Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Endotoxin Contamination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Endotoxin, a common contaminant of bacterial origin, has biological effects that can mask the true biological effects of nanoparticles, if its presence is overlooked. In this review, we report the features of nanoparticle contamination by endotoxin, and the different biological effects of endotoxin-contaminated nanoparticles.
Soluble recombinant botulinum toxin proteins
The present invention includes recombinant proteins derived from Clostridium botulinum toxins. In particular, soluble recombinant Clostridium botulinum type A, type B and type E toxin proteins are provided. Methods which allow for the isolation of recombinant proteins free of significant endotoxin contamination are provided. The soluble, endotoxin-free recombinant proteins are used as immunogens for the production of vaccines and antitoxins. These vaccines and antitoxins are useful in the treatment of humans and other animals at risk of intoxication with clostridial toxin.
Owner:ALLERGAN INC
Method and system for assay and removal of harmful toxins during pocess of tobacco products
InactiveCN1384714AInhibitionReduce toxin levelsTobacco preparationOrganic chemistryQuality controlEndotoxin Contamination
The present invention relates to methods and systems for the continuous analysis (160) and removal (410) of toxins from tobacco. Products, such as tobacco contaminated with mycotoxins, especially aflatoxins and benzopyrene and their precursors, are treated, usually in a solvent medium, to remove the toxin contamination from the tobacco. As an example, all noxious toxins eluted from the wash solvent (150) are continuously monitored (160) by immunoantibody UV fluorescence analysis. A quality control process ensures that harmful toxins are removed from the tobacco before further processing. Purification of extraction solvent streams and readditives ensures safe reuse or disposal of solvents or readditives.
Owner:凯丽·斯科特·莱恩
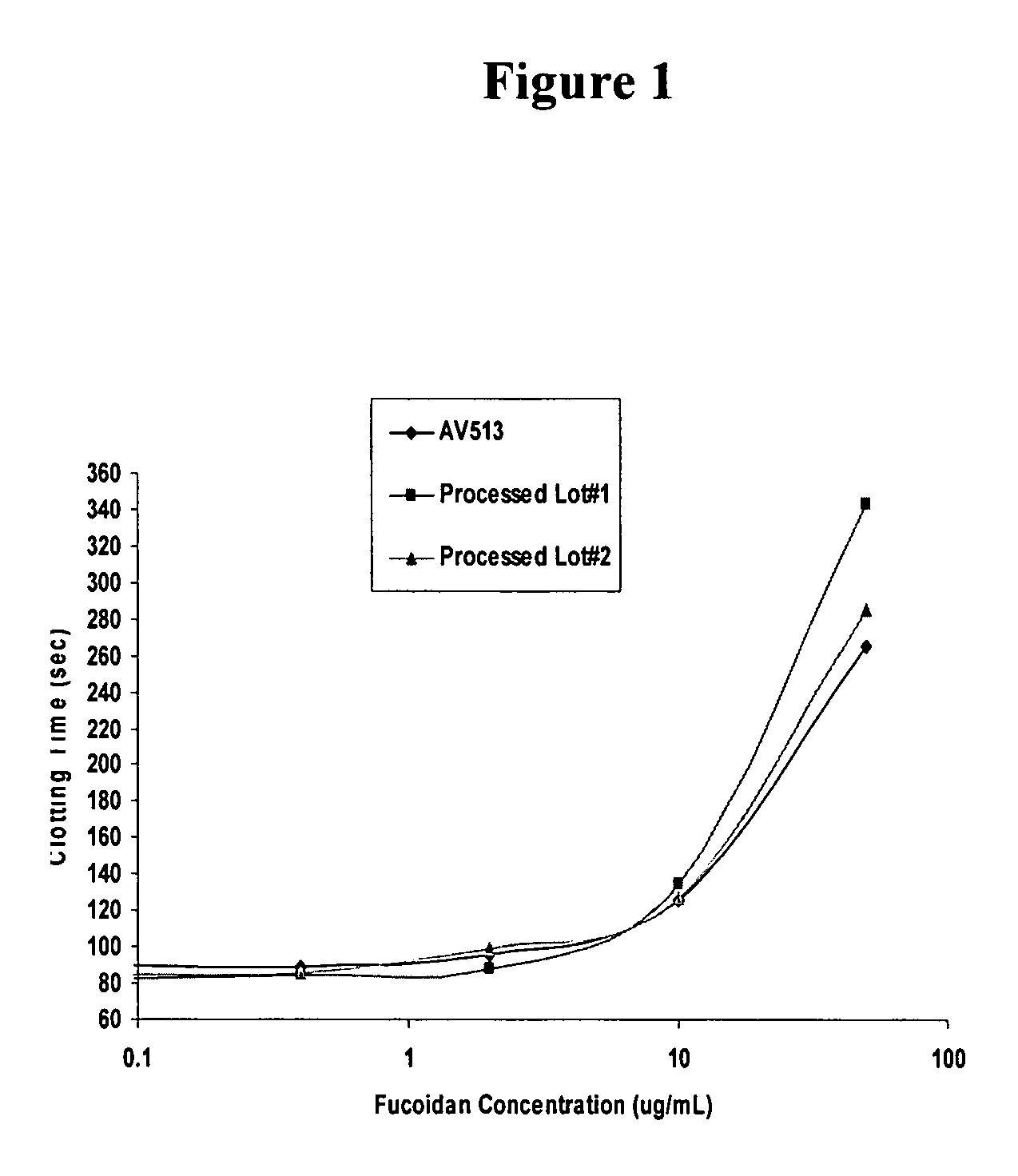
Methods for fucoidan purification from sea weed extracts
Methods for purifying fucoidan in extracts from brown seaweed are disclosed. In particular, methods of purifying fucoidan in the extract to remove heavy metal ions, bacterial and endotoxin contaminants, and other impurities are disclosed. The methods include the use of a chelating agent, selective precipitation, and filtration.
