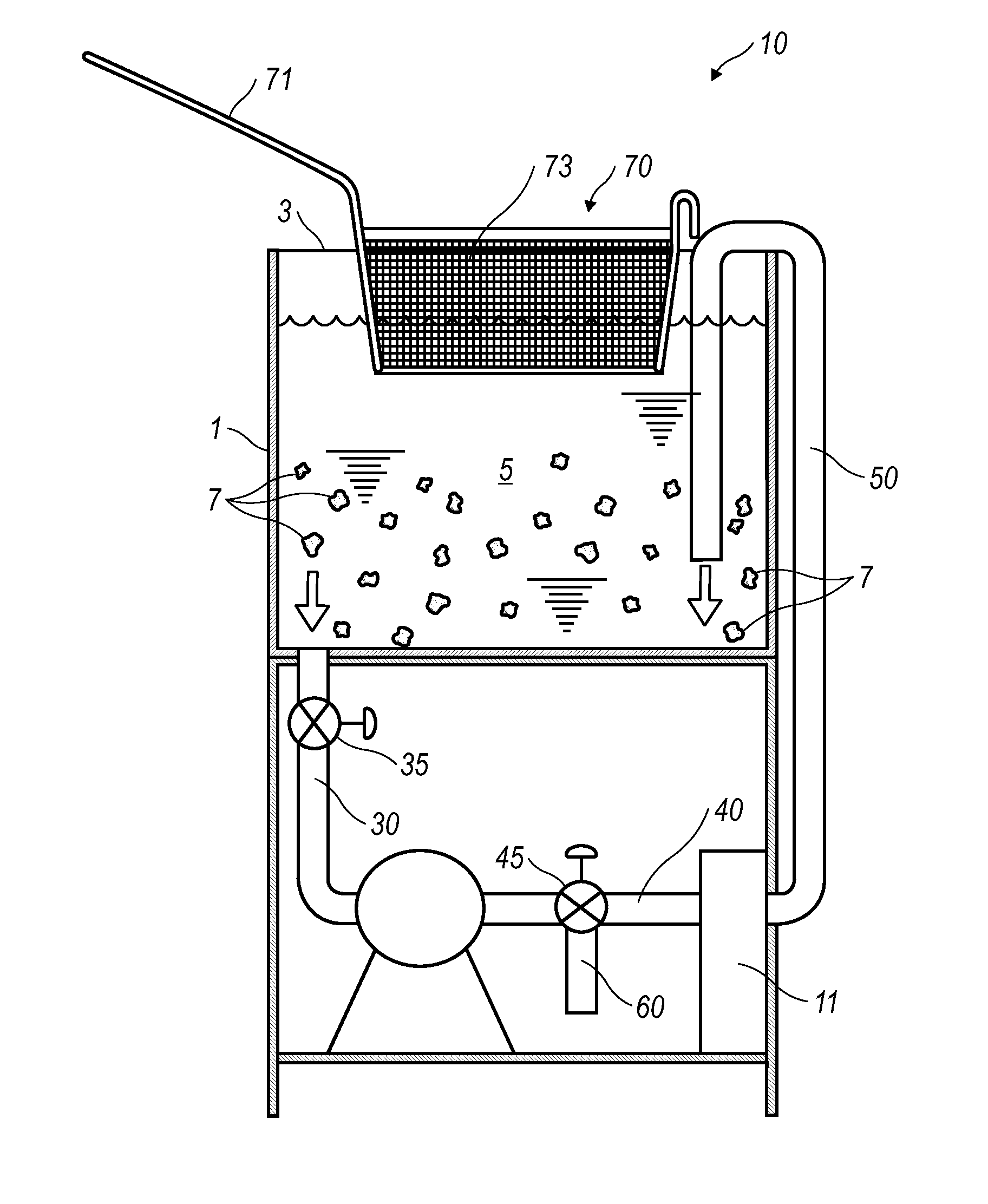
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
107 results about "Fda approval" patented technology
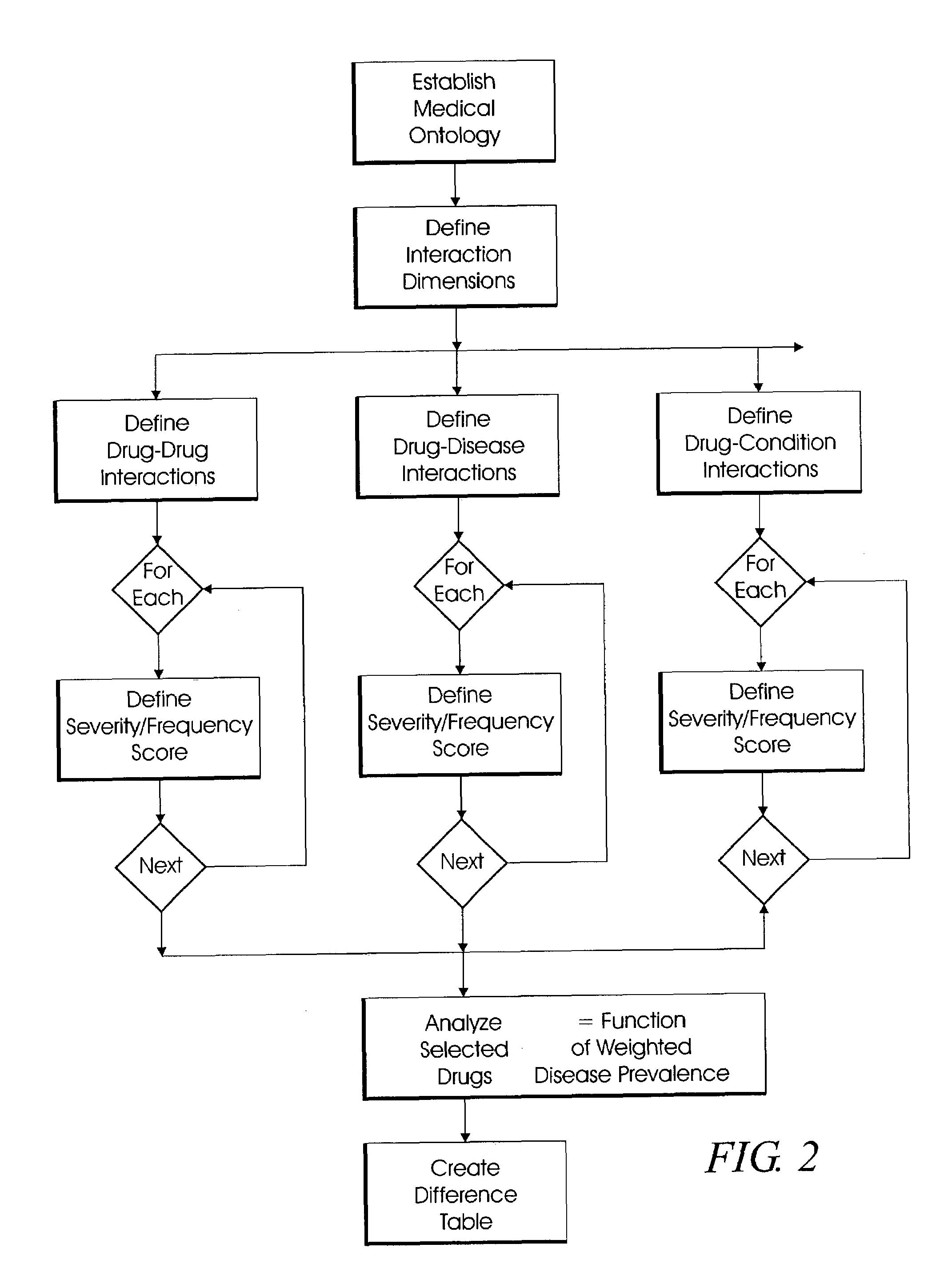
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
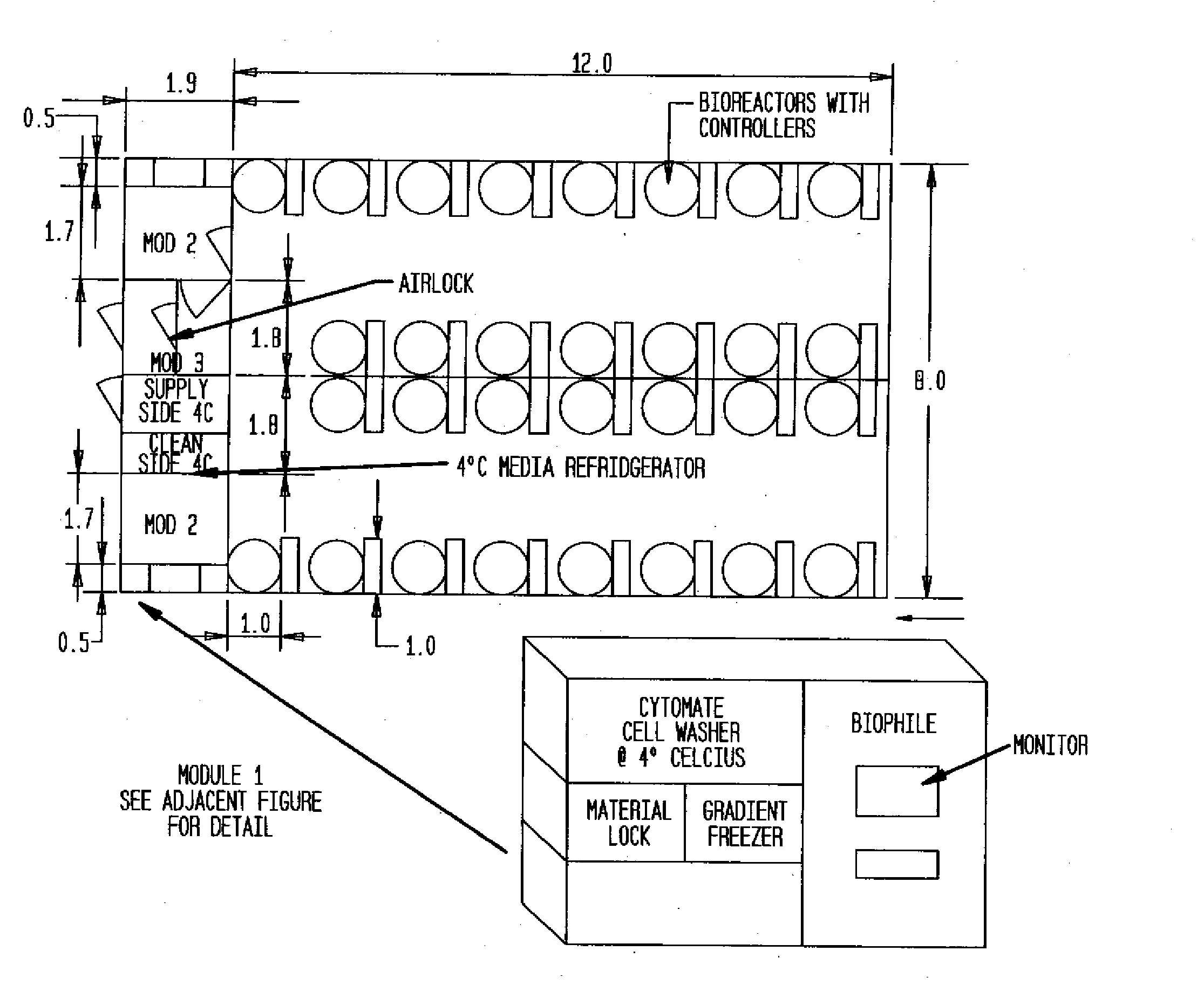
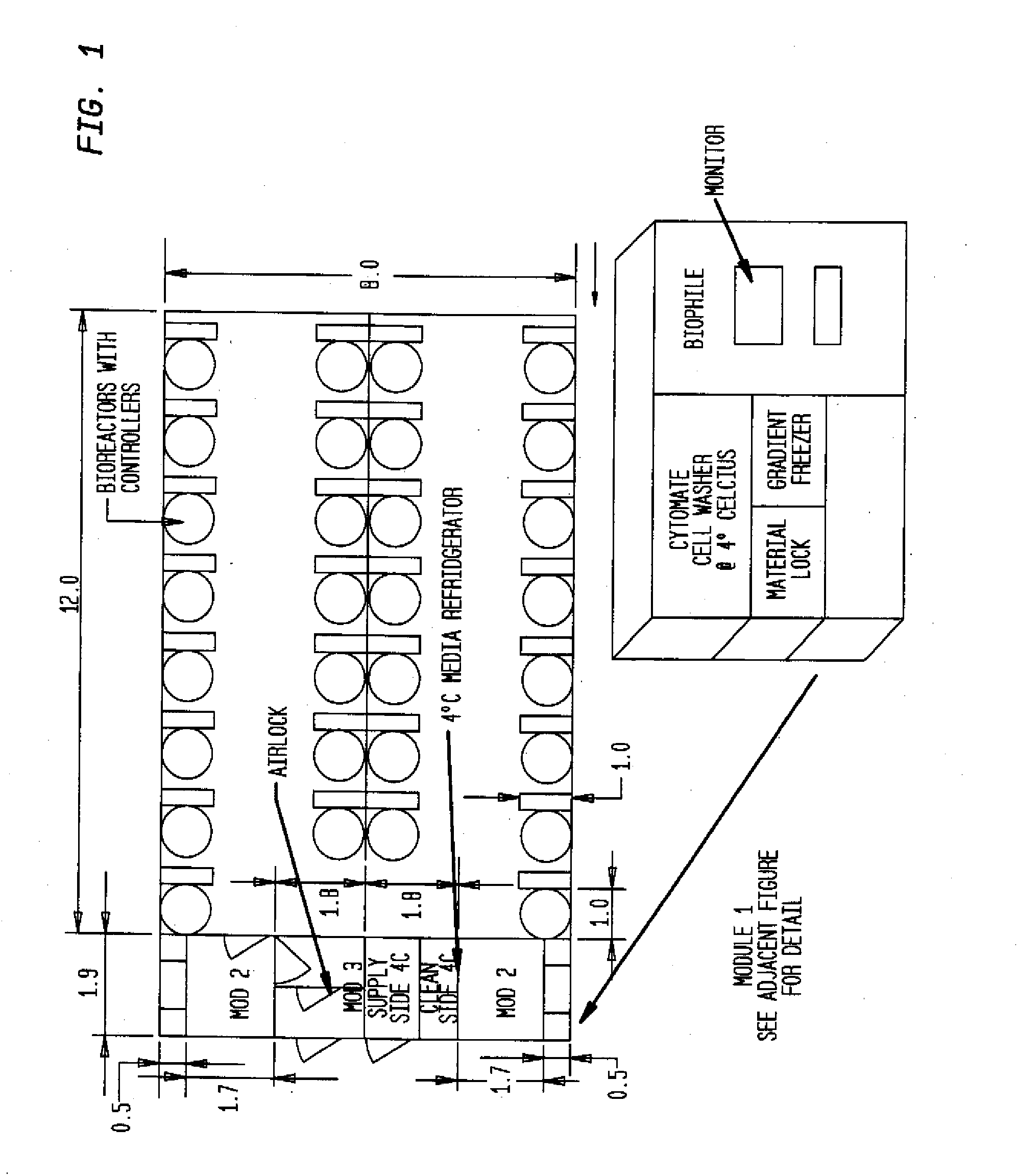
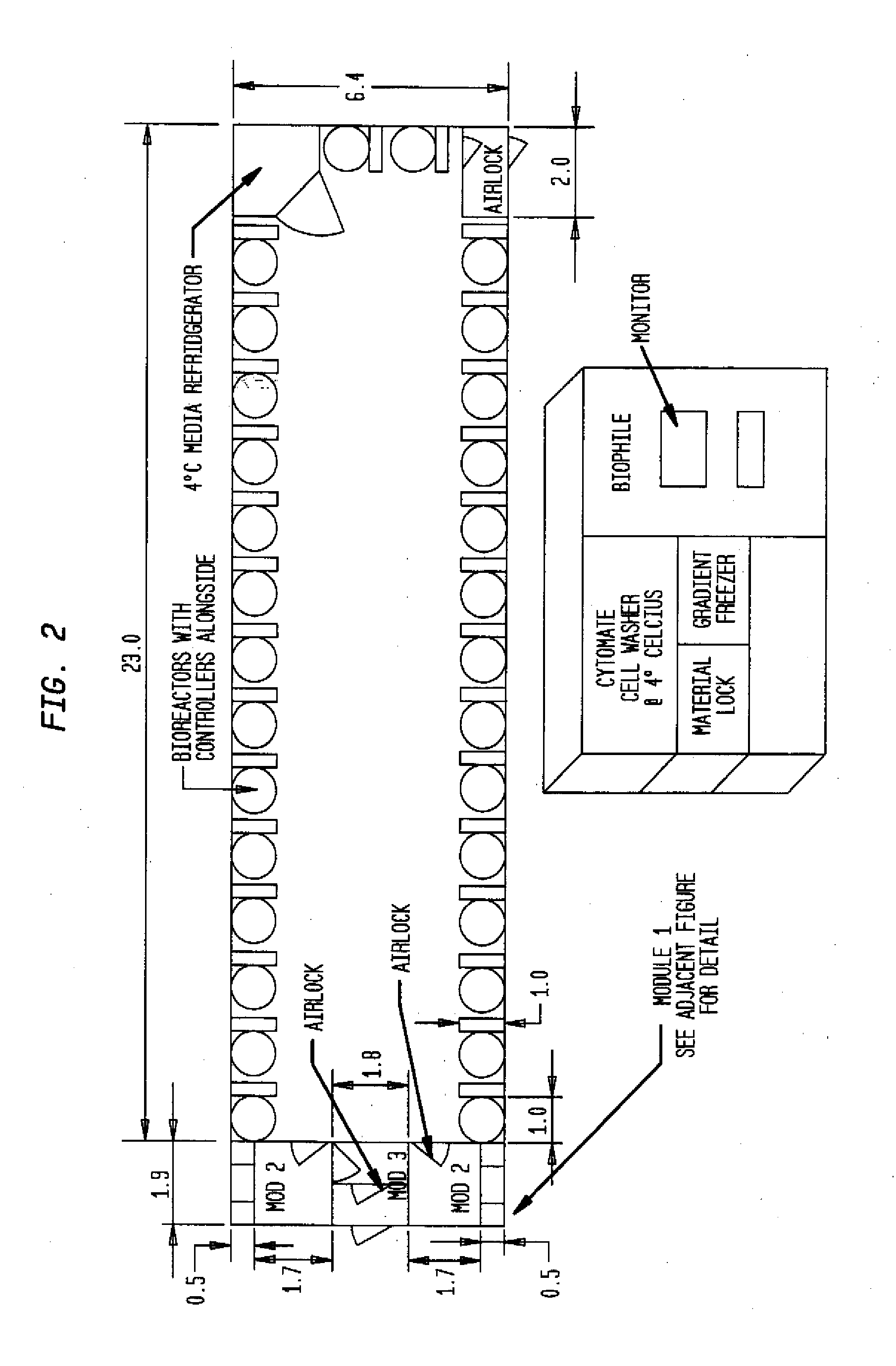
Prevalidated, modular good manufacturing practice-compliant facility
InactiveUS20090305626A1Precious timeShorten the timeApparatus sterilizationDust-free enclosuresInterior spaceGood laboratory practice
The invention is directed to a ready-to-use modular cleanroom and facility, in particular for the production of drugs and biological substances, which is equipped with pre-approved manufacturing equipment cores. The modular cleanroom is implemented in the interior space of a container, such as a standard shipping container, and includes at least one bioreactor station. The modular facility can be installed on-site from pre-approved cleanroom modules without further regulatory approval. The cleanroom and facility comply with FDA-approved good manufacturing practices (GMP) and good laboratory practices (GLP).
Owner:HOPE ERNEST G
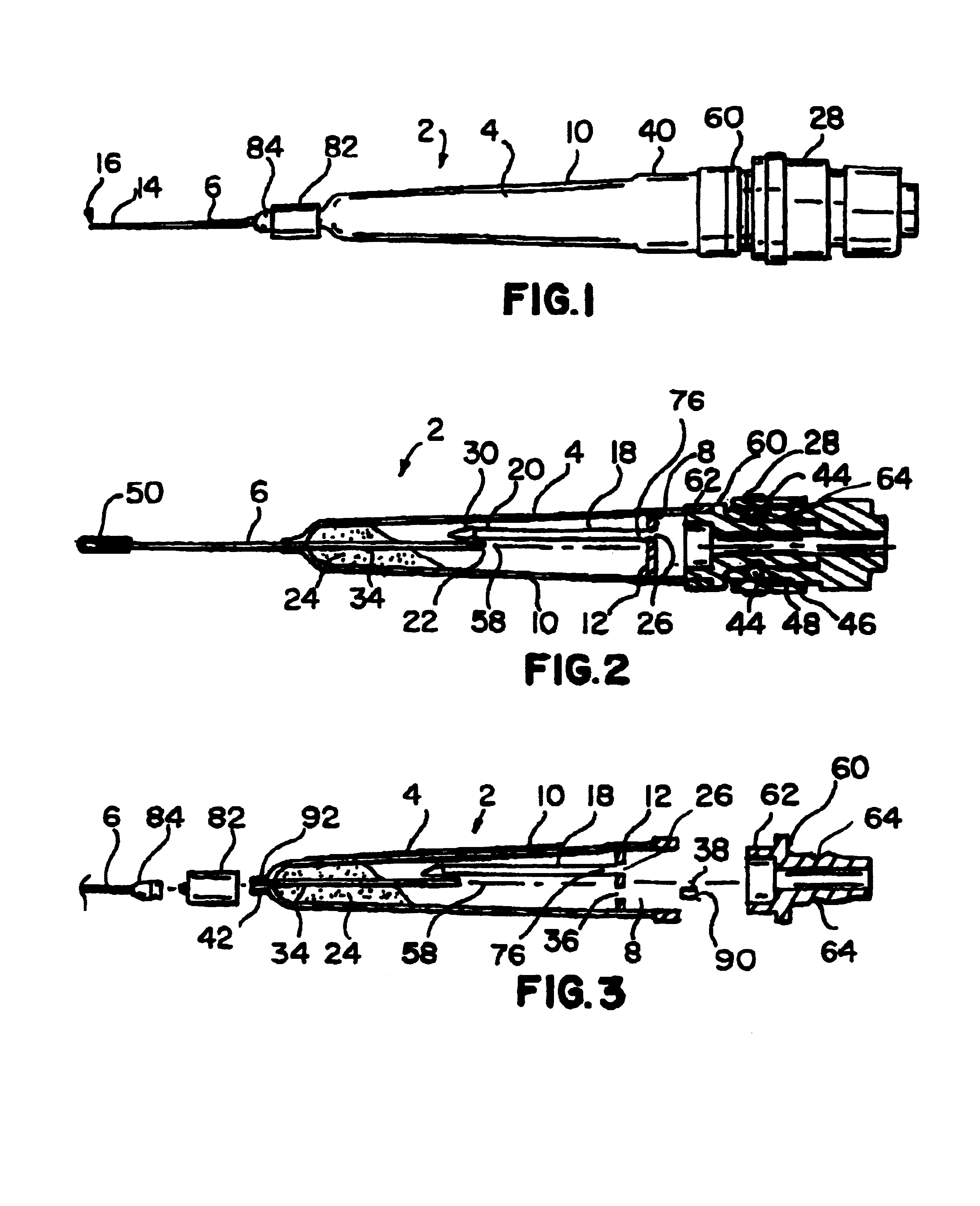
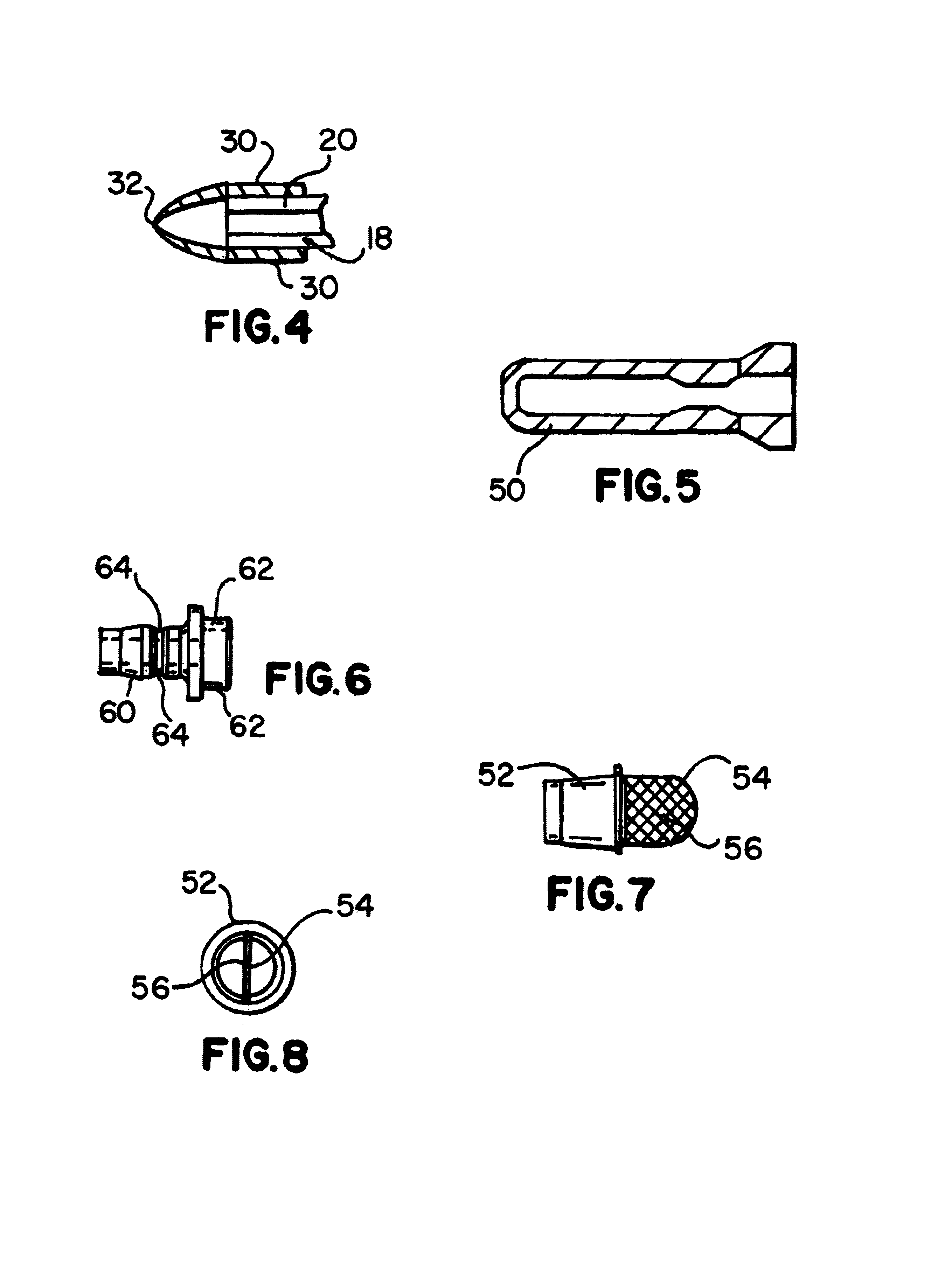
Surgical retractor
ActiveUS20110301421A1Improve performanceImprove visibilitySurgical forcepsLess invasive surgeryImage resolution
A surgical retractor and a method of minimally invasive surgery, wherein the surgical retractor includes ribs and a mechanism for microns-resolution transferring of linear and rotational movements of the ribs and wherein each rib can be easily replaced without use of any additional tools.According to an embodiment of the present invention the transmission is simpler. Likewise, an auxiliary handle is added to facilitate the insertion of the ribs into the body of the operated patient, and the vast majority of components of the surgical retractor are made with biocompatible, and FDA approved material ULTEM HU 1000 RESIN transparent (radiolucent) to X-ray radiation and are designated for single use.
Owner:MENI MED
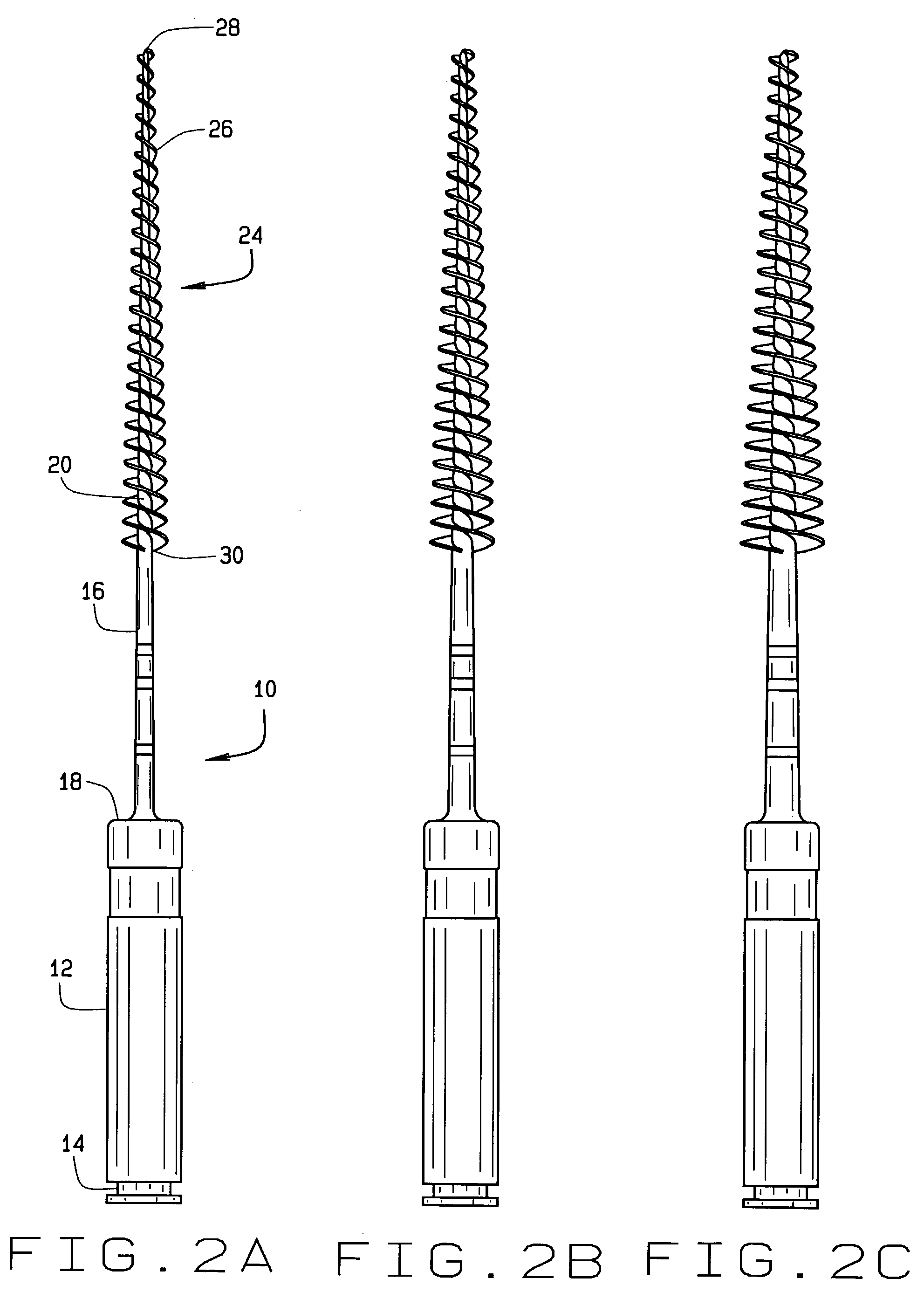
Injection molded endodontic brush
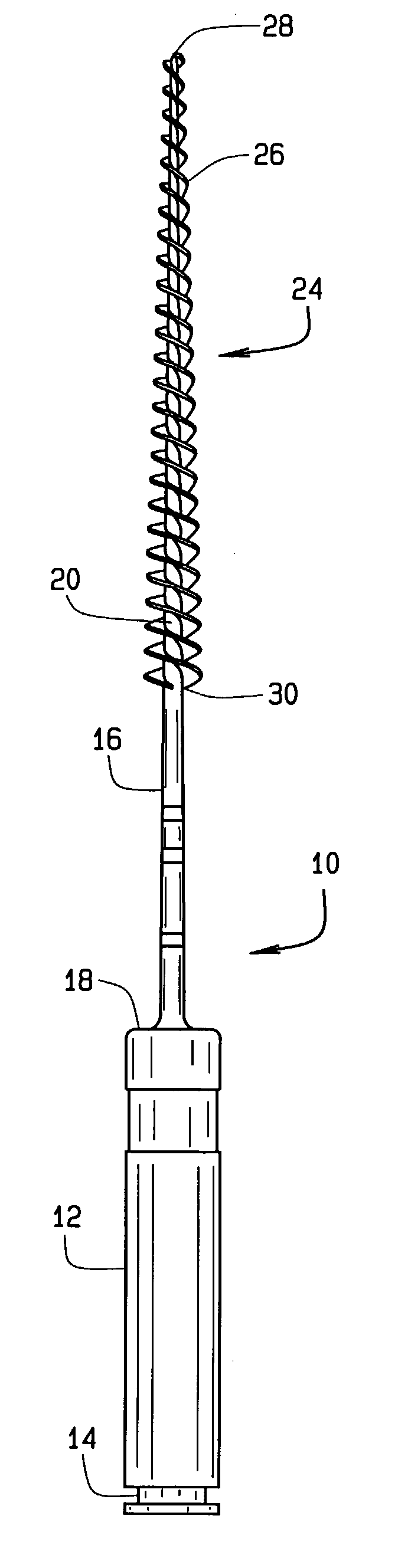
InactiveUS6981869B2Easy to disassembleAccurate diameterBrush bodiesWheelchairs/patient conveyanceBristleEngineering
Unitary, one-piece plastic molded micro-brushes are provided to remove the smear layer that remains in the root canal and access chamber after the pulp, bacteria, and related irritants have been mechanically and chemically removed from the root canal and access chamber using rotary cutting burs, files and various chemical reagents. The brushes include a handle, shaft or shank and a tapered brush section extending from the distal end of the shank. The brush section includes a plurality of bristles extending radially from a core. The bristles of the root canal brush can be formed in a reverse thread pattern. The brush section of the root canal brush has a diameter of between about 0.1 mm and about 0.2 mm at a tip end and a diameter of between about 0.5 mm and 3 mm at a coronal-most end. The brush section of the root canal brush is about 16 mm long, and has a taper of about 2% to about 12%. In the access chamber brush, the core can be formed as to be spherical, football-shaped, or tapered. The brushes are injection molded from an FDA approved resin.
Owner:CLIFFORD J RUDDLE D D S P C
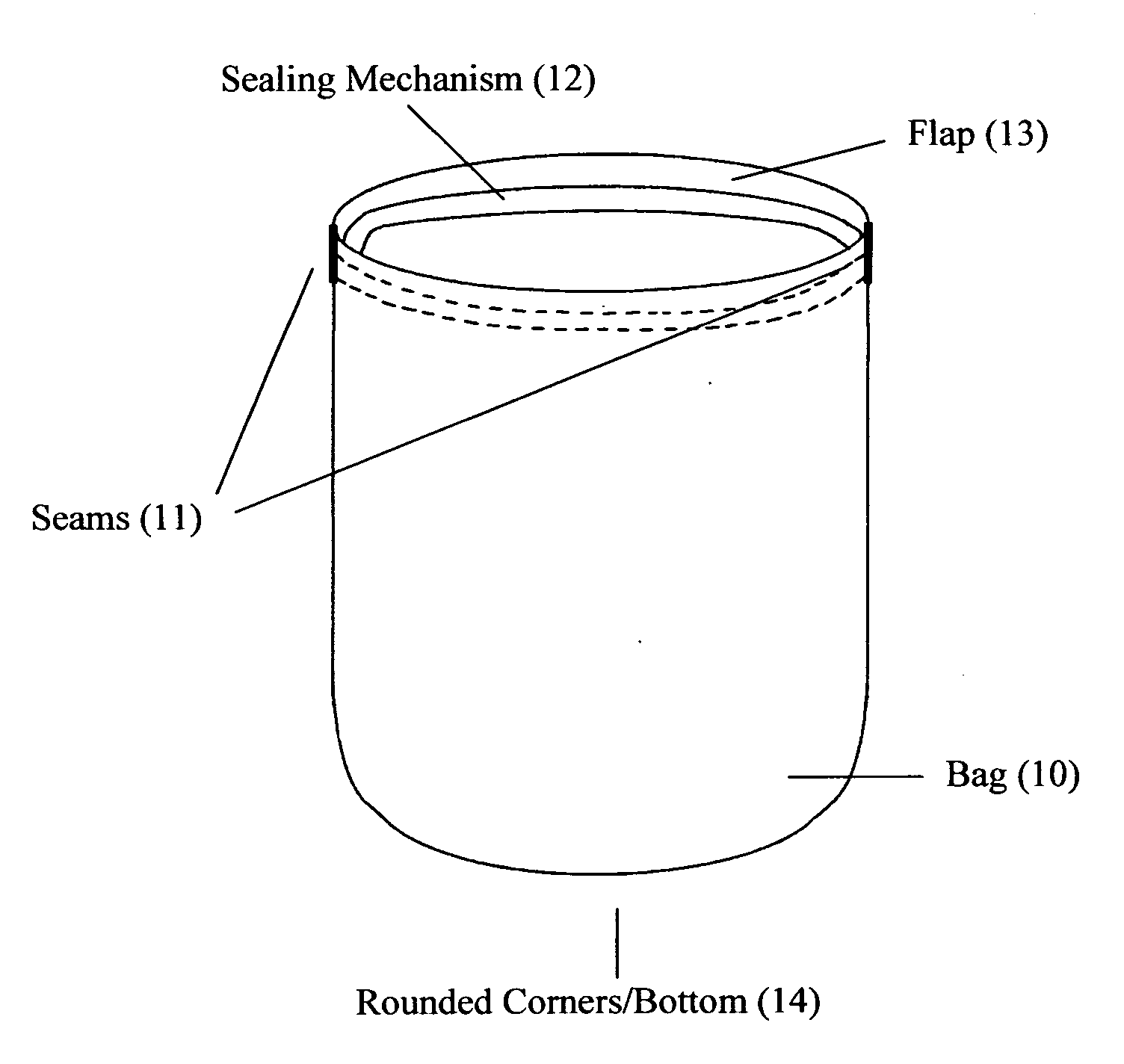
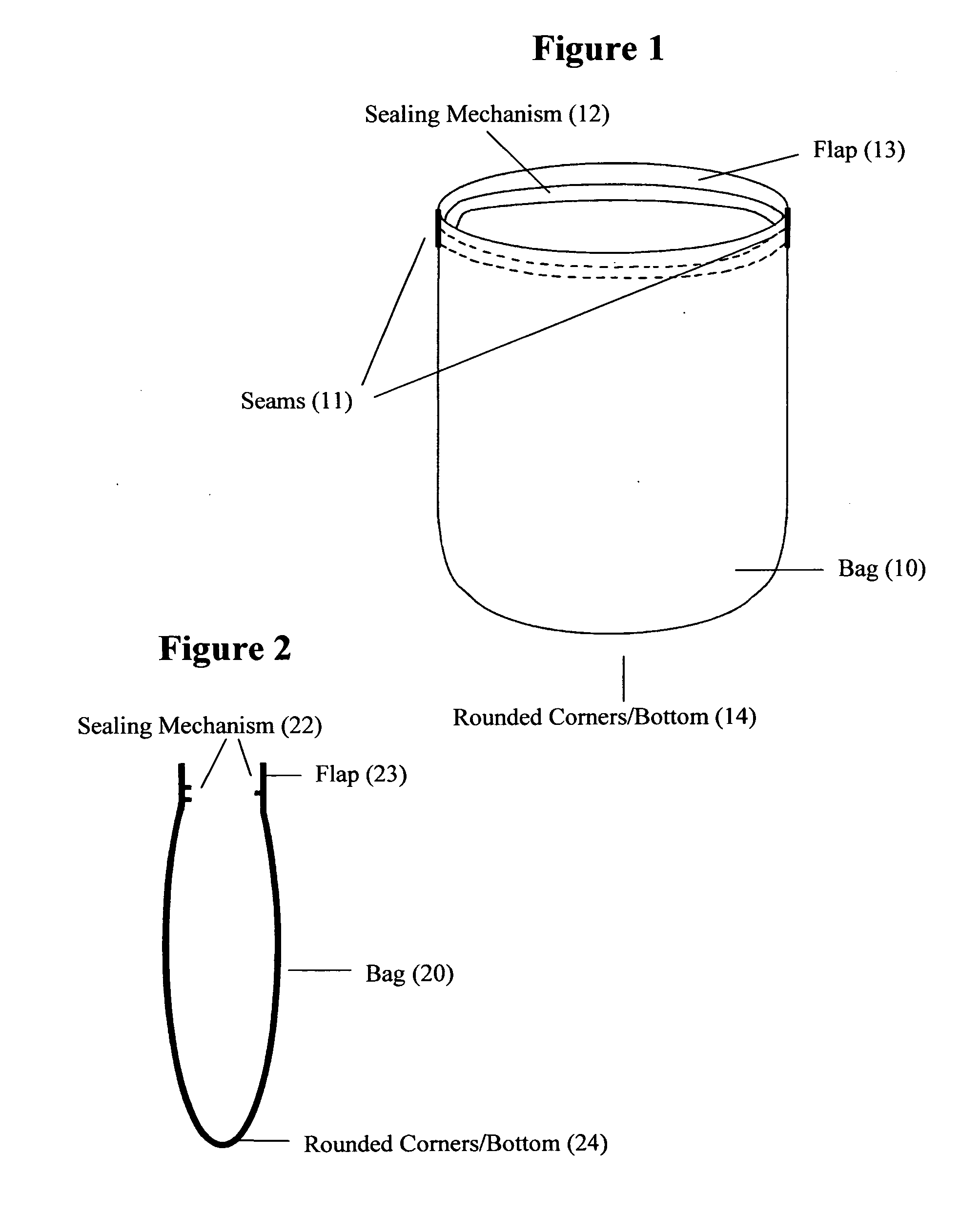
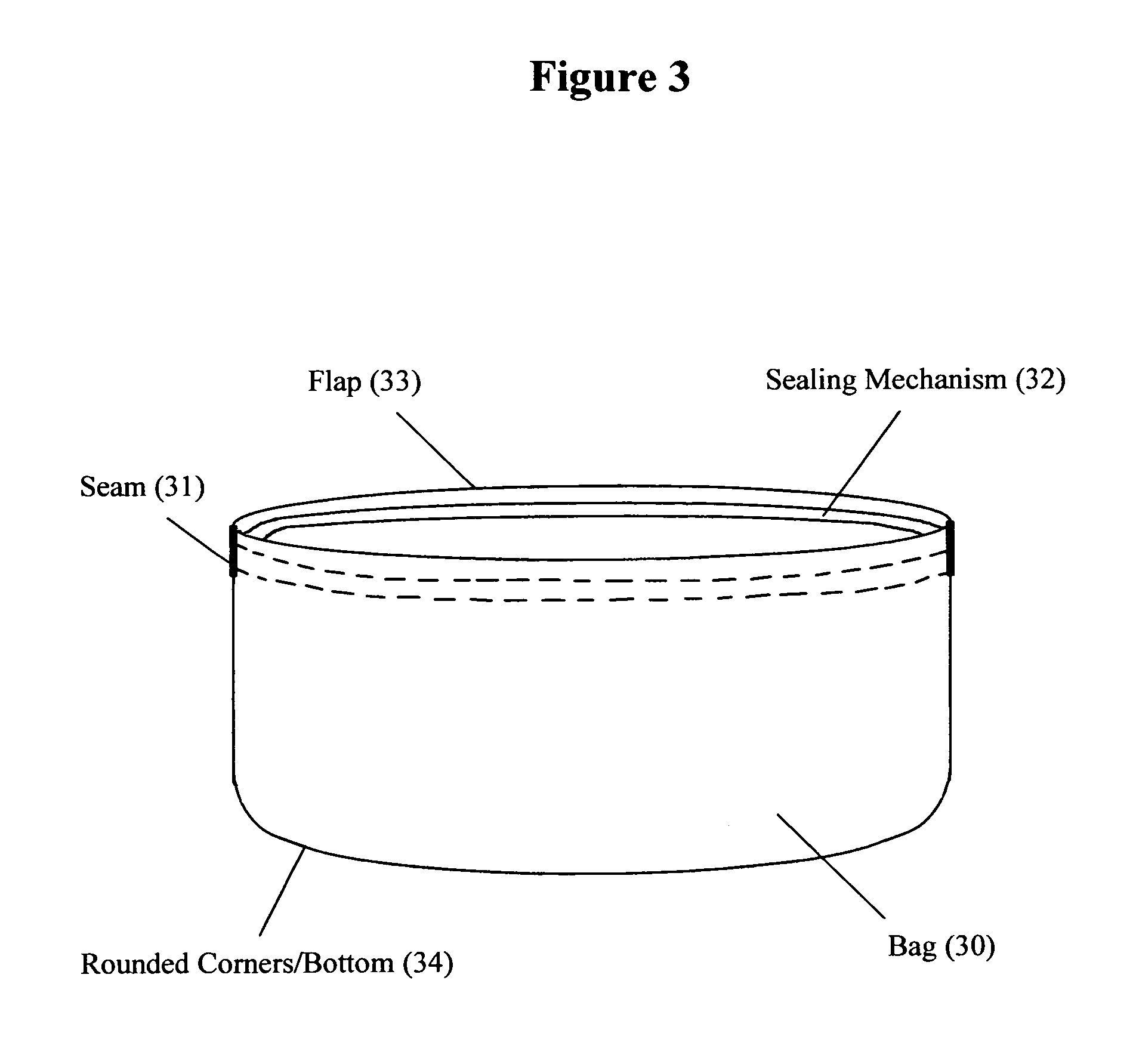
Reusable seamless multipurpose bag
InactiveUS20130105352A1Improve securityImprove reusabilityPackage recyclingBagsExtreme temperatureEngineering
A reusable bag (10) made of materials, such as an FDA approved food-grade silicone, which may or may not include fillers, that can withstand the extreme temperatures used in the entire food preparation, cooking, and cleaning processes, including, but not limited to, storing, freezing, defrosting, marinating, baking, and washing for reuse. The bag (10) may be seamless and may have rounded corners and / or a rounded bottom (14), which allows for reversibility for the purpose of cleaning and prevents the accumulation of food, bacteria, or other contaminants thereby ensuring the safety and reusability of the invention. This bag may be closed and opened repeatedly by a sealing mechanism (12) that allows for an air and water tight seal.