Owner:TAKEDA PHARMA CO LTD
Multi-copy metallothionein recombinant expression vector and method thereof for high-efficiency expression of metallothionein
InactiveCN103898153AReduce outputIncrease production costMicroorganism based processesVector-based foreign material introductionEndotoxin ContaminationZinc metallothionein
The invention provides a multi-copy metallothionein recombinant expression vector and a method thereof for high-efficiency expression of metallothionein, and belongs to the technical field of biological chemistry. The method is as follows: constructing the multi-copy metallothionein recombinant expression vector; converting the constructed multi-copy metallothionein recombinant expression vector in pichia pastoris GS115 or KM71, and screening on a histidine-free culture medium to obtain recombinant bacteria; inoculating the screened recombinant bacteria in a BMGY culture solution, rapidly oscillating for culture, centrifugally collecting bacteria, resuspending with the culture solution, diluting, rapidly oscillating for culture until log phase, adding methanol, and feeding the methanol in batches to continue to cultivate; collecting the bacteria, ultrasonically crushing, collecting supernatant by centrifugation, and separating and purifying the supernatant to obtain the metallothionein. The method overcomes the defects of a traditional metallothionein preparation method, can effectively avoid the waste of a lot of animal resources and bacteria sourced endotoxin contamination, reduces the production cost, and improves the protein yield.
Owner:山东东兴生物科技股份有限公司
Soluble recombinant botulinum toxin proteins
The present invention includes recombinant proteins derived from Clostridium botulinum toxins. In particular, soluble recombinant Clostridium botulinum type A, type B and type E toxin proteins are provided. Methods which allow for the isolation of recombinant proteins free of significant endotoxin contamination are provided. The soluble, endotoxin-free recombinant proteins are used as immunogens for the production of vaccines and antitoxins. These vaccines and antitoxins are useful in the treatment of humans and other animals at risk of intoxication with clostridial toxin.