Owner:MUNGUIA MARK
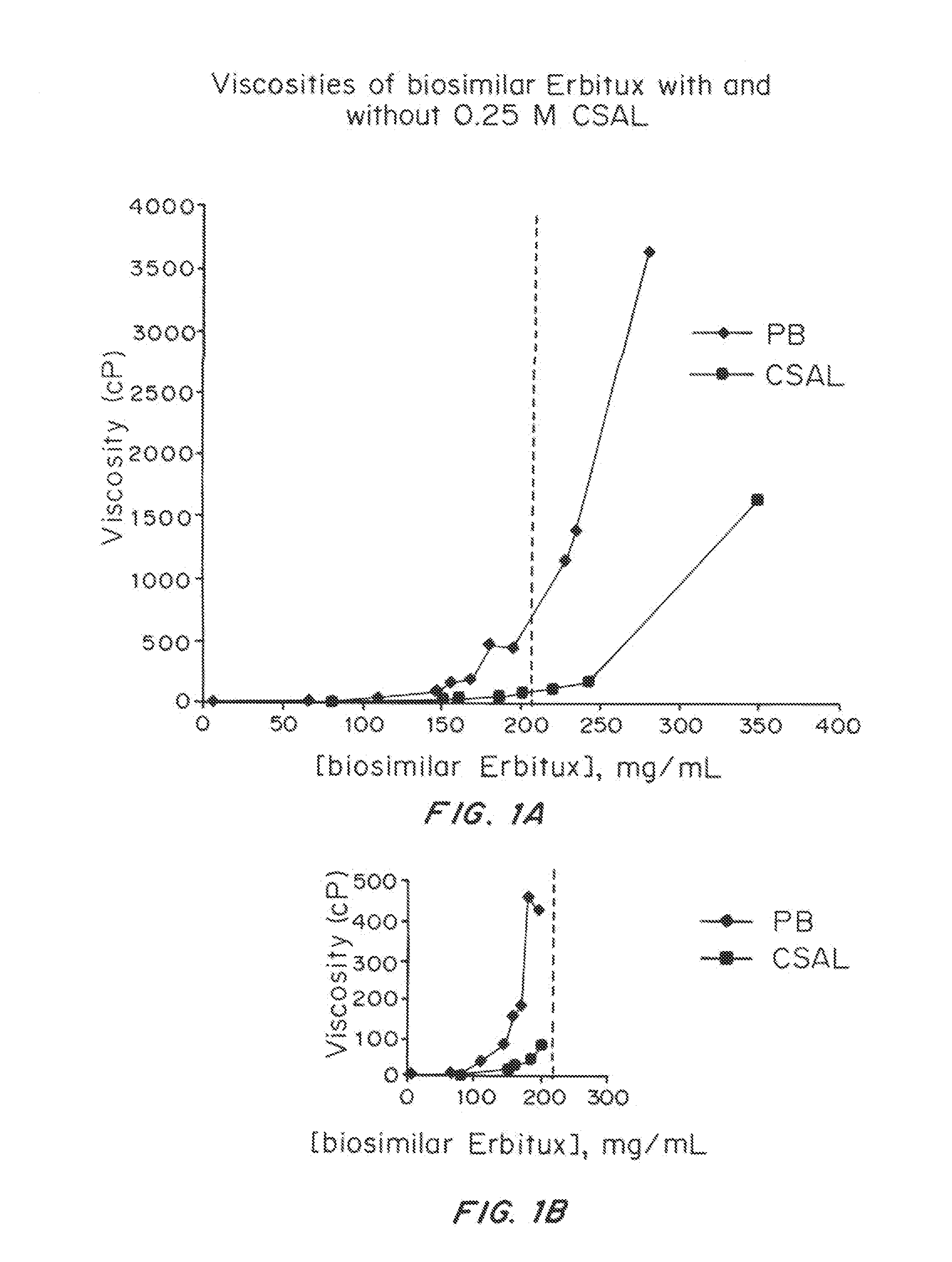
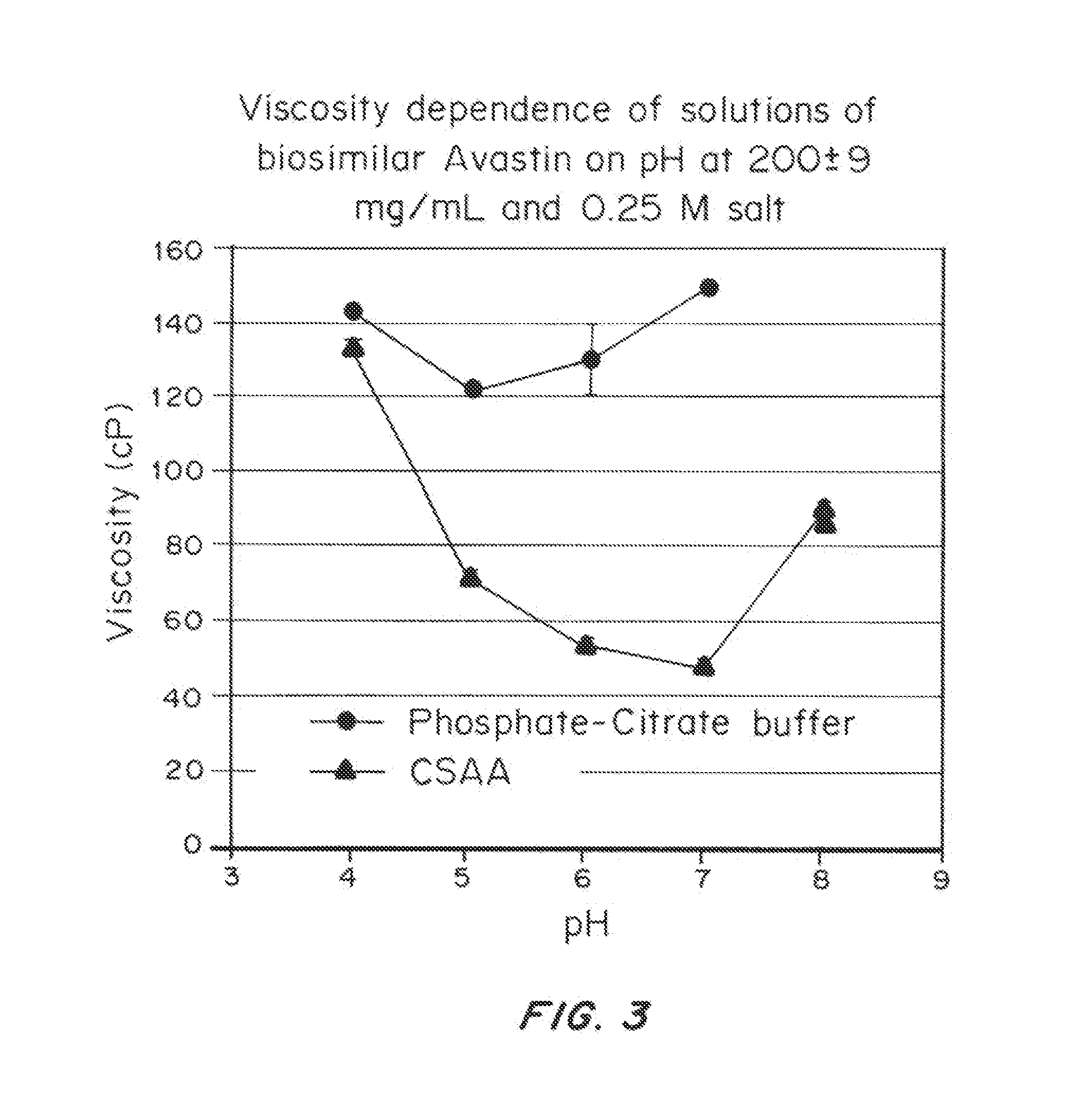
Liquid protein formulations containing viscosity-lowering agents
ActiveUS20150071925A1Facilitates and accelerates reconstitutionEasy to processNervous disorderPeptide/protein ingredientsIntramuscular injectionAdditive ingredient
Concentrated, low-viscosity, low-volume liquid pharmaceutical formulations of proteins have been developed. Such formulations can be rapidly and conveniently administered by subcutaneous or intramuscular injection, rather than by lengthy intravenous infusion. These formulations include low-molecular-weight and / or high-molecular-weight proteins, such as mAbs, and viscosity-lowering agents that are typically bulky polar organic compounds, such as many of the GRAS (US Food and Drug Administration List of compounds generally regarded as safe) and inactive injectable ingredients and FDA approved therapeutics.
Owner:EAGLE BIOLOGICS INC
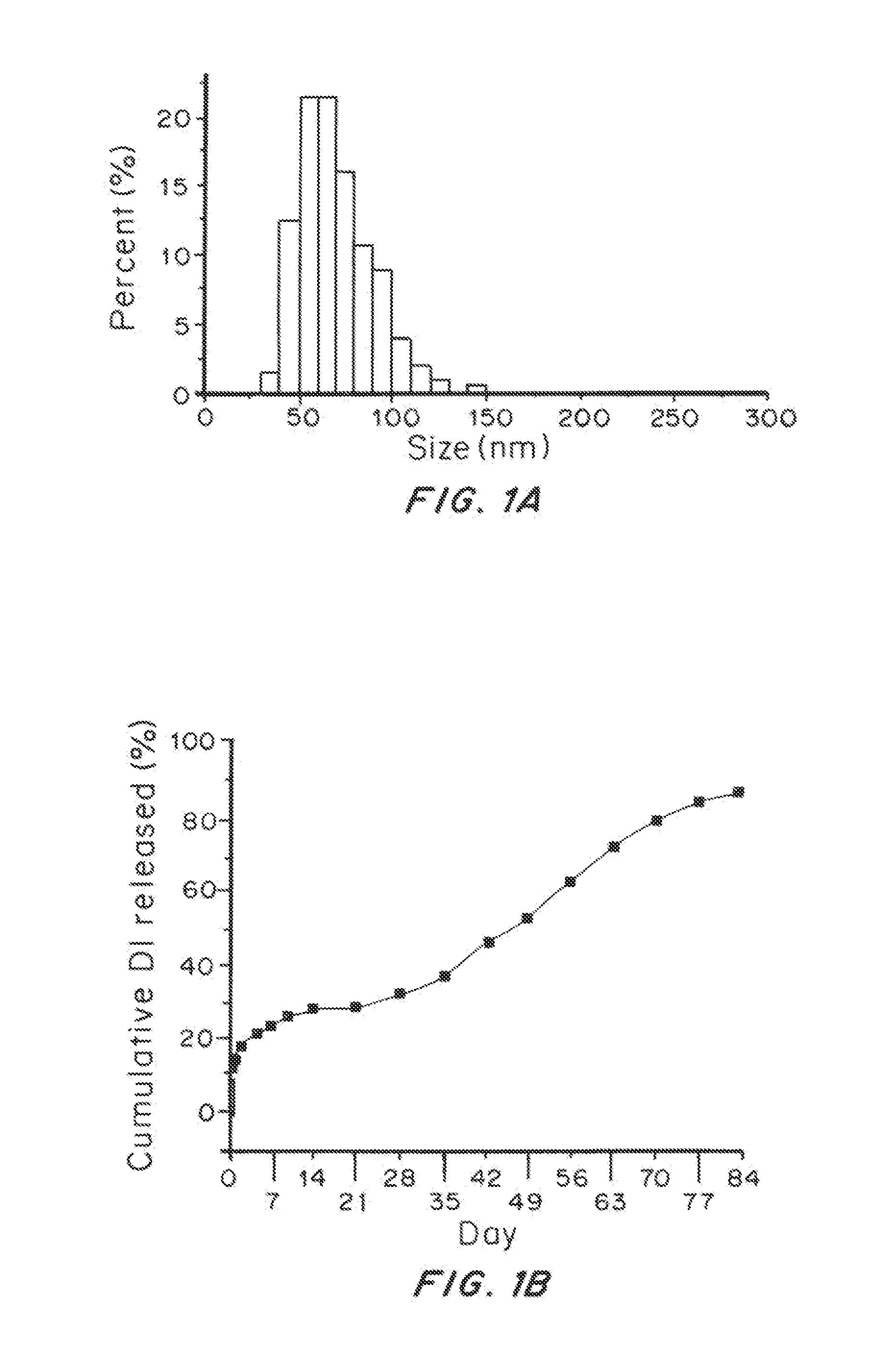
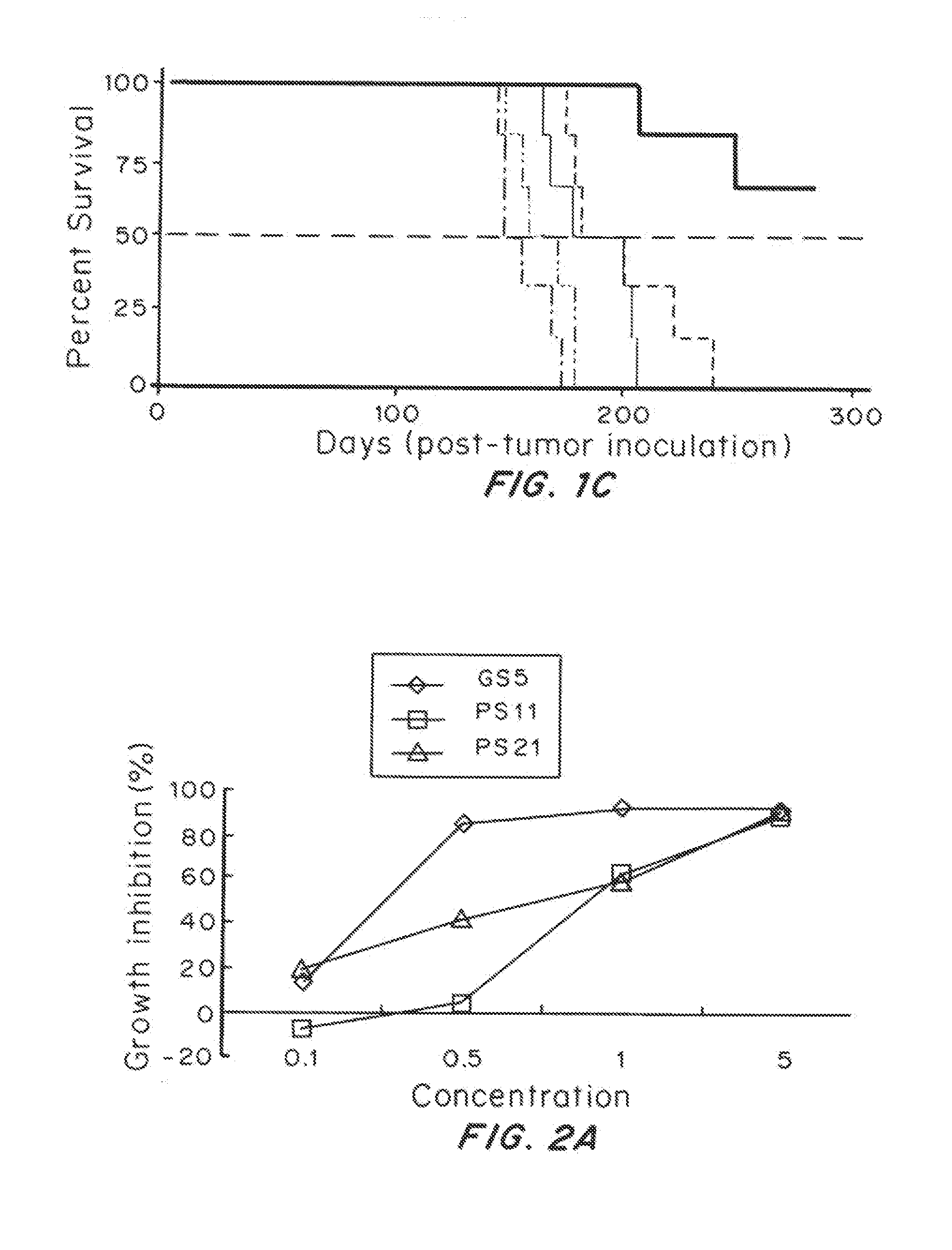
Highly Penetrative Nanocarriers for Treatment of CNS Disease
ActiveUS20150118311A1Improve survivalTreatment of brain tumorsBiocidePowder deliveryDrugs solutionNanocarriers
Brain-penetrating polymeric nanoparticles that can be loaded with drugs and are optimized for intracranial convection-enhanced delivery (CED) have been developed. In the preferred embodiment, these are loaded with FDA-approved compounds, identified through library screening to target brain cancer stem cells (BSCSs). The particles are formed by emulsifying a polymer-drug solution, then removing solvent and centrifuging at a first force to remove the larger particles, then collecting the smaller particles using a second higher force to sediment the smaller particles having a diameter of less than 100 nm, more preferably less than 90 nanometers average diameter, able to penetrate brain interstitial spaces.
Owner:YALE UNIV
Modified release formulations of Anti-irritability drugs
InactiveUS20090017110A1Provide flexibilityBiocideOrganic active ingredientsFda approvalAnti allergic drug
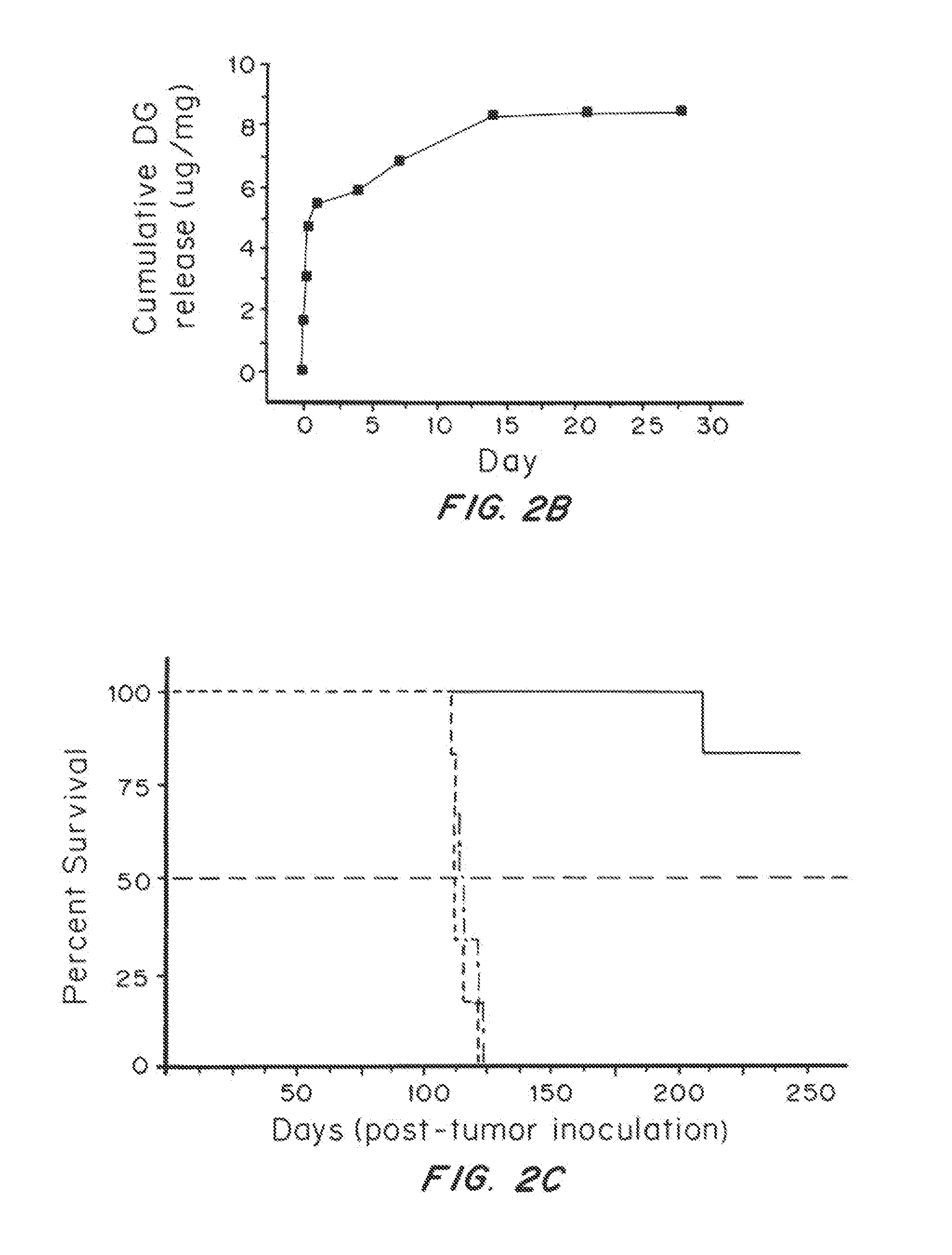
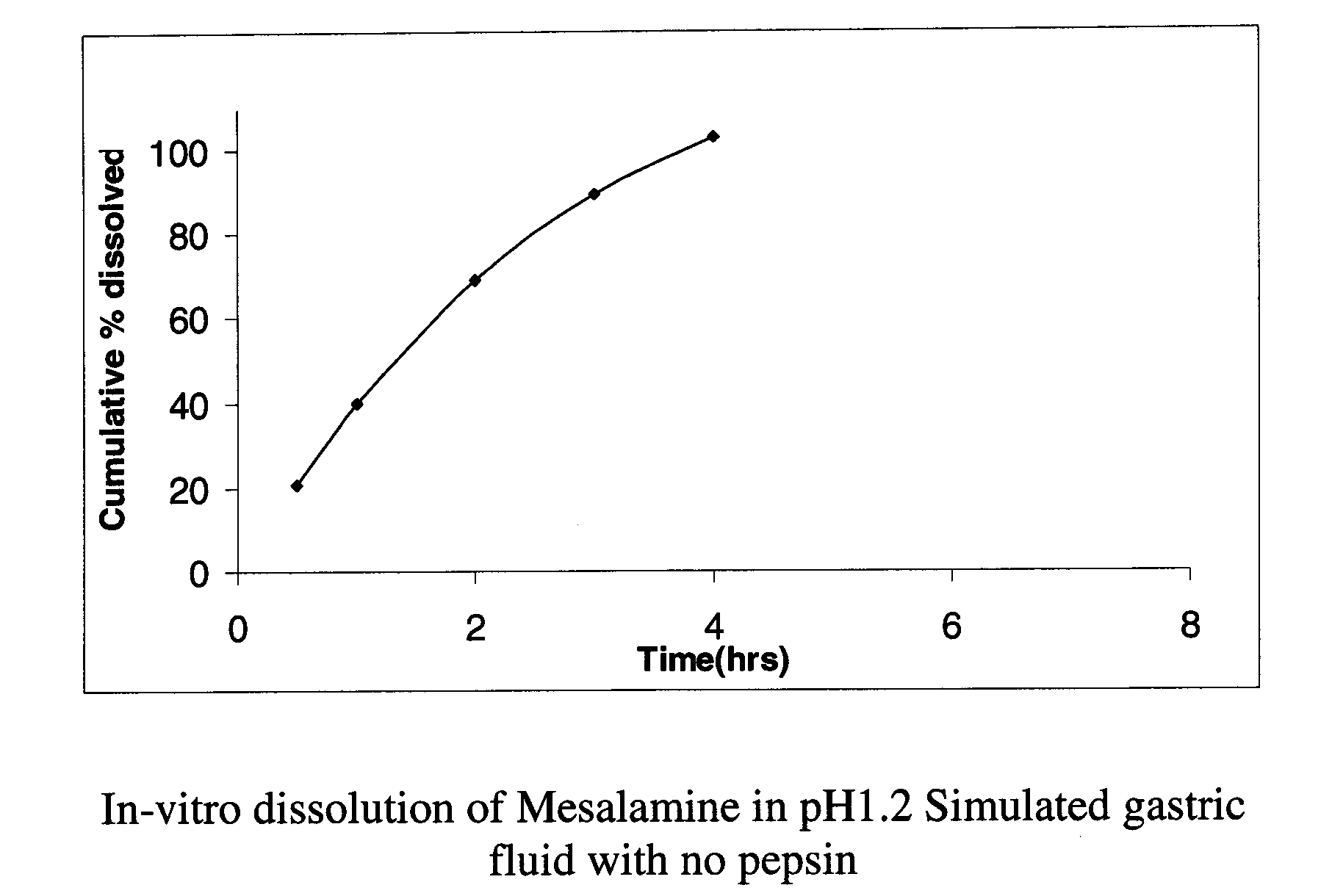
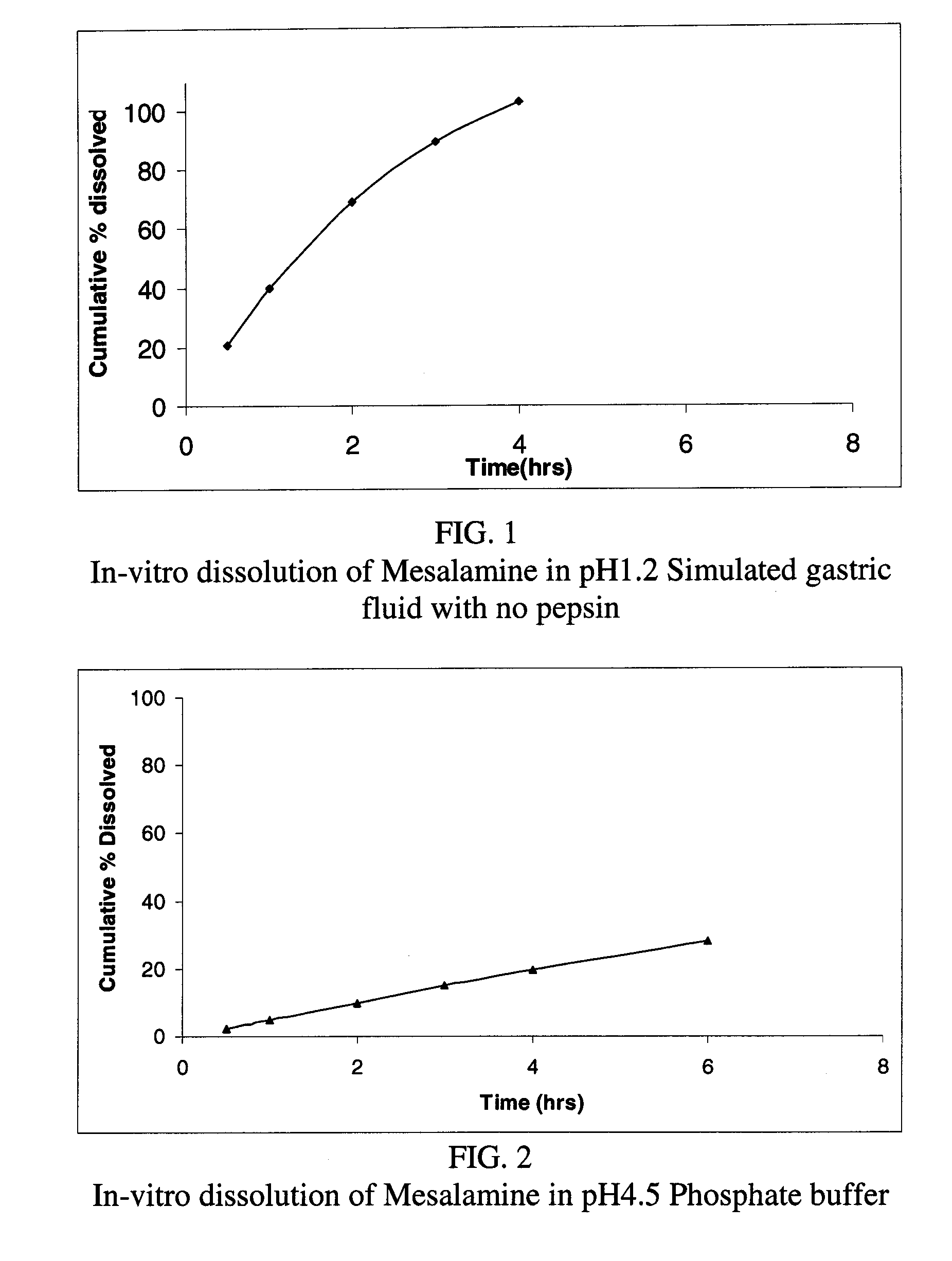
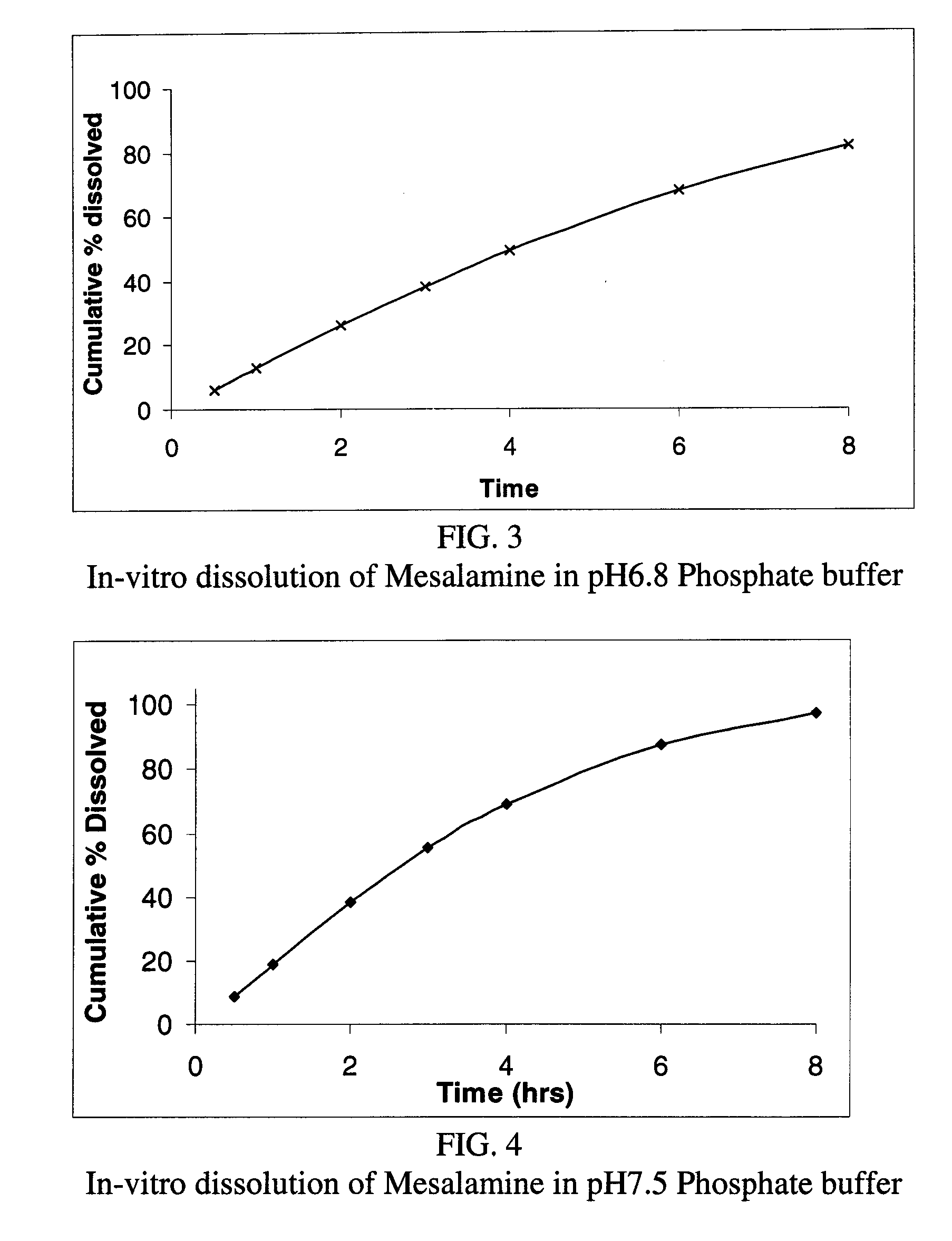
Modified or extended release formulations containing mesalamine compounds and associated methods are disclosed and described. In some aspects, such formulations may be substantially bioequivalent to known FDA approved mesalamine formulations such as PENTASA®.
Owner:CAPRICORN PHARMA INC
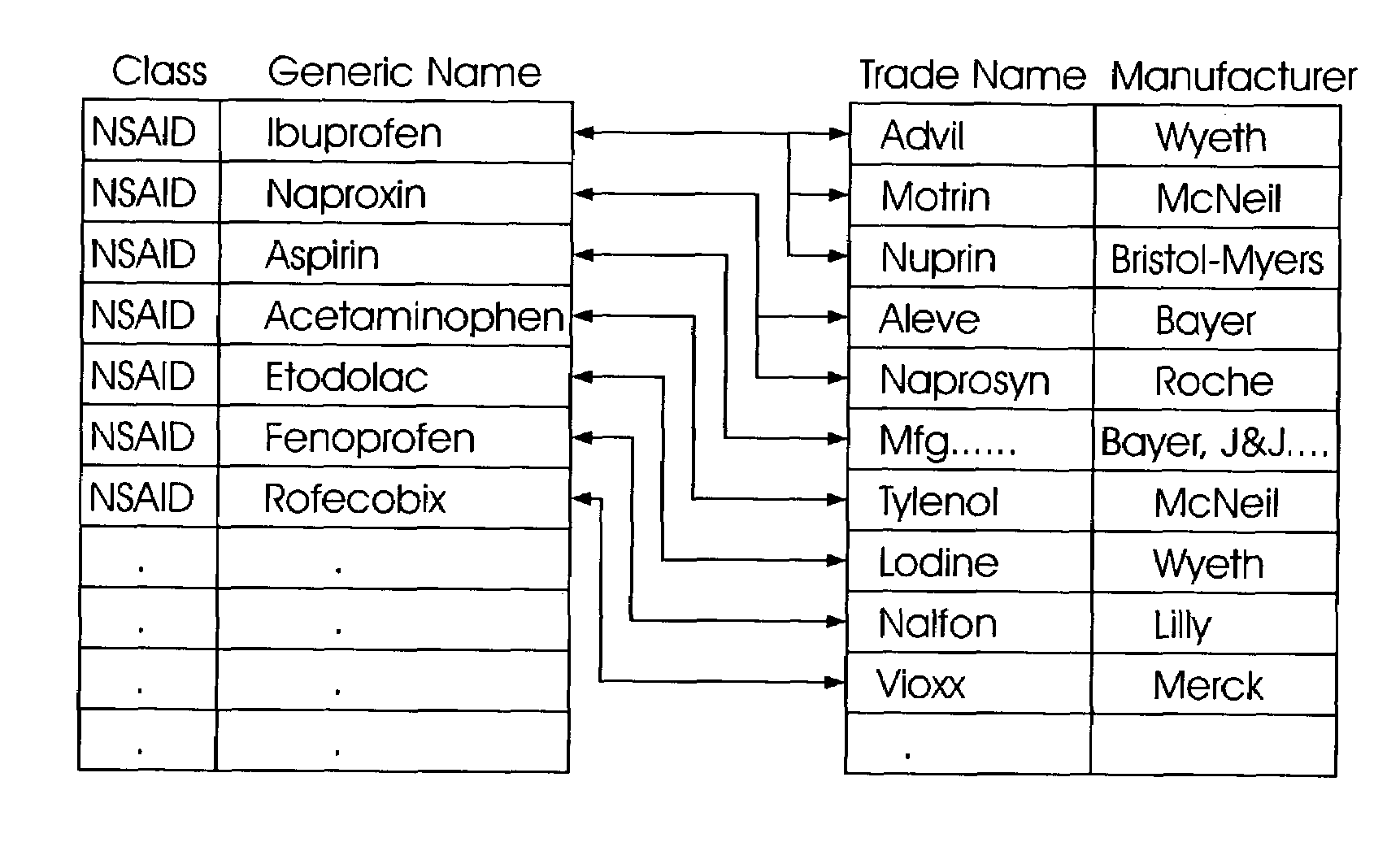
Computerized system and method for rapid data entry of past medical diagnoses
ActiveUS7624029B1Minimal costMinimal time commitmentMultiple digital computer combinationsDiagnostic recording/measuringDiseaseData set
A system and method for rapid entry of past medical diagnoses allows for development of specific, clinically relevant diagnoses from a simple input medication through use of a developed database that maps an input medication to a specific medical diagnosis through an intermediate indications data set. Input medications are normalized to their generic or chemical name and the normalized medication data is associated to an intermediate diagnosis through a medications indications listing contained in a database. Each medication develops a short list of possible specific and / or macro-diagnoses (indications) consistent with the medications and a selected indication is concept-matched to a specific medical diagnosis in order to develop a proper diagnosis in clinically meaningful terms, from a concept-mapped portion of the database. The concept-mapped portion is developed by grouping medical terms into clinically meaningful groups and developing pointers within those groups between identified diseases and a developed indications taxonomy, itself developed by forming a composite of FDA-approved and non-FDA approved indications with respect to particular normalized medications.
Owner:HUMANA INC
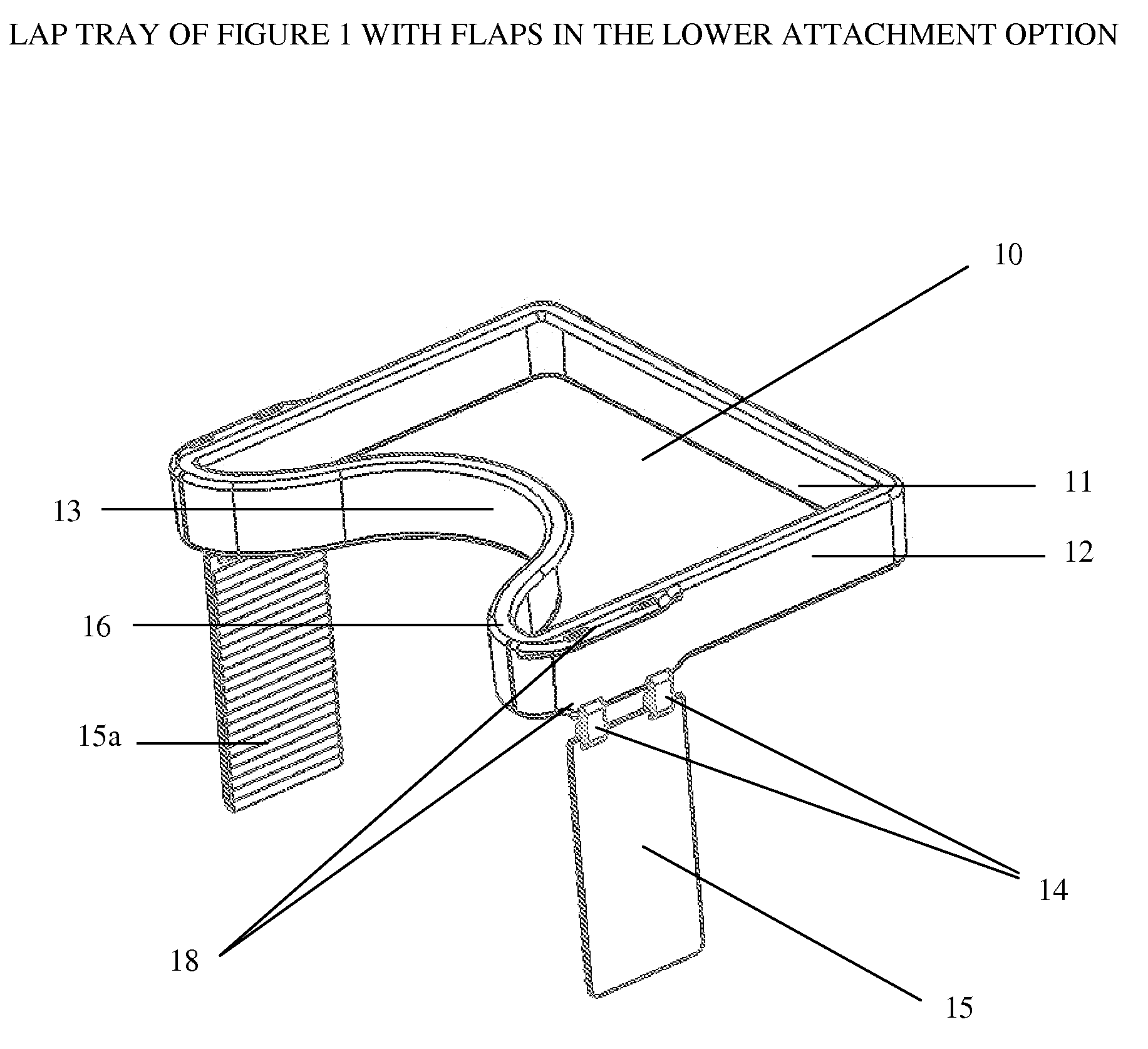
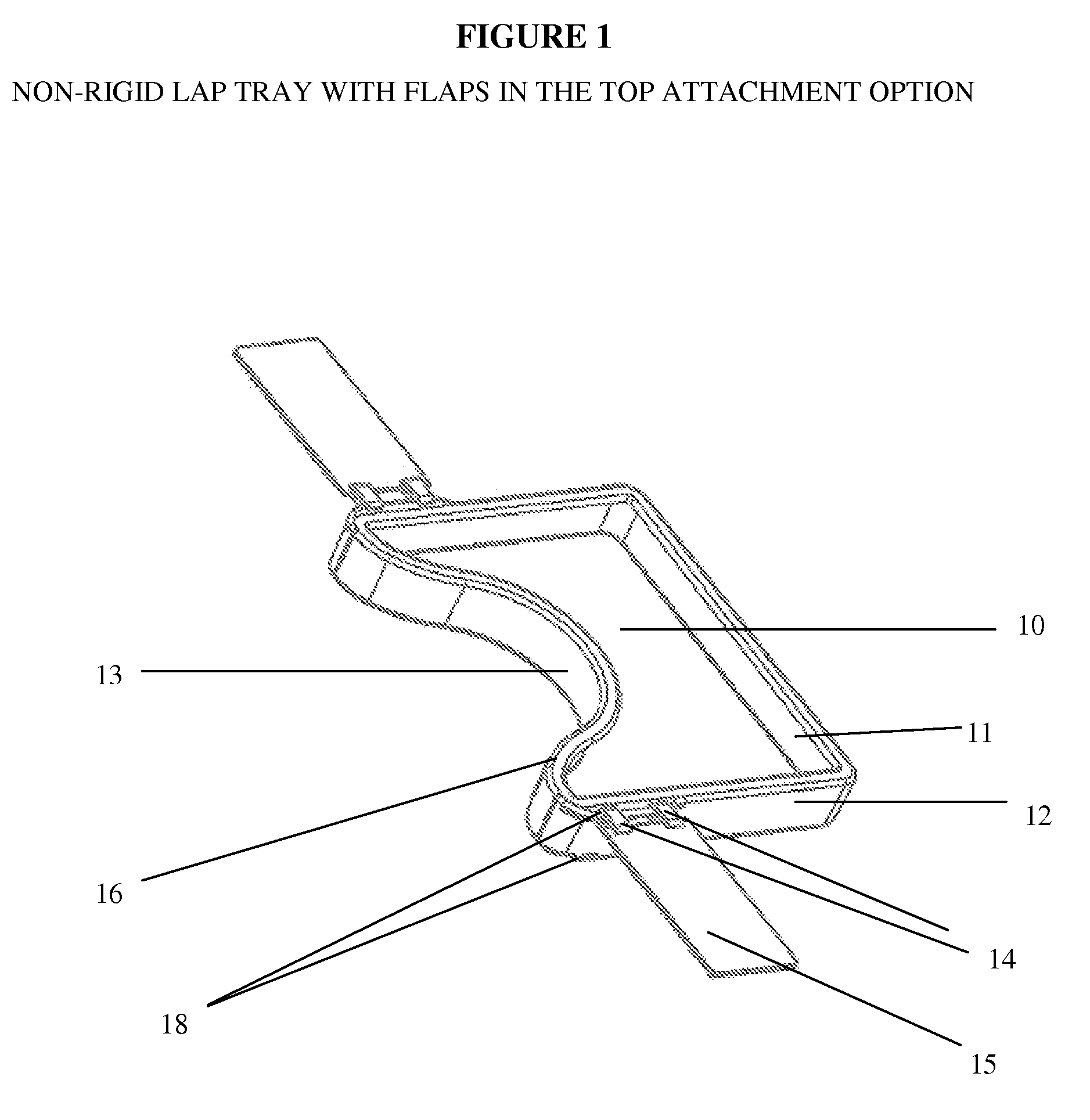
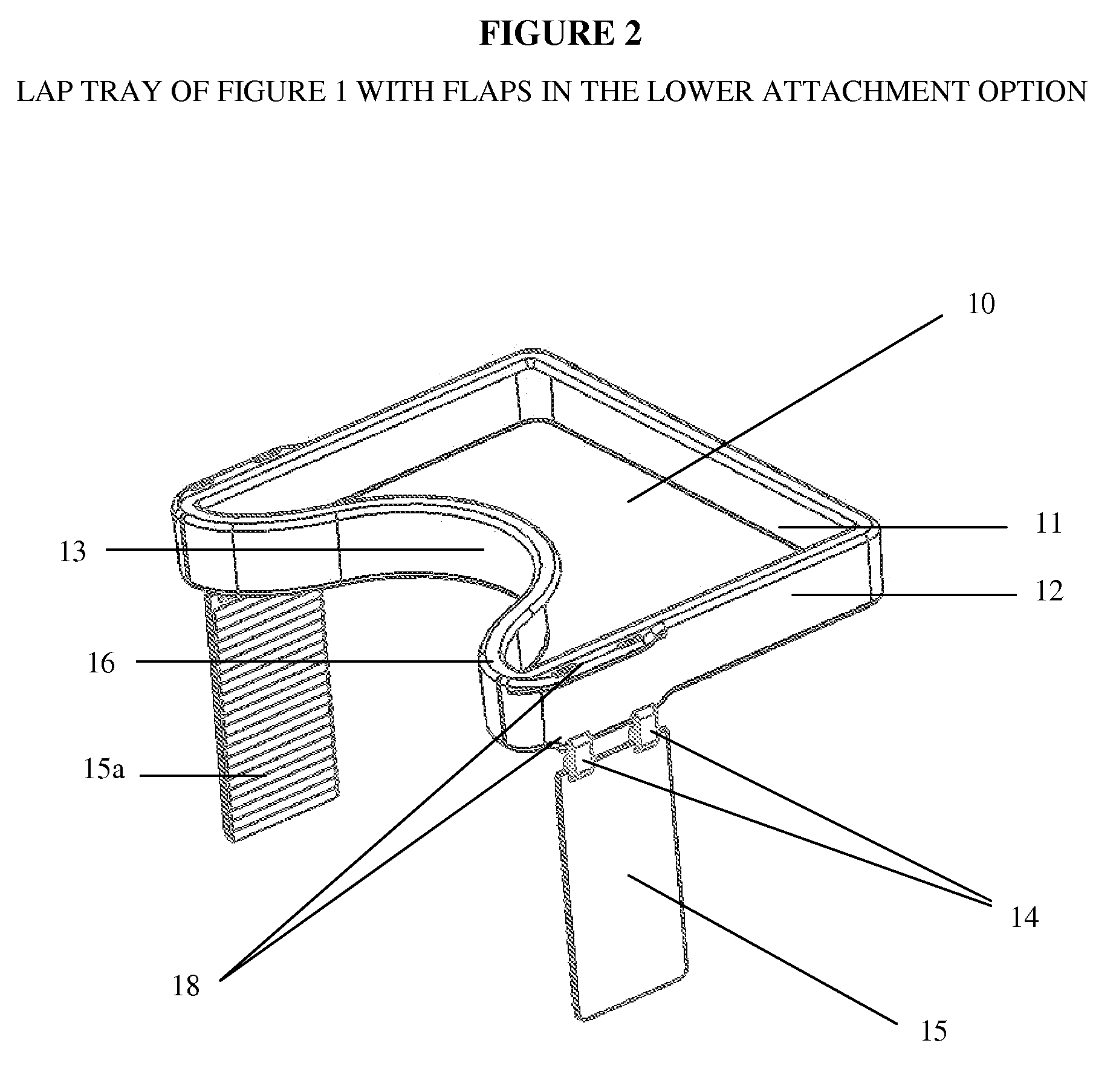
Impact collapsible non-rigid lap tray with four surrounding high sides
The present invention relates to lap trays, specifically to non-rigid automobile lap trays with four surrounding high sides, constructed from FDA-approved materials for safe contact with food, having properties available to withstand temperatures greater than 200° F. The non-rigid automobile lap tray is formed through one of several processes: they can be compression molded or transfer molded if a rubber or silicone material is used, or injection molded with non-rigid plastic materials.