Owner:ALLERGAN SALES ALLERGAN BOTOX
Scalable process for protein purification
ActiveUS7888098B2High purityEfficient removalVirus peptidesDepsipeptidesIon exchangeEndotoxin Contamination
Owner:KUROS US LLC
Method for establishing a system of dual-promoter methanol yeast efficiently expressing recombinant human metallothionein, and preparation and purification method of the protein
InactiveCN102660574AHigh expressionImprove filtration efficiencyFungiMicroorganism based processesDual promoterHarvesting organs
The invention discloses a method which is feasible in pharmaceutical industry for efficient secretion expressing, preparing and purifying of gene recombinant human metallothionein or analogues thereof. The method is characterized by introducing exogenous genes containing dual-promoters into relevant host yeast cells in transformation or double crossover recombination manner by using plasmid vector pPICZ alpha-MT of exogenous metal sulfur protein genes which contain methanol response element AOX and contain or not contain metal response element MRE to construction expression engineering cells containing the dual-promoters in the methanol yeast host cells. According to the invention, several problems with traditional preparation method of metallothionein, such as high production cost, slaughter and organ harvesting to large number of animals, virus pollution derived from animal, endotoxin pollution derived from bacterial, immunogen of non-humanized protein and the like, are avoided effectively,.
Owner:汪志友
Scalable Process for Protein Purification
ActiveUS20090048433A1High purityEfficient removalVirus peptidesDepsipeptidesEndotoxin ContaminationEngineering
The invention provides a process for the purification recombinantly expressed, self-assembled VLP from the homogenate of a bacterial host, wherein the process can be scaled up to a commercial production scale in a cost effective manner. The process comprises a first chromatography using an anion exchange matrix, a second chromatography using hydroxyapatite and, optionally, a size exclusion chromatography. VLP preparations obtained by the process of the invention are essentially free of endotoxin contaminations.
Owner:KUROS US LLC
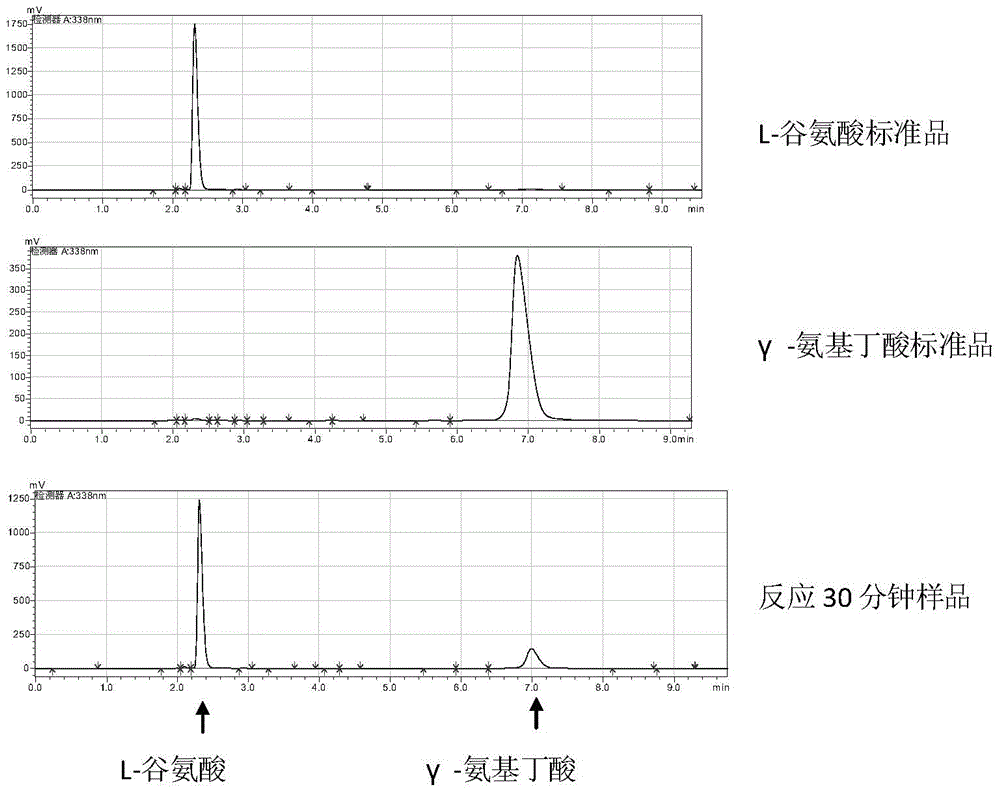
Engineering bacteria for producing gamma-aminobutyric acid and construction method and application thereof
ActiveCN104894043AHigh activityGood genetic stabilityBacteriaMicroorganism based processesGlutamate decarboxylaseDecomposition
The invention discloses engineering bacteria for producing gamma-aminobutyric acid and a construction method and application thereof, and belongs to the technical field of bioengineering. The engineering bacteria are obtained by integrating genes of L-glutamic acid decarboxylase and promoters thereof on chromosomes of host bacteria, and the integration loca are located in gamma-aminobutyric acid transaminase genes. The constructed engineering bacteria are good in heredity stability; the expressed L-glutamic acid decarboxylase is high in activity; excess protein expression, especially antibiotic resistant protein expression does not exist, and safety is high. Meanwhile, gamma-aminobutyric acid transaminase of the degradation product gamma-aminobutyric acid is inactivated in the engineering bacteria, so that decomposition of products is avoided in the production process and the yield of products is improved. The raw materials of the engineering bacteria are L-glutamic acid; the gamma-aminobutyric acid is produced efficiently through a biotransformation method, and the produced gamma-aminobutyric acid has no threat of endotoxin pollution, is safe and reliable and can be used in the field of food and heath care products.
Owner:SUZHOU RENBEN PHARMA
Method for removing endotoxin out of polypeptide
InactiveCN104031900AReduce operating volumeConducive to toxinsHormone peptidesCorticotropinEndotoxin ContaminationUltrafiltration
The invention discloses a method for removing endotoxin out of polypeptide. The method comprises the steps of dissolving the crude polypeptide in water, and carrying out ultrafiltration concentration to obtain a concentrated solution, sequentially adding an aqueous solution of calcium salt and an aqueous solution of phosphate to adjust the pH value to 8-9; and centrifuging, wherein the crude polypeptide is crude hyaluronidase, crude chorionic gonadotrophin, crude menotrophin or crude corticotropin. The method for removing endotoxin in the production of polypeptide disclosed by the invention; under the premise that the activity and yield of the polypeptide are not affected, endotoxin contaminants contained in the production of the polypeptide are effectively removed; at present, the method has been used in the manufacture of the polypeptide crude drugs, and the content of endotoxin is in line with the requirements of Chinese Pharmacopoeia (2010 Edition).
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Dual-promoter inducible secretable shuttle plasmid and construction method thereof
InactiveCN101948865APromote secretionConvenient shuttleAntibacterial agentsAntimycoticsEscherichia coliDual promoter
The invention belongs to the field of biological genetic engineering, and provides a dual-promoter inducible secretable shuttle plasmid. The shuttle plasmid consists of an enzyme cutting Escherichia coli pET-28a plasmid and an enzyme cutting bacillus subtilis pE194 plasmid, wherein a lysozyme-antibacterial peptide functional fragment and an antibacterial peptide gene are inserted into the enzyme cutting Escherichia coli pET-28a plasmid. The invention also provides a method for constructing the dual-promoter inducible secretable shuttle plasmid. The shuttle plasmid is transferred to a prokaryote to express proteins such as toxalbumin, cecropin and the like in a fusion mode without toxicity to a host. The shuttle plasmid constructed by the method can be induced by IPTG or lactose to improve gene expression level; the shuttle plasmid is also suitable for exocrine expression, and a target protein for the exocrine expression is convenient to process and is not polluted by endotoxin; and because the shuttle plasmid has dual functional promoters, different genes can be inserted in appropriate correct directions for gene expression.