Owner:GRANT MARGARET JEAN
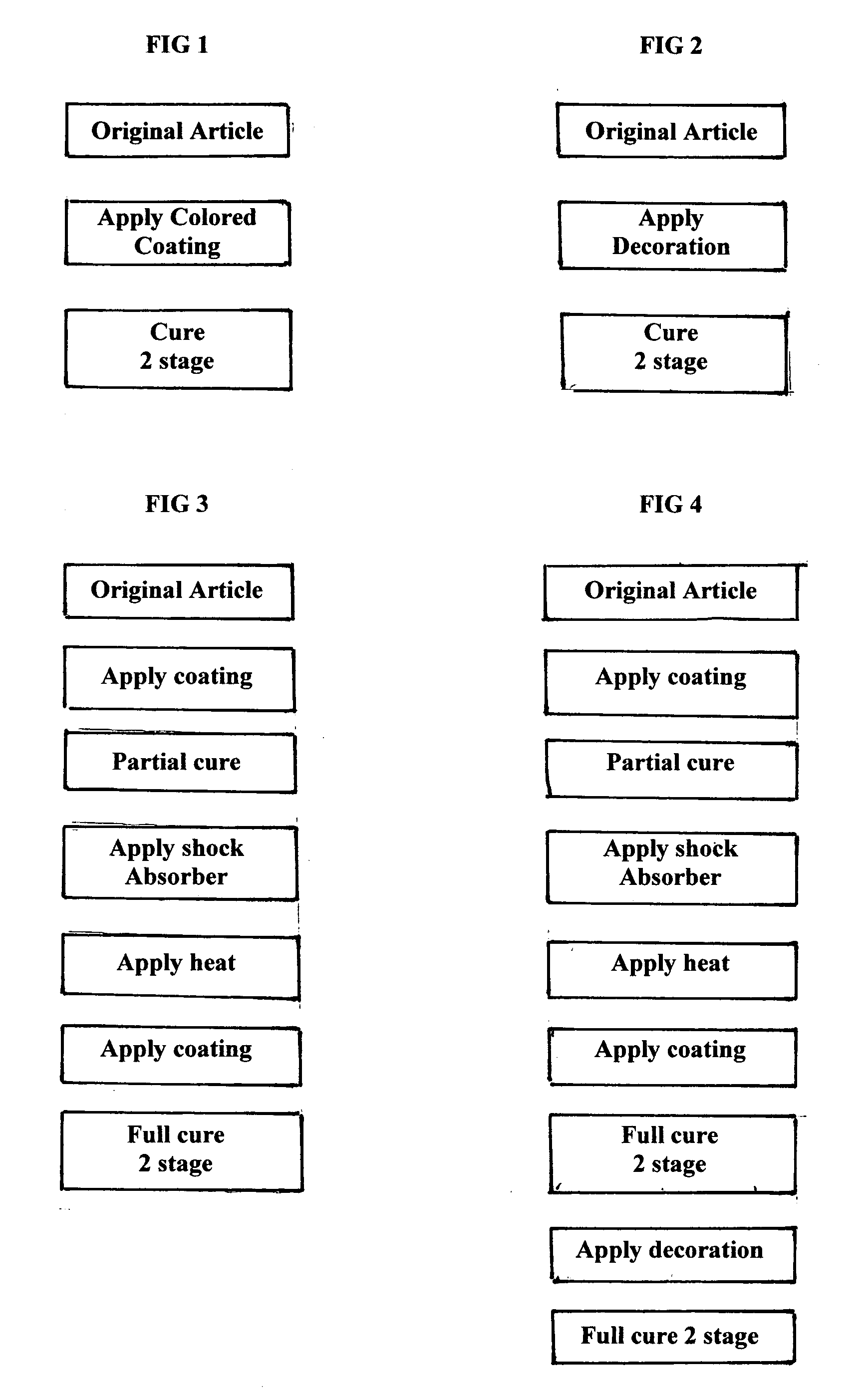
Decorative and protective system for wares
InactiveUS20110250405A1Good adhesion to glassImprove fracture resistanceLayered productsPretreated surfacesCross-linkProduction rate
A copolymer Tradenamed HexiLok is applied in the form of an ink as a decorative and protective outer covering to glassware and other tableware. It serves also as an adhesion coating to which a cross-linked ethylene acrylic acid copolymer is applied, to serve as a shock absorber and shard retention agent, in the event of breakage of the wares. The impact resistance of drinking glasses can be improved ten-fold with a 5 mil outer coating. The use of UV light as a curing agent in a 2-stage curing process, enables high production rates. The tough HexiLok ink serves to hermetically seal the decorated surface of the wares, enabling the use of non-FDA approved decorating agents.
Owner:SAWATSKY HANK
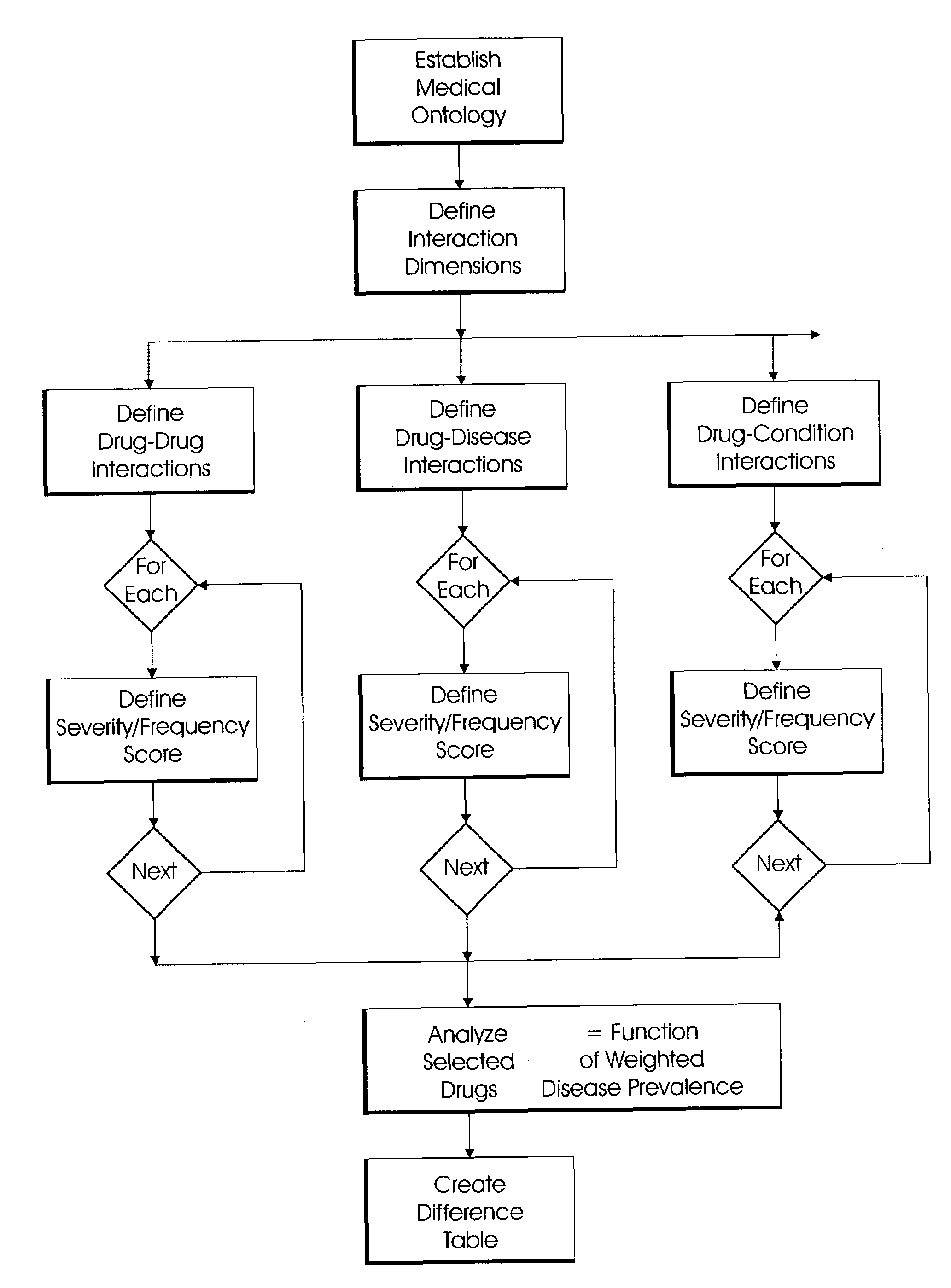
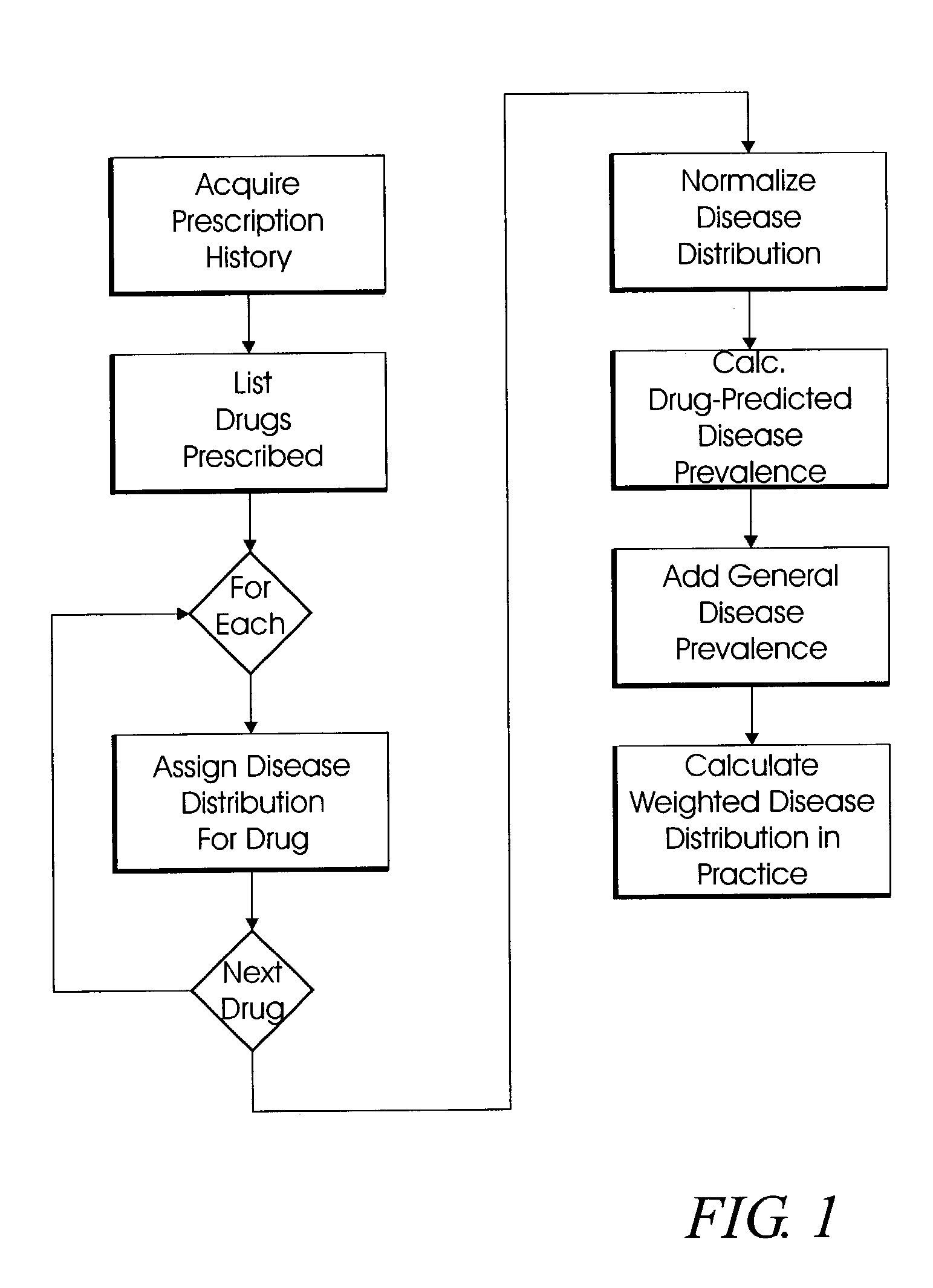
System and method for multi-dimensional physician-specific data mining for pharmaceutical sales and marketing
ActiveUS7698157B2Facilitate electronicFacilitate algorithmic computationDigital data processing detailsDrug and medicationsDiseaseSide effect
The present invention relates to a system and method for electronic and algorithmic data mining of an individual physician's prescribing history to determine the approximate distribution of diseases within their practice population for optimizing pharmaceutical sales and marketing. Rapid and large-scale determination of specific clinical safety and efficacy attributes of a marketed drug which are most pertinent and relevant to a given physician, when compared to a competitor's drug, are defined and tabularized. Major clinical characteristics taken into account include a drug's safety, efficacy, cost, dosing convenience, formulary insurance coverage, side effect profiles, and FDA approval for the intended use. A symbolic representation of knowledge is employed in which the marketed drug and each competitor's drug are compared algorithmically against each other with a scoring system that is based upon machine analysis of each major clinical characteristic. The score is further refined according to the number and severity of safety interactions which are relevant to the comparison, and also based upon predicted prevalence of such interactions within a specific physician's practice.
Owner:HUMANA INC
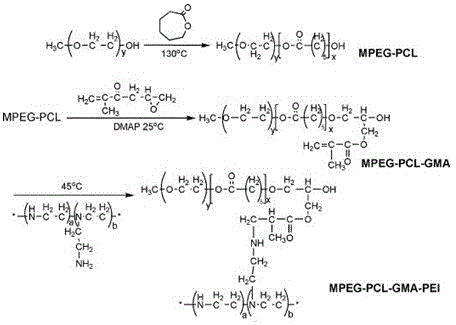
Cationic polymer-loaded paclitaxel/indocyanine green co-delivery micelle and preparation method thereof
InactiveCN105194670AAchieve compositeOrganic active ingredientsEnergy modified materialsCationic polymerizationSurface charges
The invention provides a cationic polymer-loaded paclitaxel / indocyanine green (ICG) co-delivery micelle and a preparation method thereof. To simultaneously load the chemotherapeutic paclitaxel and the photosensitizer ICG, an amphiphilic degradable cationic nano-micelle MPEG-PCL-PEI is used as a loading medium for the chemotherapeutic paclitaxel; a cationic polymer block is introduced into a polymer MPEG-PCL which forms the nano-micelle, so surface charges of the micelle can be changed; negatively-charged ICG molecules are adsorbed through interaction between positive and negative charges, and sustained release of paclitaxel and ICG is realized; so a novel method is provided for integrated diagnosis and treatment of tumors. According to the invention, the triblock cationic polymer synthesized from an amphiphilic polymer-grafted cationic fragment can simultaneously load the chemotherapeutic paclitaxel and the photosensitizer ICG approved by FDA, so compounding of the chemotherapeutic and fluorescence molecules is realized, and fluorescent imaging of a tumor site as well as target drug delivery for a tumor is complished.
Owner:WENZHOU MEDICAL UNIV
Universal improved particulate matter delivery device
InactiveUS7101265B1Simple internal structureEffective fluidizationBlast gunsAbrasive feedersParticulatesMedicine
The present invention includes an apparatus for delivery of pressurized particulate matter against a surface or target to abrade, texture, sandblast, etch, erase, cut, penetrate, smooth, clean, polish, harden and / or deburr the surface or target. The invention is expected to be used in slightly different embodiments, both by dentists and oral hygienists to clean teeth, and by hobbyists, although numerous other uses are within the contemplation of the inventors. The dental embodiment features a prefilled, sealed, and disposable fluidizing chamber and cannula assembly that avoids contamination and which has been approved by the FDA for dental use. The general utility embodiment features a refillable fluidizing chamber and detachable cannula.Included is a fluidizing chamber having a discharge end of an inlet tube that is disposed below or overlaps the intake end of the outlet tube such that the discharge of the inlet tube blows the particulate matter into the fluid above the intake end of the outlet tube, thereby suspending it therein, without clogging. The invention further provides for a duckbill check valve to prevent backflow of particulate matter when the chamber is disconnected from the pressurized fluid source or there is a drop in pressure from said source. Included with the general utility embodiment are a refill aperture and removable refill aperture plug to facilitate recharging the fluidizing chamber with particulate matter using a filling cartridge.