Owner:FUDAN UNIV
Method for preparing inositol by multi-enzyme reaction system expressed by edible microorganisms
ActiveCN109913489AAvoid the possibility of contaminationReduce manufacturing costHydrolasesTransferasesEscherichia coliInositol monophosphatase
The invention discloses a method for preparing inositol by a multi-enzyme reaction system expressed by edible microorganisms. The method has the advantages that strains for the food industry serve asmulti-enzyme expression systems to produce isoamylase, glucan phosphorylase, glucose phosphate mutase, inositol-3-phosphate synthase and inositol monophosphatase, which are required for catalyzing starch and derivatives thereof, so that the possibility of contaminating the inositol by toxic protein, antigenic protein or endotoxin generated by escherichia coli in a production process is avoided fundamentally, a strict and complicated purification process is avoided, and the production cost is reduced.
Owner:CHENGDU BOHAODA BIOLOGICAL TECH CO LTD
Recombinant protein, pharmaceutical composition containing the same, and method of biosynthesizing
ActiveUS9233999B2Increase concentrationHigh yieldNervous disorderPeptide/protein ingredientsHigh concentrationYeast
The present invention provides a method of biosynthesizing a recombinant protein containing the biologically active peptide, the pharmaceutical composition containing the recombinant protein as well as the preparation of the recombinant protein. Simply speaking the consecutively multiple copies of the bioactive peptide are replaced in the amino acid sequence of a recombinant protein (so called peptide-protein). Then, a high concentration and high yield of the peptide-protein containing the consecutively multiple copies of the bioactive peptide is produced by the biosafety and edible strain of yeast without any endotoxin contamination.
Owner:ROSY DAWN BIOMEDICAL LTD
Porcine circovirus 3 Cap protein, nucleic acid, virus-like particles, vaccine, and preparation method and application of porcine circovirus 3 Cap protein
ActiveCN111253477AImprove expression efficiencyGood immune effectViral antigen ingredientsVirus peptidesProtein targetPorcine Circoviruses
The invention provides a porcine circovirus 3 Cap protein, nucleic acid, virus-like particles, vaccine, and a preparation method and application of the porcine circovirus 3 Cap protein, and relates tothe technical field of molecular biology. The porcine circovirus 3 Cap protein provided by the invention has an amino acid sequence shown as SEQ ID NO.2, can be expressed in a soluble manner in a yeast expression system, and effectively improves yeast expression efficiency of the porcine circovirus 3 Cap protein. In addition, the porcine circovirus 3 Cap protein provided by the invention can be self-assembled into the virus-like particles, the vaccine prepared by using the virus-like particles has the characteristics of cell immunity and humoral immunity, and immunologic related experiments show that the immune effect is good. The method for expressing the porcine circovirus type 3 Cap protein by utilizing the yeast expression system has the advantages that production cost is low, the production process is simple, and a large amount of target proteins can be obtained through high-density fermentation, has no endotoxin pollution, and can be used for large-scale production.
Owner:天康制药股份有限公司
Methods for fucoidan purification from sea weed extracts
Methods for purifying fucoidan in extracts from brown seaweed are disclosed. In particular, methods of purifying fucoidan in the extract to remove heavy metal ions, bacterial and endotoxin contaminants, and other impurities are disclosed. The methods include the use of a chelating agent, selective precipitation, and filtration.
Owner:TAKEDA PHARMA CO LTD
Pichia pastoris with endotoxin removal activity and application of pichia pastoris
The invention discloses pichia pastoris with endotoxin removal activity and an application of the pichia pastoris in endotoxin removal. The pichia pastoris with endotoxin removal activity is screenedby high-throughput breeding, and the pichia pastoris is preserved in CCTCC (China Center for Type Culture Collection) with the preservation number being CCTCC NO: M2017723. The invention further discloses the application of the pichia pastoris in the endotoxin removal. Whole cells, cell wall components or fermentation liquor of the pichia pastoris are added to endotoxin polluted feed, food or fermentation liquor, and residual level of endotoxin is reduced to 60% or lower. The pichia pastoris can be applied to the endotoxin removal of products such as feed, food, biological products and the like.
Owner:SHANDONG BIO SUNKEEN
Recombinant protein, pharmaceutical composition containing the same, and method of biosynthesizing
ActiveUS20140186429A1Increase concentrationReduce manufacturing costNervous disorderPeptide/protein ingredientsHigh concentrationYeast
Owner:ROSY DAWN BIOMEDICAL LTD
Method for removing endotoxin from influenza vaccine formulation
InactiveCN1621089AKeep active ingredientsEfficient removalAntiviralsPeptide preparation methodsMedicineFiltration
The present invention is method of eliminating endotoxin from influenza vaccine. Affinity medium is filled to form chromatographic column or membrane filter, which is used in processing influenza vaccine to eliminate endotoxin through static adsorption or filtration. The present invention features that the affinity medium is polypropylene. The method of eliminating endotoxin from influenza vaccine can eliminate endotoxin pollutant from influenza vaccine preparation effectively while maintaining the effective components in the vaccine and without altering the properties of the vaccine. The present invention may be used in the influenza vaccine producing process to raise the product quality.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A kind of engineering bacteria producing γ-aminobutyric acid and its construction and application
ActiveCN104894043BHigh activityGood genetic stabilityBacteriaMicroorganism based processesGlutamate decarboxylaseEngineering
The invention discloses engineering bacteria for producing gamma-aminobutyric acid and a construction method and application thereof, and belongs to the technical field of bioengineering. The engineering bacteria are obtained by integrating genes of L-glutamic acid decarboxylase and promoters thereof on chromosomes of host bacteria, and the integration loca are located in gamma-aminobutyric acid transaminase genes. The constructed engineering bacteria are good in heredity stability; the expressed L-glutamic acid decarboxylase is high in activity; excess protein expression, especially antibiotic resistant protein expression does not exist, and safety is high. Meanwhile, gamma-aminobutyric acid transaminase of the degradation product gamma-aminobutyric acid is inactivated in the engineering bacteria, so that decomposition of products is avoided in the production process and the yield of products is improved. The raw materials of the engineering bacteria are L-glutamic acid; the gamma-aminobutyric acid is produced efficiently through a biotransformation method, and the produced gamma-aminobutyric acid has no threat of endotoxin pollution, is safe and reliable and can be used in the field of food and heath care products.
Owner:山东蓝康药业有限公司
Method for preparing inositol by multi-enzyme reaction system expressed by edible microorganism
ActiveCN109913489BAvoid the possibility of contaminationReduce manufacturing costHydrolasesTransferasesEscherichia coliInositol monophosphatase
The invention discloses a method for preparing inositol by a multi-enzyme reaction system expressed by edible microorganisms. The method has the advantages that strains for the food industry serve asmulti-enzyme expression systems to produce isoamylase, glucan phosphorylase, glucose phosphate mutase, inositol-3-phosphate synthase and inositol monophosphatase, which are required for catalyzing starch and derivatives thereof, so that the possibility of contaminating the inositol by toxic protein, antigenic protein or endotoxin generated by escherichia coli in a production process is avoided fundamentally, a strict and complicated purification process is avoided, and the production cost is reduced.