Owner:RED MOUNTAIN
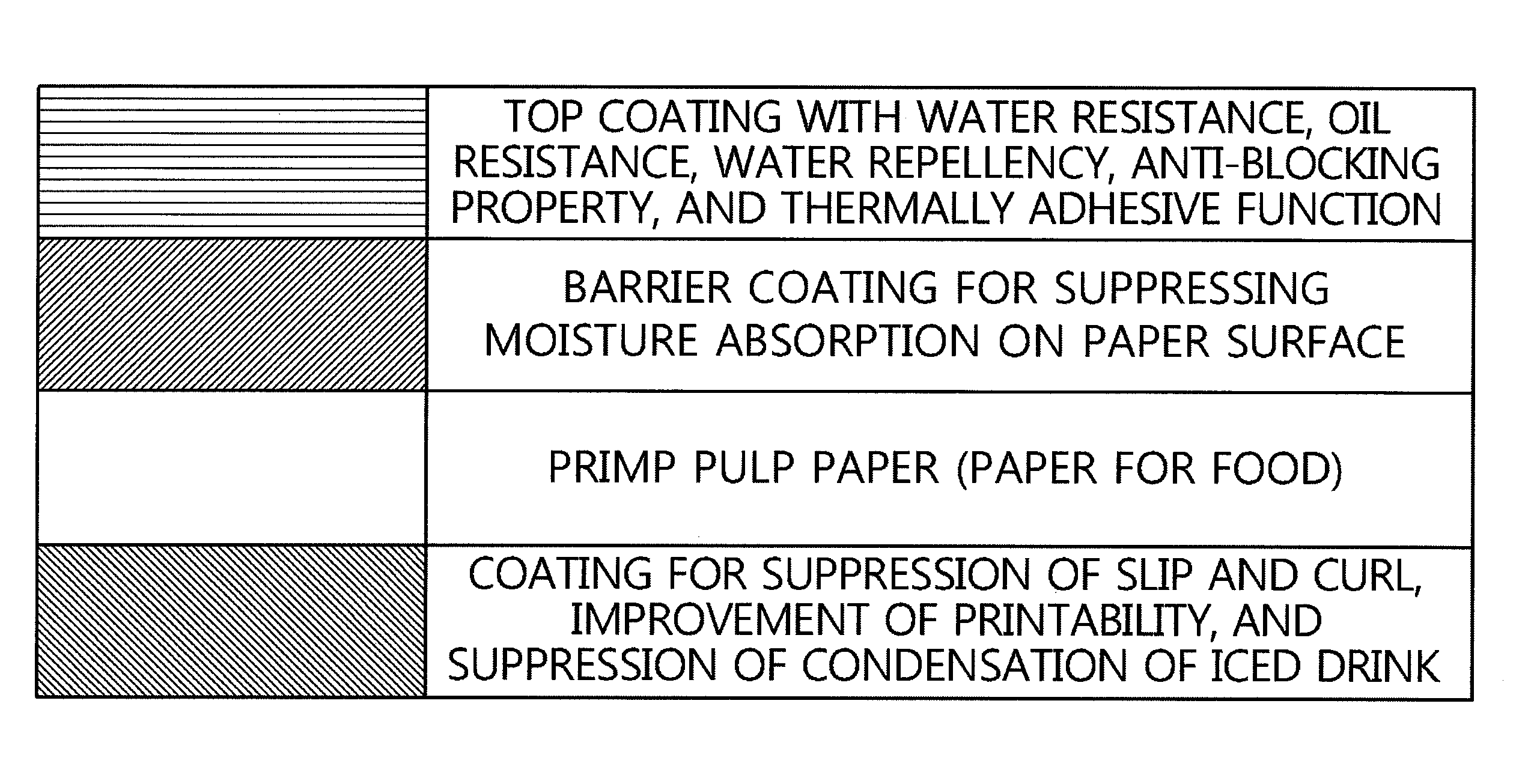
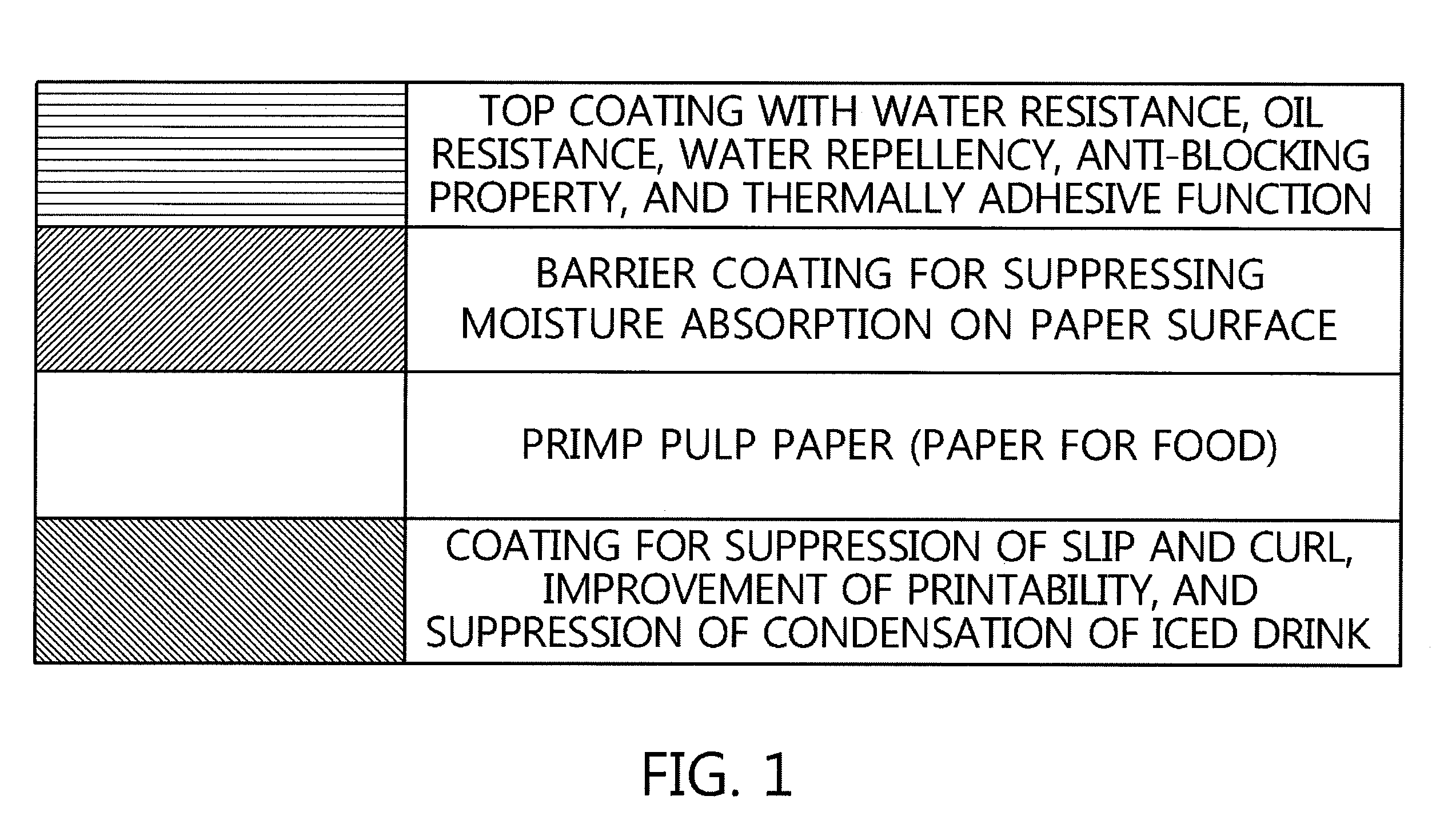
Method for preparing environmentally friendly paper coating agent by using water dispersible resin of ethylene-(METH)acrylic acid polymer, and use thereof
InactiveUS20160145806A1Good dispersionImprove uniformityWrappersSpecial paperWater dispersibleAlkali metal
There is provided a method of manufacturing a paper-forming product formed of an environmentally friendly paper coating agent, which is excellent in water resistance, oil resistance, thermally adhesive property, and alkaline water-dissociative property, the method including mixing alkali metal hydroxide, amines, ammonia water, or the like with each of an ethylene-acrylic acid copolymer or an ethylene-(meth)acrylic acid copolymer or a mixture of these polymers, and using a water-dispersible coating solution prepared by adding FDA-approved matting agent, slip agent, and anti-foaming agent into a water-dispersible resin solution prepared by neutralizing the mixed product in an aqueous medium.
Owner:RHEE BYEONG SEOK
Growth factor nanotube slow-release system used for osseointegration and preparation method and application thereof
InactiveCN104436313AIncrease drug loadEasy to operateSurgerySurface reaction electrolytic coatingFda approvalTitanium alloy
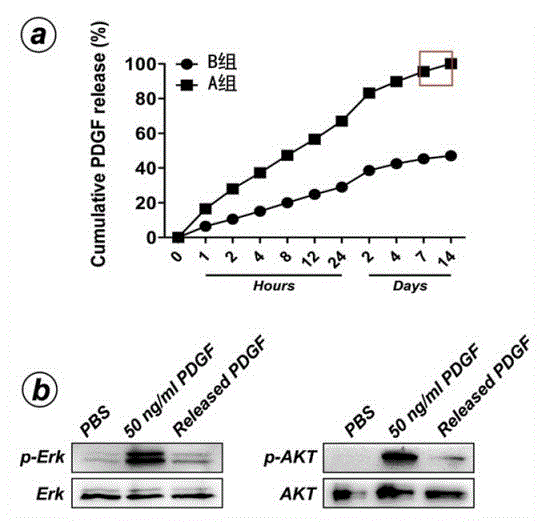
The invention discloses a growth factor nanotube slow-release system used for osseointegration and a preparation method and application thereof, the nanotube slow-release system includes a titanium alloy implant, a titanium dioxide nanotube coating is prepared on the titanium alloy implant surface by the method of anodic oxidation, and the titanium alloy implant is loaded with a growth factor. The growth factor nanotube slow-release system has the advantages that: (1) the growth factor nanotube slow-release system can improve the nanotube drug loading amount by a simple and effective method of vacuum aspiration; (2) the invention also provides a rhPDGF- BB nanotube slow-release system which can significantly promote the osseointegration in bone osteoporosis state, rhPDGF-BB is approved by the FDA to be used in clinical osteanagenesis therapies, and clinical application of the nanotube slow-release system may be provided; and (3) including nanotube preparation, therowth factor nanotube slow-release system is in no need of special, complex and expensive equipment, simple in operation process, and conducive to the promotion and use.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
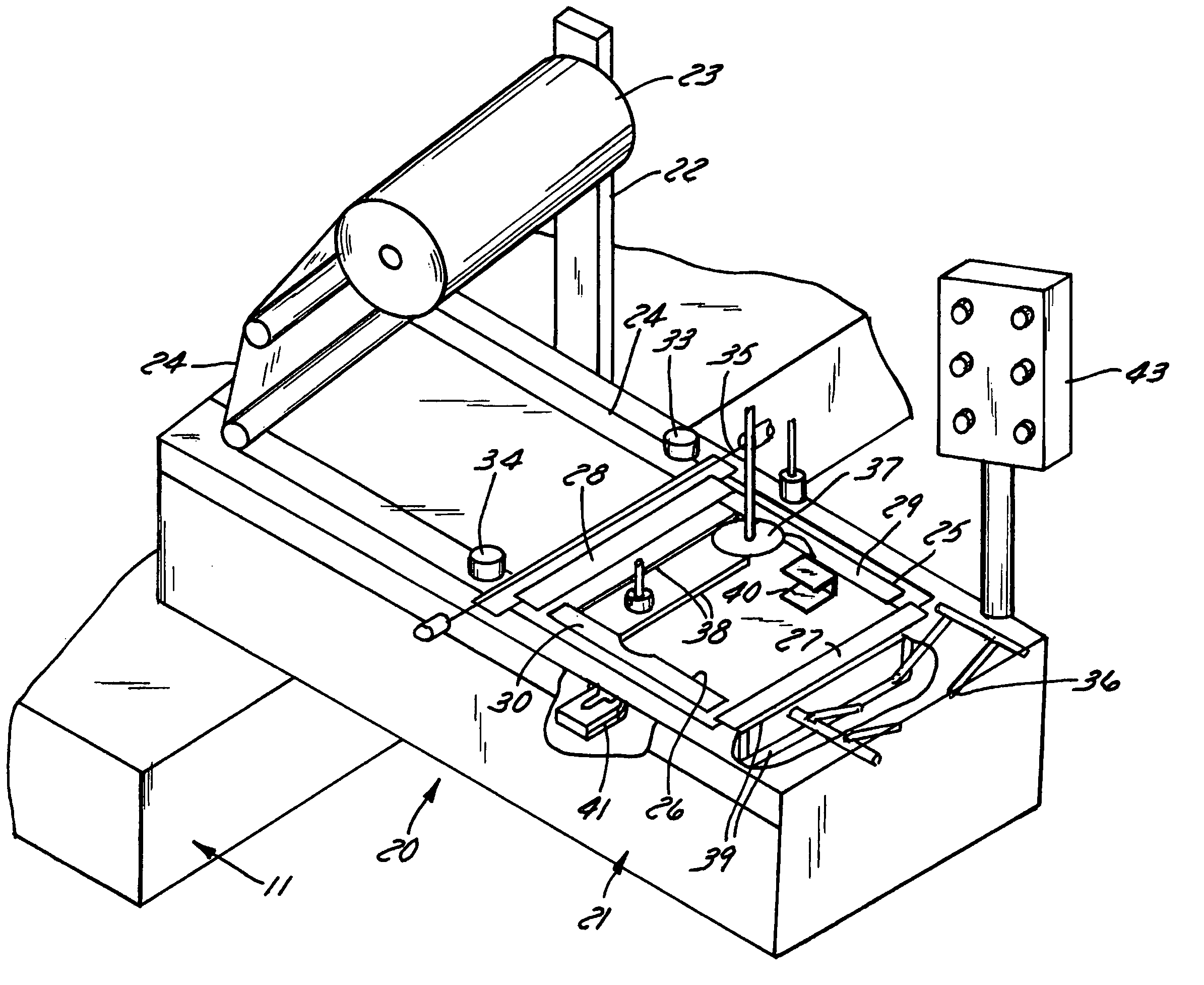
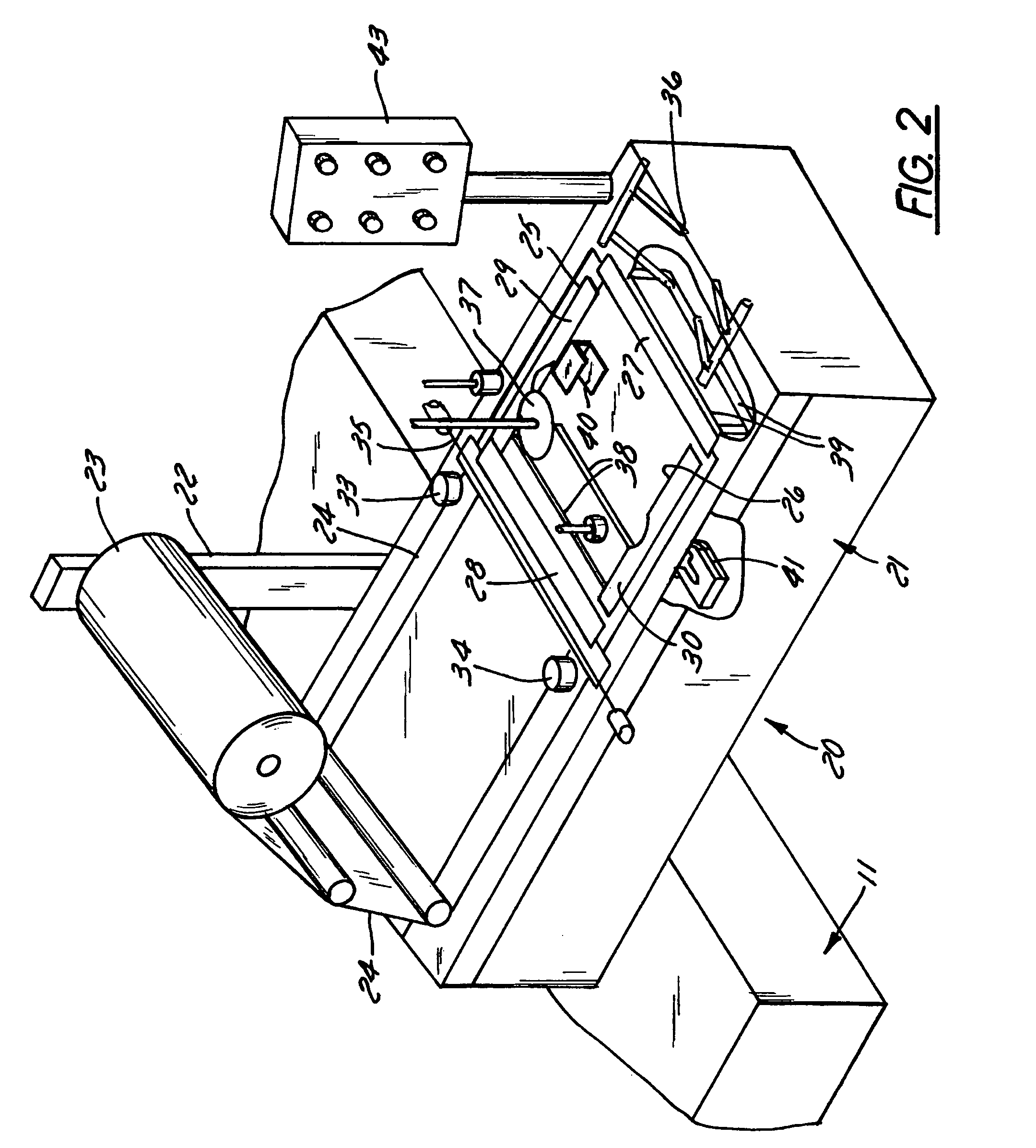
Apparatus and method for stretch-wrapping articles
InactiveUS20050022475A1Improve productivityReduce labor costsWrappers shrinkageWrapper folding/bending apparatusLeading edgeThin membrane
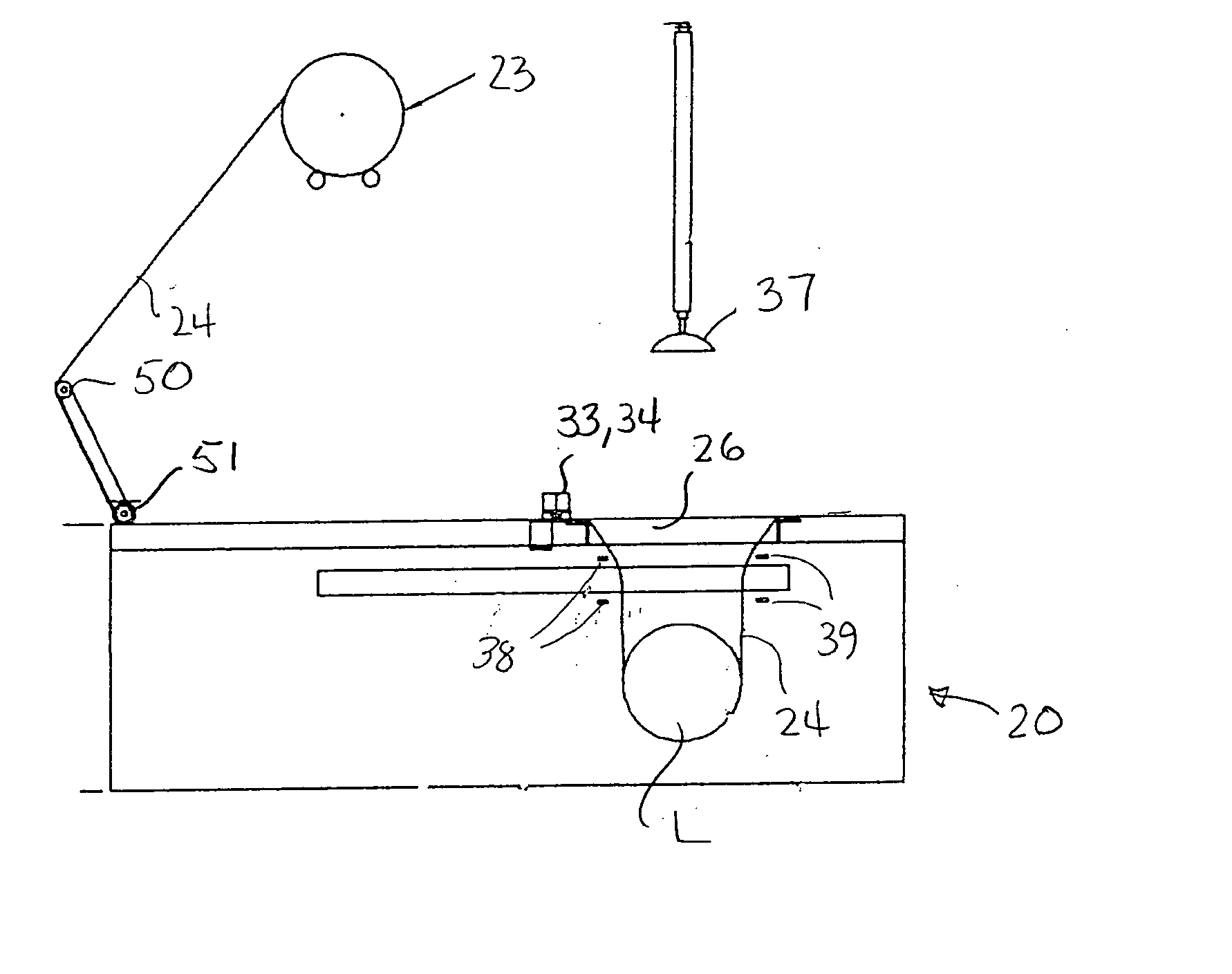
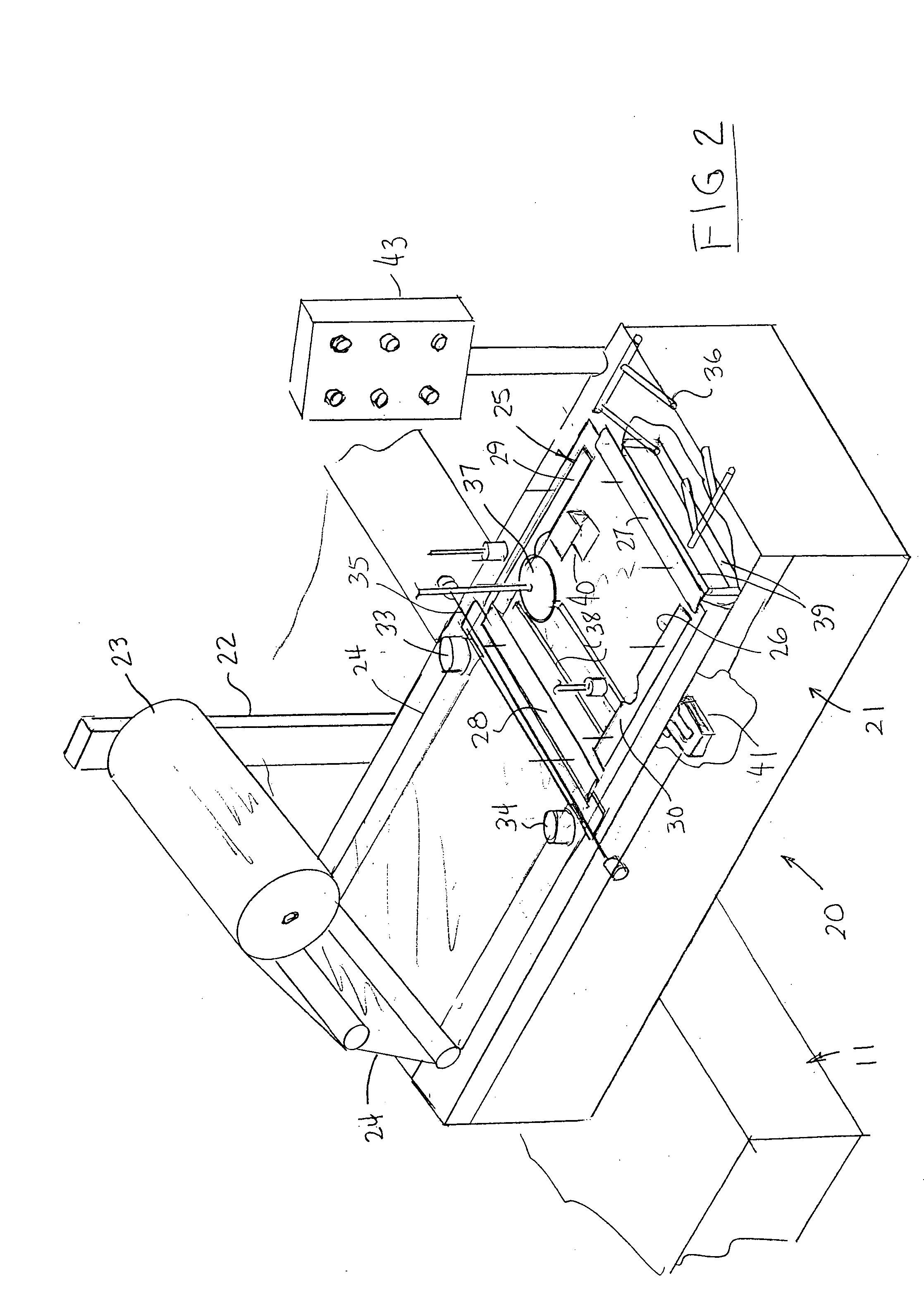
Apparatus and process for stretch wrapping articles, especially heads of lettuce, wherein a roll (23) of film (24) is supported on a housing (21), and the film is supplied from the roll around a dancer roller (50) and film feed roller (51) to a pair of film feed grippers (33, 34) which grip a leading edge of the film and advance it past an opening (26), whereupon a clamp (27) engages the leading edge of the film to hold it while the grippers release and return to their home position. Additional clamps (28-32) then engage the film to hold it in place over the opening. A head of lettuce (L) is placed on the film in centered relation to the opening, and a ram (37) engages the lettuce and pushes it against the film to stretch the film through and below the opening. Gathering bars (38, 39) engage the film stretched between the opening and the head of lettuce to gather the film in a first direction, and a U-channel (40) and sealing and trimming mechanism (41) gather the film in a second direction to produce a narrow necked down section of gathered film, fuse and seal the necked down section to produce a wrapped head of lettuce, trim excess film from the lettuce, and release the wrapped head of lettuce to drop onto a take away conveyor. The film (24) is a polyethylene based multi-layer film with an outer polypropylene layer for added gloss, slip and clarity, has a 300% stretch capability, is FDA approved for direct food contact, has an anti-fog additive for moisture dissipation during temperature fluctuations, is thirteen microns thick, and has a width of sixteen inches.
Owner:INT PAPER CO
Molded or Extruded Catnip Composite and Method of Manufacturing Catnip Pet Toys and Pet Toys Made from the Same
A domestic pet toy is provided having a housing element formed of a moldable or extrudable catnip composite made of a solid catnip attractant, a lignocellulose cork agglomerate filler material, and an FDA approved binding agent adhesive. The housing material provides sufficient delivery aromatic olfactory attractant to be effective in having an interactive response with a domestic feline. A sound chip assembly can also be positioned in a retention cavity and emits a prerecorded sound as an audible attractant in response to one or both of commencement and continuation of movement the pet toy.
Owner:OUR PETS
Encapsulated agent guided imaging and therapies
InactiveUS20110311455A1Extended circulation timeHigh level of monodiversityUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsAnimal virusFluorescence
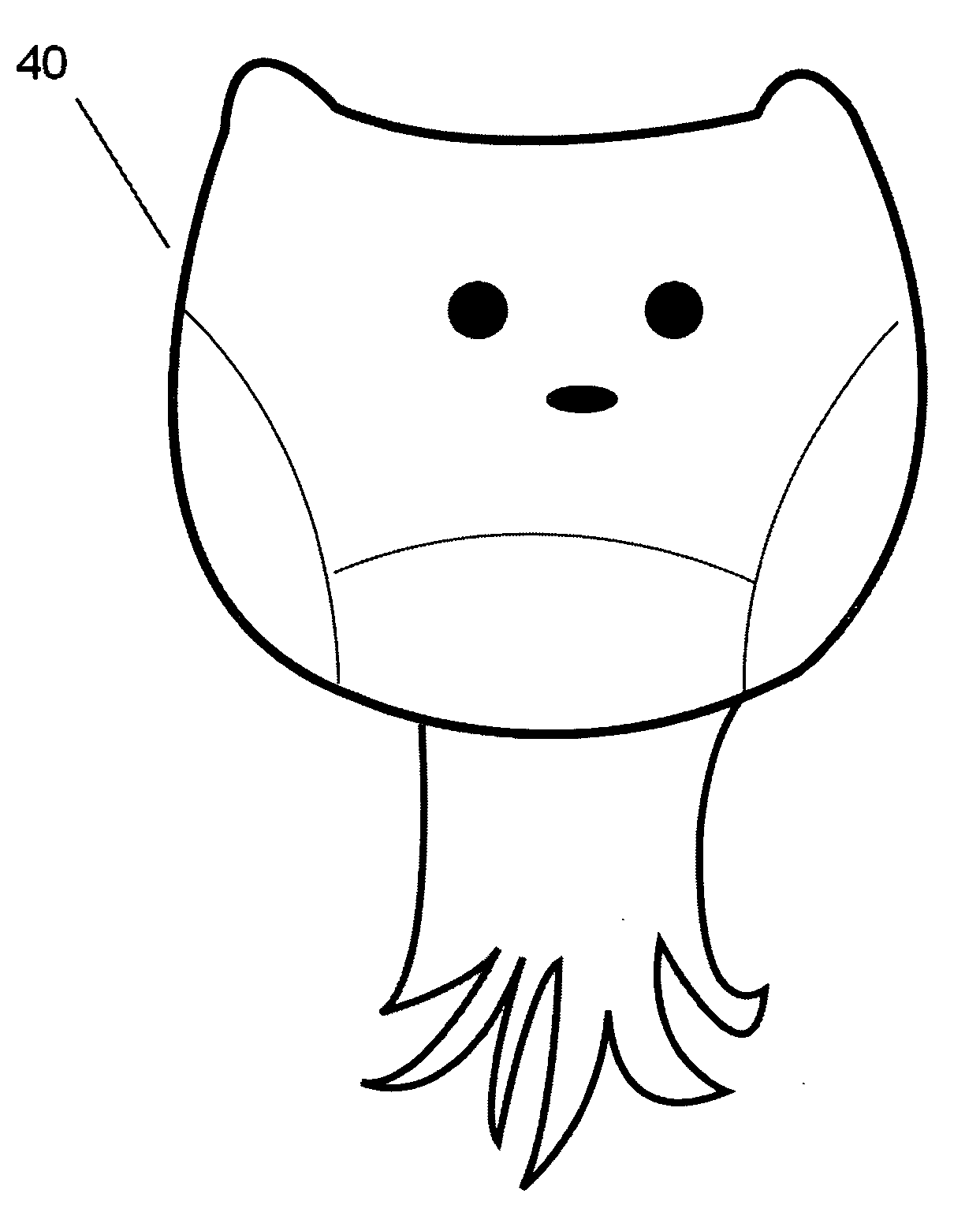
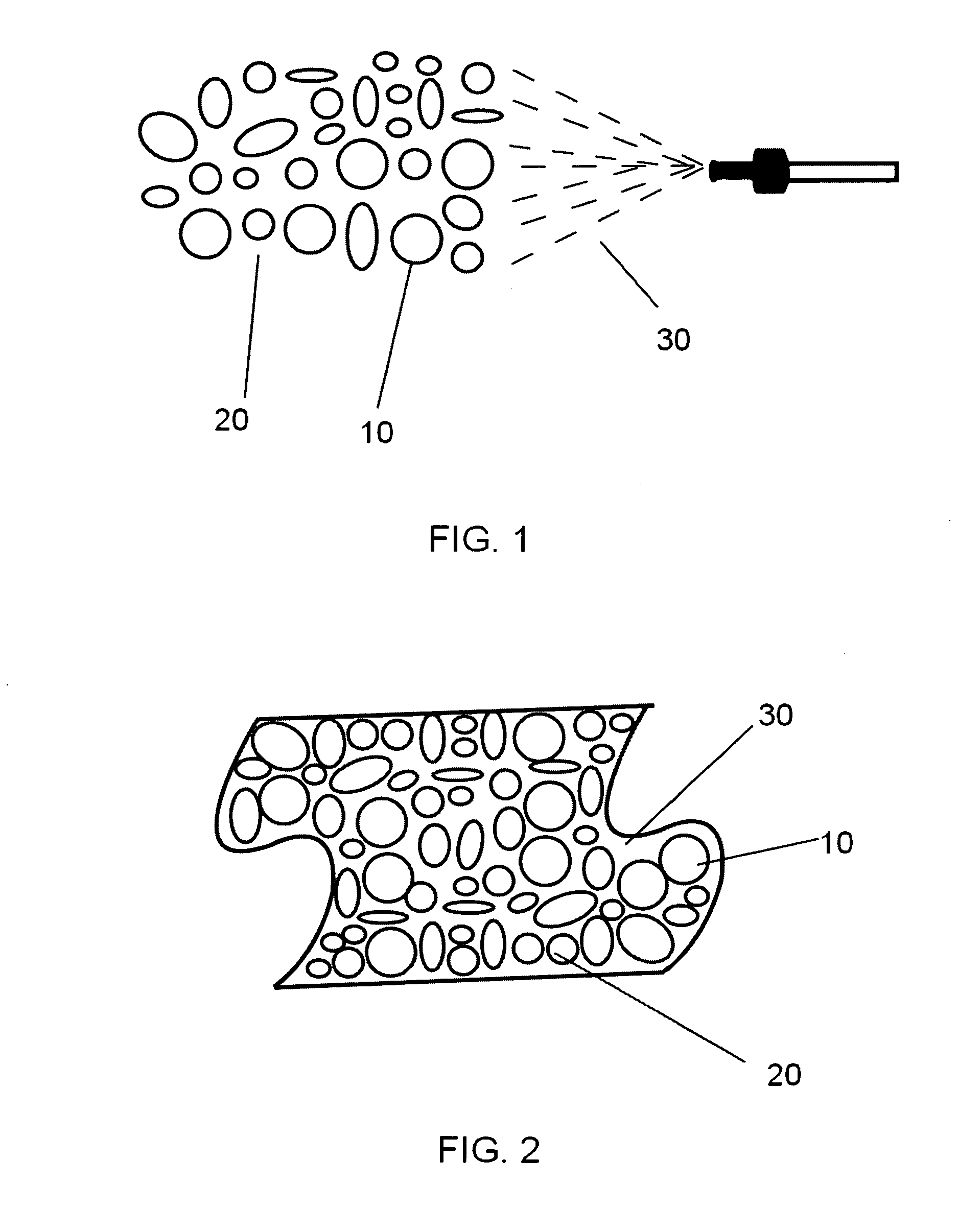
A nano-capsule construct for imaging and therapeutic uses and method for production are provided. One nano-probe embodiment based on genome-depleted plant brome mosaic virus (BMV) whose interior is doped with indocyanine green (ICG), an FDA-approved near infrared fluorescent dye, is used to illustrate the invention. The material encapsulated in viral shell components may be coated with functionalized coatings such as branched, dendritic polymer coatings to improve longevity and distribution in the body as well as antibody conjugation for increased target specificity. The constructs can also be coated with ferromagnetic iron oxide nanoparticles, enabling the ICG-containing capsules to be used as nano-probes with the capability of being detected in both optical and magnetic resonance imaging. The capsules may be produced by purifying a plant or animal viruses and disassembling the viruses to provide virus shell components. The virus shell components are reassembled in the presence of a material for encapsulation thereby encapsulating said material within the core of the construct in one embodiment.
Owner:RGT UNIV OF CALIFORNIA
Utilization of non-nutritive adsorbents to sequester mycotoxins during extraction of protein or other value added components from mycotoxin contaminated cereal or seed oil meal
A method for the removal of mycotoxins from cereal or oil seed meal that includes the use of a mycotoxin sequestrant to form a food grade composition for human consumption wherein said composition contains no more or less than an FDA approved level of mycotoxin for a human food product.
Owner:NORTH CAROLINA STATE UNIV +1
Application of cobimetinib in preparation of medicaments for treating tuberculosis
InactiveCN110151763AImprove securityGood development valueAntibacterial agentsOrganic active ingredientsCobimetinibFda approval
The invention discloses application of cobimetinib in preparation of medicaments for treating tuberculosis. The application is high in safety, and the efficiency of killing mycobacterium tuberculosisis high. According to the application of cobimetinib in preparation of the medicaments for treating tuberculosis, the medicaments for treating tuberculosis are resistant to mycobacterium tuberculosis.The invention also provides a pharmaceutical composition for treating tuberculosis. The pharmaceutical composition comprises cobimetinib. Cobimetinib serving as a potential medicament for targeting HDT has been approved by FDA, is high in safety and has a great development value, so that by applying cobimetinib as a main component in anti-tuberculosis medicaments, the safety is high. Experimentsshow that cobimetinib in macrophages can obviously enhance the activity of the macrophages to kill tubercle bacillus.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
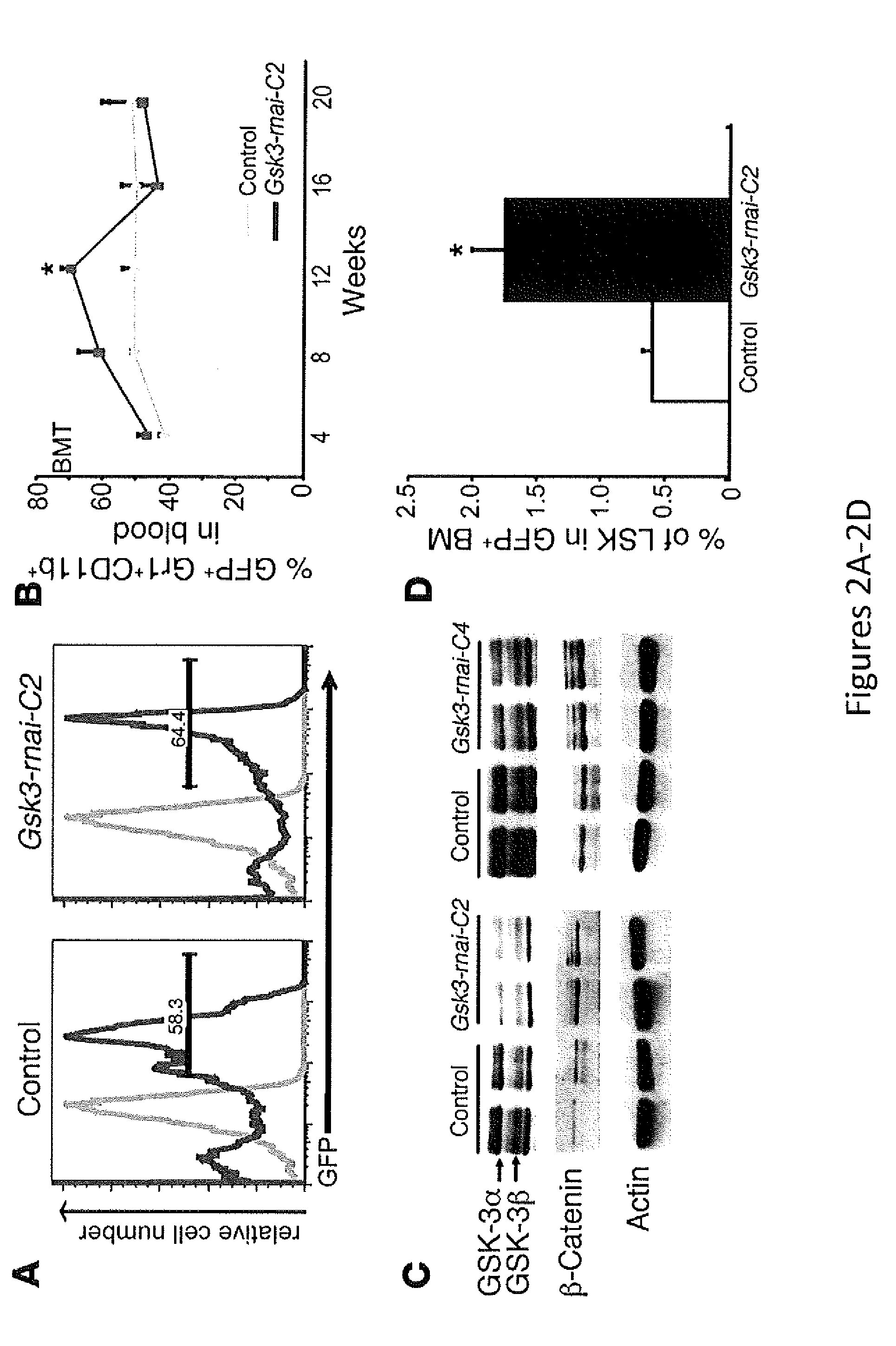
In Vivo and Ex Vivo Expansion of Hematopoietic Stem Cells With a Targeted Combination of Clinically Tested, FDA Approved Drugs
The present invention provides a therapeutic approach to maintain and expand HSCs in vivo using currently available medications that target GSK-3 and mTOR. The present invention also provides a system and method for the ex vivo culturing of HSCs, where an mTOR inhibitor is combined with a GSK-3 inhibitor within the culturing conditions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Surgical retractor
ActiveUS8974380B2Improve performanceImprove visibilitySurgical forcepsLess invasive surgeryImage resolution
A surgical retractor and a method of minimally invasive surgery, wherein the surgical retractor includes ribs and a mechanism for microns-resolution transferring of linear and rotational movements of the ribs and wherein each rib can be easily replaced without use of any additional tools.According to an embodiment of the present invention the transmission is simpler. Likewise, an auxiliary handle is added to facilitate the insertion of the ribs into the body of the operated patient, and the vast majority of components of the surgical retractor are made with biocompatible, and FDA approved material ULTEM HU 1000 RESIN transparent (radiolucent) to X-ray radiation and are designated for single use.
Owner:MENI MED
Application of Lenvatinib to preparation of medicine for preventing and curing macular degeneration
The invention relates to application of Lenvatinib to preparation of medicine for preventing and curing macular degeneration, and belongs to the field of medicine. The problem that the conventional therapeutic medicine for macular degeneration is low in curative effect and low in patient compliance is solved, and the technical scheme is to provide application of Lenvatinib to preparation of medicine for preventing and curing macular degeneration. Lenvatinib is a multiple target point micromolecularly targeted inhibitor resist to VEGFR, PDGFR and bFGFR, and has been approved by FDA in the market for the treatment of tumors. The invention shows that Lenvatinib has potential for curing macular degeneration, and the application of Lenvatinib is verified by establishing an in vitro and vivo evaluation model, and the application has preferable clinic and social meanings.
Owner:GUANGDONG ZHONGSHENG PHARMA
Utilization of Non-Nutritive Adsorbents to Sequester Mycotoxins During Extraction of Protein or Other Value Added Components From Mycotoxin Contaminated Cereal or Seed Oil Meal
A method for the removal of mycotoxins from cereal or oil seed meal that includes the use of a mycotoxin sequestrant to form a food grade composition for human consumption wherein said composition contains no more or less than an FDA approved level of mycotoxin for a human food product.
Owner:UNITED STATES OF AMERICA
Coating for drinking water tube/container liners, preparation method and construction method
InactiveCN101638552AFast curingEasy constructionLiquid surface applicatorsPolyurea/polyurethane coatingsSolventPigment
The invention provides a coating for drinking water tube / container liners, a preparation method and a construction method, which can solve the problems in the prior art, such as toxicity and low construction efficiency, etc. The coating of the invention contains no solvent, and all the pigments and auxiliaries pass the FDA approval. Through detection, the products pass the BS6920 standard absolutely and can be safely applied to the drinking water duct liners. Meanwhile, the coating of the invention features rapid curing rate, convenient and fast construction and thick one-time forming film, and is extremely suitable for large-scale drinking water duct construction.
Owner:青岛佳联化工新材料有限公司
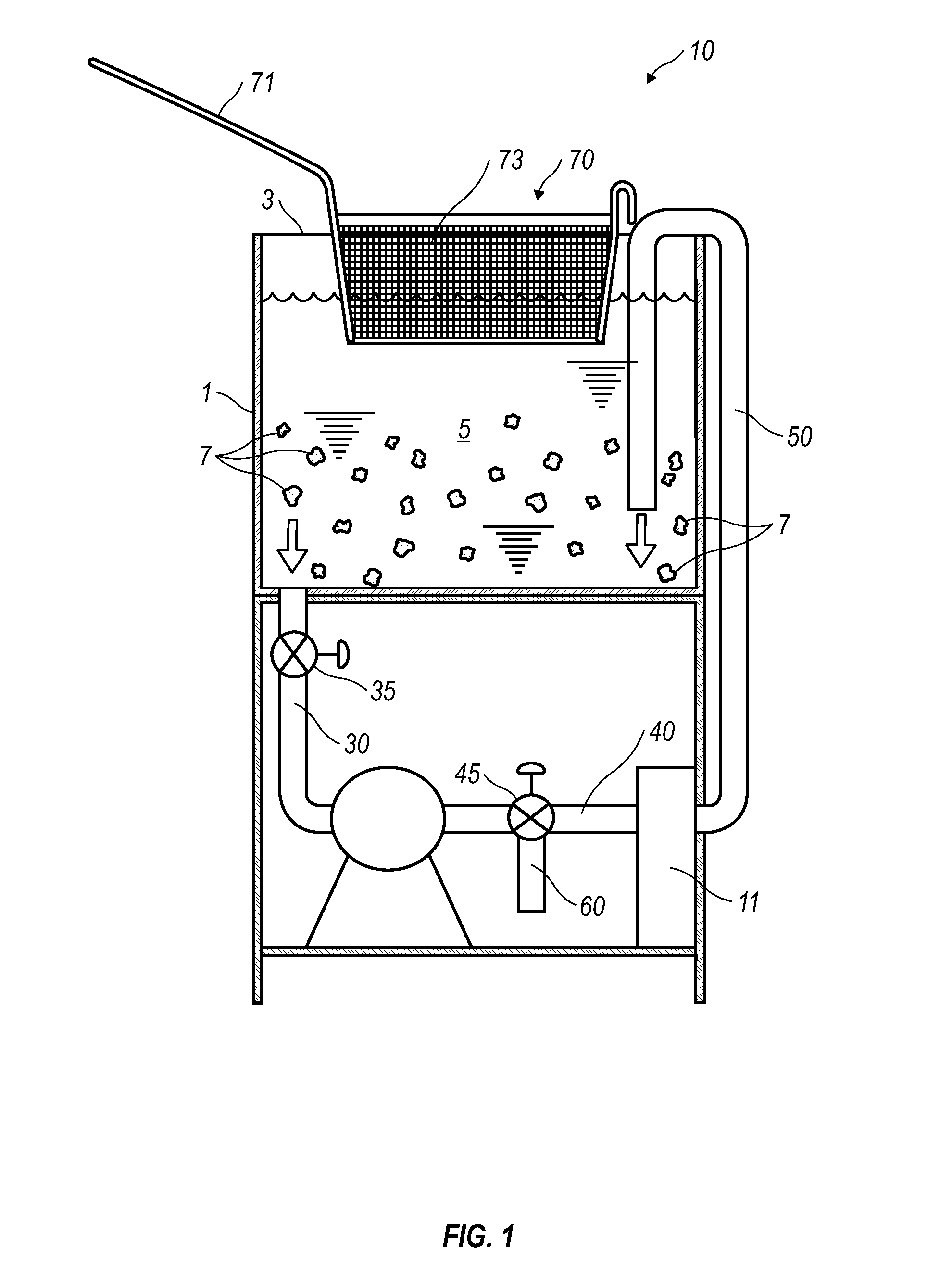
Cooking Oil Filtration System
InactiveUS20130008320A1Eliminates purification problemReduced likelihoodDeep fat fryersFiltrationFuel tank
A cooking oil filtration system for a cooking apparatus wherein organic contaminants suspended in hot cooking oil are separated and trapped in / on a hollow cylindrical filter made of high temperature food grade and / or FDA approved polymer material as the hot cooking oil is pumped through a filter housing fluidly connected to a cooking oil reservoir. A fryer basket with walls and a bottom constructed of fine wire mesh provide surface filtration of organic contaminants suspended in the hot cooking oil before passing through the filter housing.