Owner:CHENGDU BOHAODA BIOLOGICAL TECH CO LTD
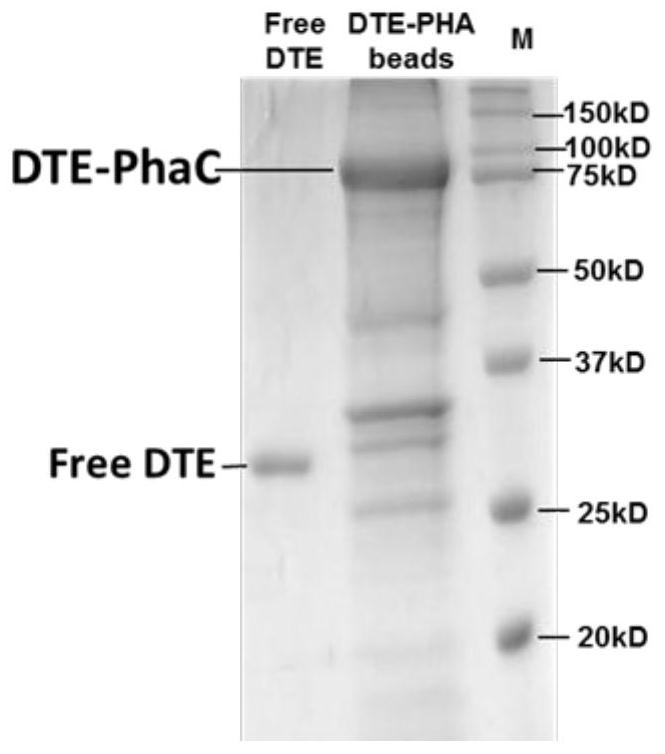
A kind of dte-immobilized nano-microspheres and preparation method thereof and method for producing d-psicose based thereon
ActiveCN110016472BIncrease production capacityAchieve fixationRacemaces/epimerasesIsomerasesTagatoseFood grade
Owner:XI AN JIAOTONG UNIV +1
Assay and kit for detection of endotoxin
ActiveUS10969388B2Quick checkImprove accuracyBiological material analysisBiological testingAssayNanoparticle
The present invention relates to a membrane based assay method, device and kit for rapid detection and / or quantification of endotoxins in aqueous solutions and test samples. The kit as per the present invention comprises lipopolysaccharide (LPS) affinity ligand conjugated with gold nanoparticles (GNPs); a membrane device comprising an endotoxin affinity membrane positioned parallelly to one or more layer(s) of a hydrophilic material, which are optionally secured in an enclosure; and optionally comprising an indicator chart for quantification of endotoxins in the sample. The method comprises placing the sample suspected of endotoxin contamination on a surface of a membrane comprised in a membrane device; placing once or more a suspension of LPS-affinity ligand conjugated with GNPs over the same area as the sample placed and detecting the presence of endotoxin if the colour signal appears and based on its intensity quantifying the endotoxin levels.
Owner:NANODX HEALTHCARE PVT LTD
Porcine circovirus type 3 cap protein, nucleic acid, virus-like particle, vaccine, preparation method and application
ActiveCN111253477BImprove expression efficiencyGood immune effectViral antigen ingredientsVirus peptidesProtein targetPorcine Circoviruses
The invention provides a porcine circovirus type 3 Cap protein, nucleic acid, virus-like particles, vaccines and preparation methods and applications, and relates to the technical field of molecular biology. The porcine circovirus type 3 Cap protein provided by the invention has such as SEQ The protein with the amino acid sequence shown in ID NO.2 can be expressed solublely in a yeast expression system, and effectively improves the yeast expression efficiency of porcine circovirus type 3 Cap protein. Moreover, the porcine circovirus type 3 Cap protein provided by the present invention can be self-assembled into virus-like particles, and the vaccine prepared by using the virus-like particles has the characteristics of both cellular immunity and humoral immunity. Immunological related experiments show that the immune effect is very good. it is good. The use of yeast expression system to express porcine circovirus type 3 Cap protein has the advantages of low production cost, simple production process, a large amount of target protein can be obtained through high-density fermentation, and no endotoxin pollution, and can be used for large-scale production.