Owner:KILMER MICHAEL CHRISTIAN
Apparatus and method for stretch-wrapping articles
InactiveUS7000370B2Reduce plastic usageImprove productivityWrappers shrinkageWrapper folding/bending apparatusLeading edgeFda approval
Owner:INT PAPER CO
Polyvinyl alcohol/chitosan composite soluble electrospun nanofibers for disinfectant Anti-bacterial and Anti-corrosion applications
PendingUS20190327966A1High antibacterial activityBiocideOrganic active ingredientsDisinfectantElectrospun nanofibers
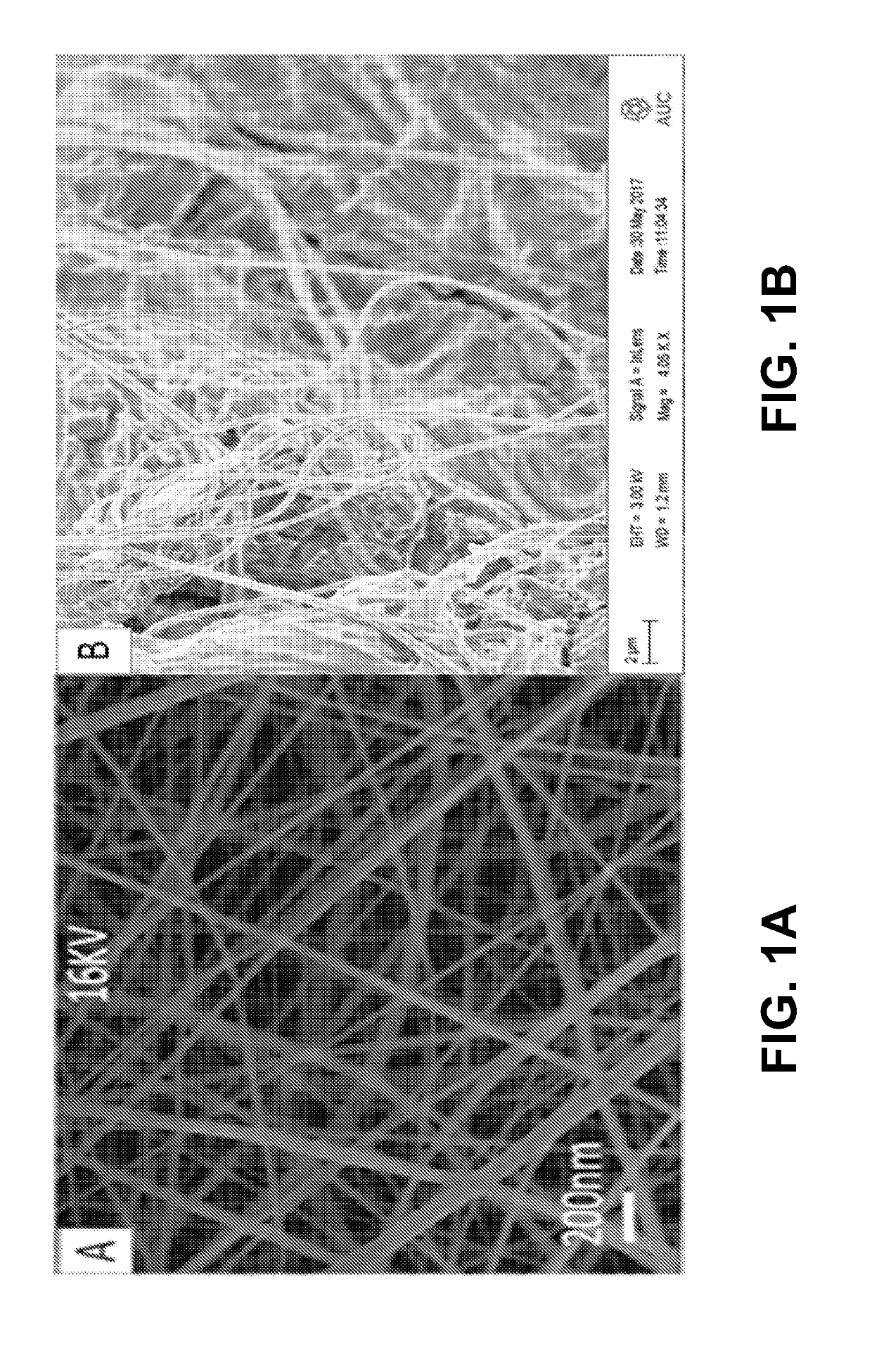
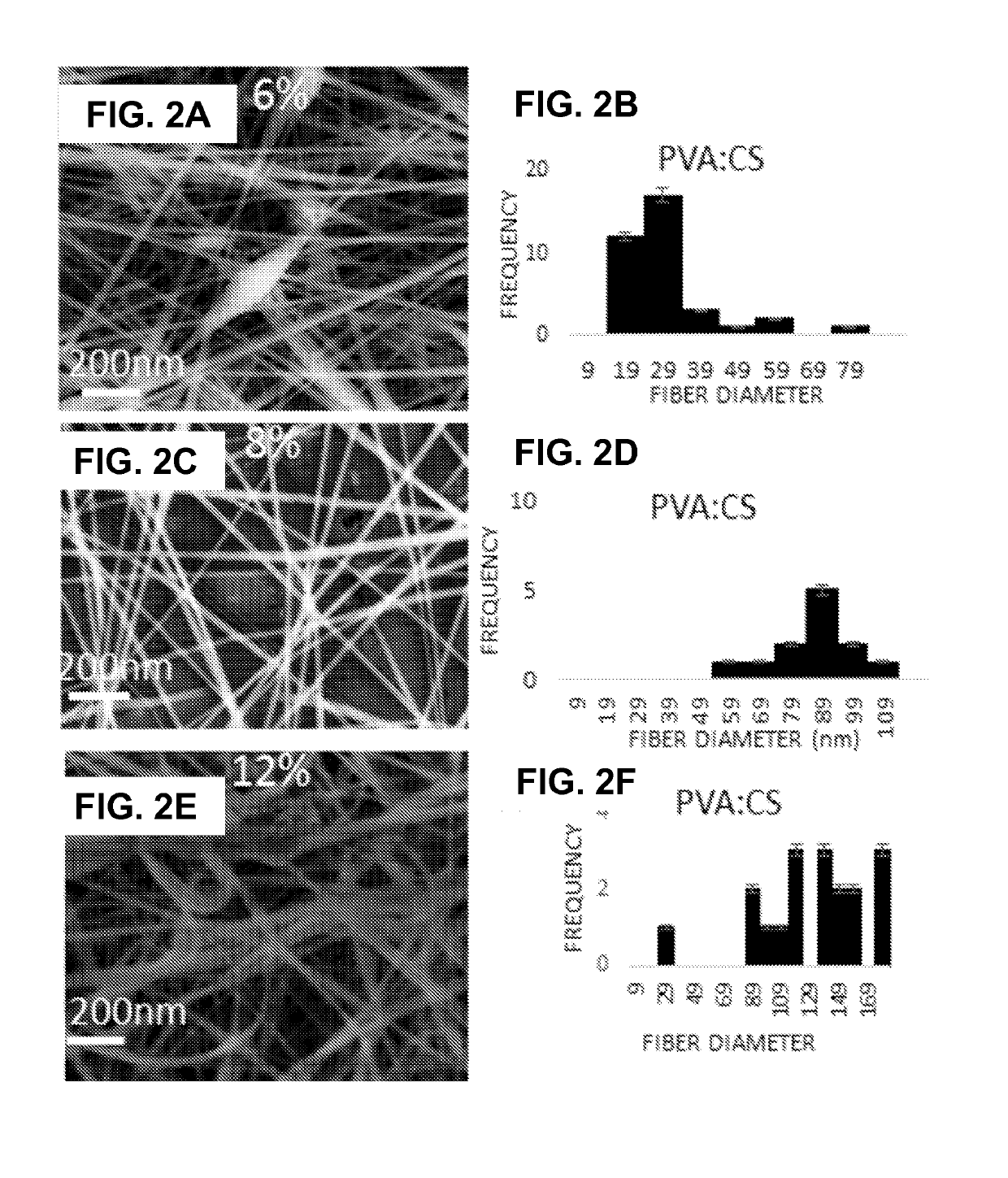
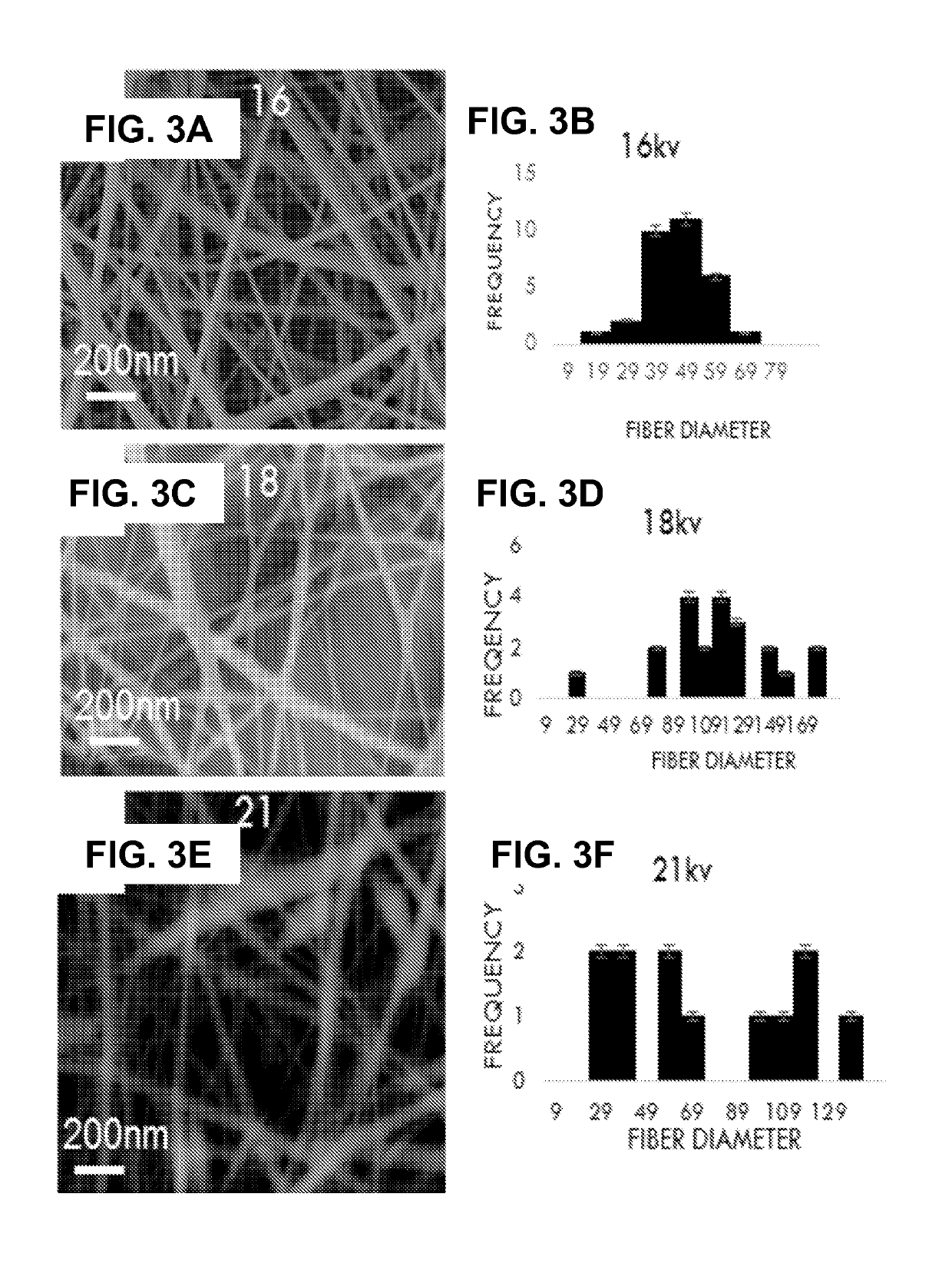
A natural liquid composition is provided for disinfectant, antibacterial or anticorrosion applications. The natural liquid composition is an electrospun solubilized composition of natural chitosan (CS) and an FDA approved polymer polyvinyl alcohol (PVA). Two fillers, citric acid (CA) and ascorbic acid (AA), added anti-corrosion property to the composite nanofiber solution without affecting the other properties (disinfectant and antibacterial) and have a final synergistic effect of all components together in a final solution form. Embodiment of the invention advances the art by providing a natural solution to overcome at least some of the problems with the currently used agents.
Owner:AMERICAN UNIVERSITY IN CAIRO
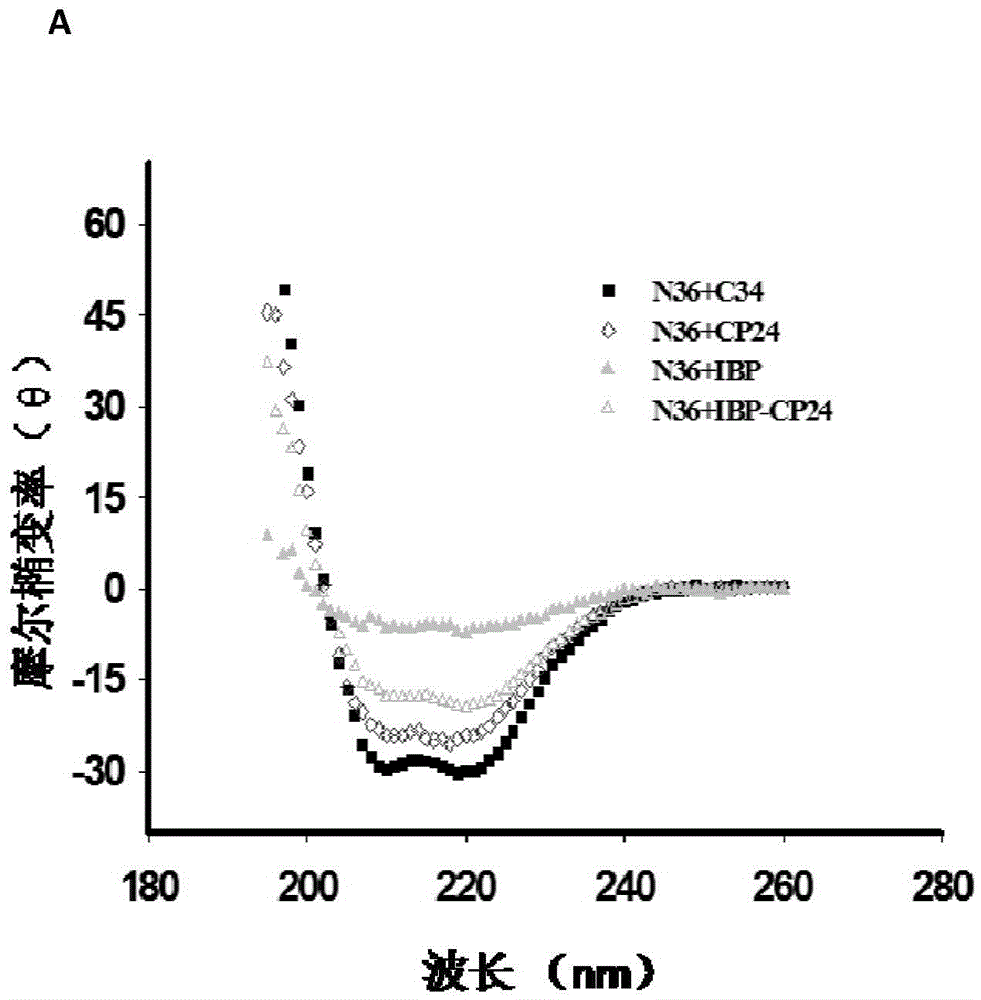
Long-acting HIV fusion inhibitor and application thereof
ActiveCN105646717APromote research and developmentExtended half-lifePeptide/protein ingredientsGenetic material ingredientsHalf-lifeTherapy HIV
Owner:FUDAN UNIV
Pediatric nasal gastric vent tube
InactiveUS7404807B2Reduce overflowPrevent overflowIsotope separationMedical syringesNoseGastric canal
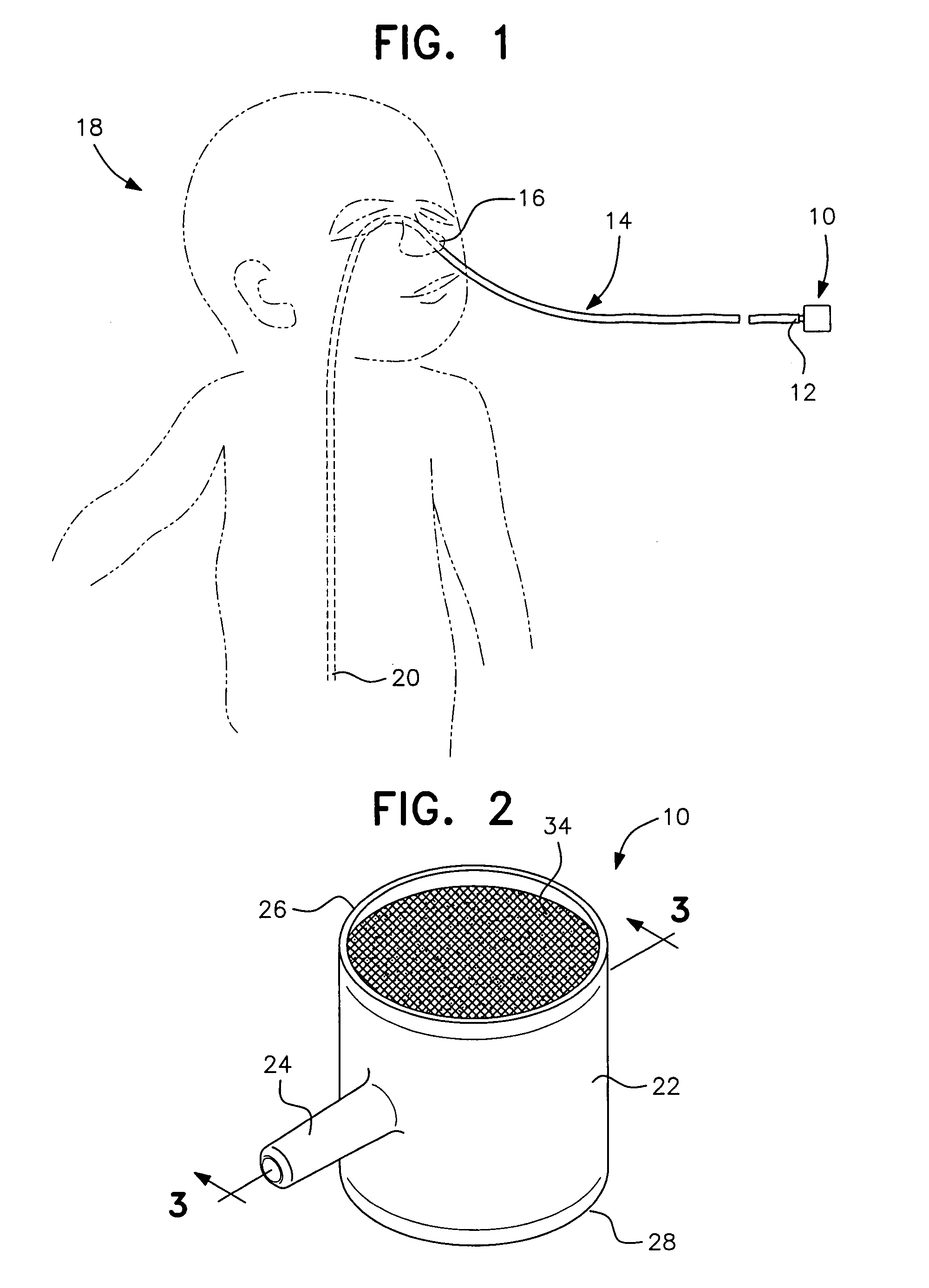
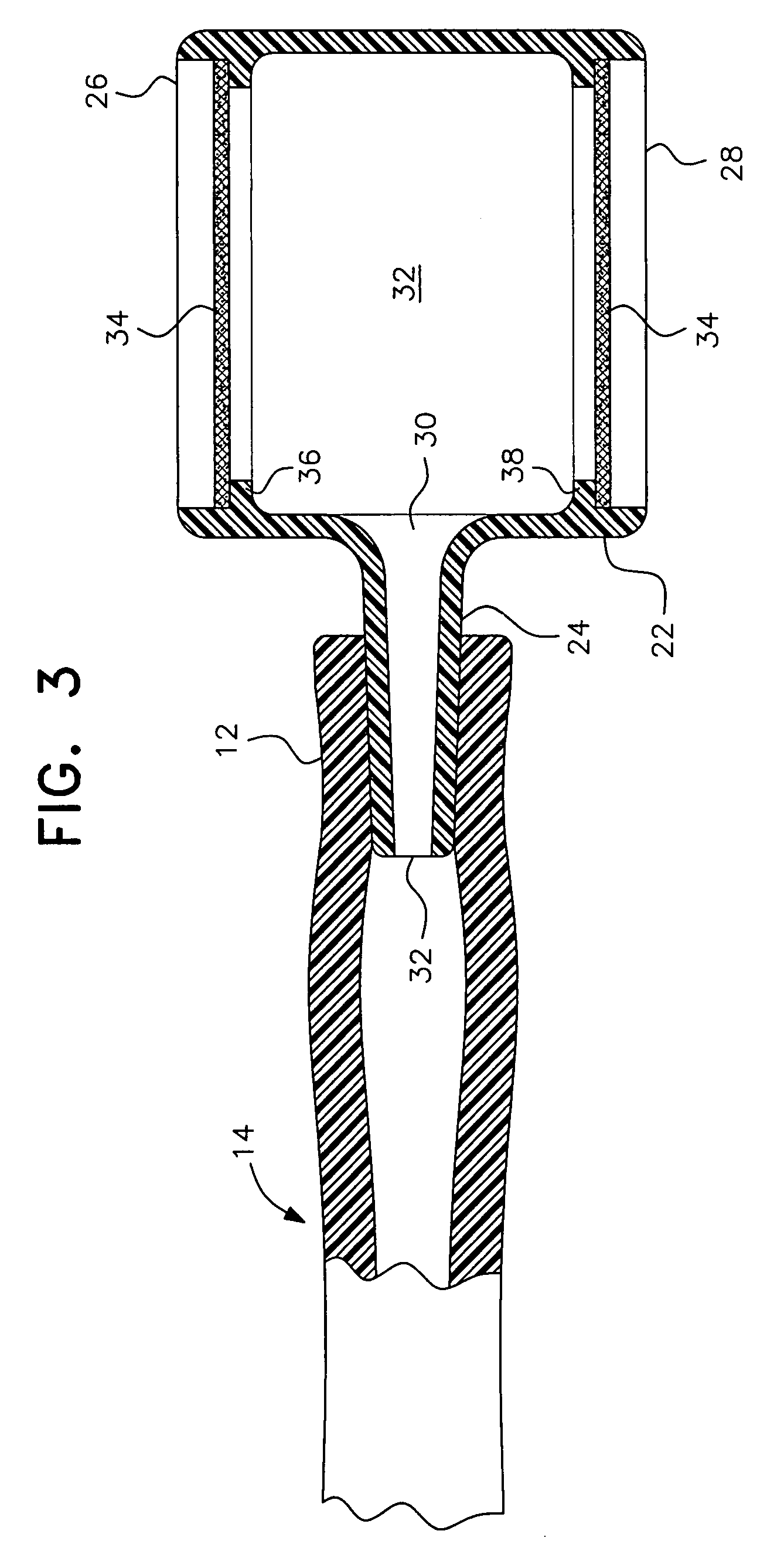
To alleviate the spillage of formula from a nasal-gastric tube, a small (5 cc size) constant diameter barrel having a side arm is inserted into the N-G tube. The equal sized open ends of the barrel are closed with a membrane that allows air to pass through but is impervious to liquids. The barrel is made of clear nylon material approved by the FDA for contact with formula for the baby.
Owner:CALLAWAY JAMES JOSIAH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com