Owner:天康制药股份有限公司
Method for removing endotoxin from influenza vaccine formulation
InactiveCN1277582CKeep active ingredientsEfficient removalAntiviralsPeptide preparation methodsMedicineFiltration
The present invention is method of eliminating endotoxin from influenza vaccine. Affinity medium is filled to form chromatographic column or membrane filter, which is used in processing influenza vaccine to eliminate endotoxin through static adsorption or filtration. The present invention features that the affinity medium is polypropylene. The method of eliminating endotoxin from influenza vaccine can eliminate endotoxin pollutant from influenza vaccine preparation effectively while maintaining the effective components in the vaccine and without altering the properties of the vaccine. The present invention may be used in the influenza vaccine producing process to raise the product quality.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Dual-promoter inducible secretable shuttle plasmid and construction method thereof
InactiveCN101948865BGood effectStrong initiativeVector-based foreign material introductionAgainst vector-borne diseasesDual promoterEscherichia coli
The invention belongs to the field of biological genetic engineering, and provides a dual-promoter inducible secretable shuttle plasmid. The shuttle plasmid consists of an enzyme cutting Escherichia coli pET-28a plasmid and an enzyme cutting bacillus subtilis pE194 plasmid, wherein a lysozyme-antibacterial peptide functional fragment and an antibacterial peptide gene are inserted into the enzyme cutting Escherichia coli pET-28a plasmid. The invention also provides a method for constructing the dual-promoter inducible secretable shuttle plasmid. The shuttle plasmid is transferred to a prokaryote to express proteins such as toxalbumin, cecropin and the like in a fusion mode without toxicity to a host. The shuttle plasmid constructed by the method can be induced by IPTG or lactose to improvegene expression level; the shuttle plasmid is also suitable for exocrine expression, and a target protein for the exocrine expression is convenient to process and is not polluted by endotoxin; and because the shuttle plasmid has dual functional promoters, different genes can be inserted in appropriate correct directions for gene expression.
Owner:FUDAN UNIV
Point of Sampling Kit and Method for Assessing Endotoxin Contamination
PendingUS20220341929A1Easy and fast measurementQuantitative results are accurateMaterial analysis by observing effect on chemical indicatorBiological material analysisOptical transparencyEndotoxin Contamination
A point-of-use kit is designed for optically detecting and quantifying bacterial endotoxin by employing specific formulations of Limulus amebocyte lysate (LAL), each formulation designed to optimize results with different sample classes. Kits are pre-certified for use with a variety of environmental, industrial, and clinical samples, each sample category having a unique kit design and containing a unique lysate reagent formulation. Pre-certification transfers time and reagent consuming tasks, such as assessment of sample compatibility and sample effect on reagent sensitivity to endotoxin from the user to the kit producer. A fixed dilution / sample treatment is employed, eliminating the need for a comparative water standard and for a sample positive control. The kit has LAL reagent prepackaged in dry polyethylene capped tubes, which retains reagent shelf life and optical clarity for accurate and reproducible results using a portable spectrophotometer / optical reader.
Owner:ATLANTIC CAPES SUSTAINABLE LYSATE LLC
Methods for Fucoidan Purification from Seaweed Extracts
Methods for purifying fucoidan in extracts from brown seaweed are disclosed. In particular, methods of purifying fucoidan in the extract to remove heavy metal ions, bacterial and endotoxin contaminants, and other impurities are disclosed. The methods include the use of a chelating agent, selective precipitation, and filtration.
Owner:TAKEDA PHARMA CO LTD
An assay and kit for detection of endotoxin
ActiveUS20190079088A1Quick checkImprove accuracyBiological material analysisNanomedicineAssayNanoparticle
The present invention relates to a membrane based assay method, device and kit for rapid detection and / or quantification of endotoxins in aqueous solutions and test samples. The kit as per the present invention comprises lipopolysaccharide (LPS) affinity ligand conjugated with gold nanoparticles (GNPs); a membrane device comprising an endotoxin affinity membrane positioned parallelly to one or more layer(s) of a hydrophilic material, which are optionally secured in an enclosure; and optionally comprising an indicator chart for quantification of endotoxins in the sample. The method comprises placing the sample suspected of endotoxin contamination on a surface of a membrane comprised in a membrane device; placing once or more a suspension of LPS-affinity ligand conjugated with GNPs over the same area as the sample placed and detecting the presence of endotoxin if the colour signal appears and based on its intensity quantifying the endotoxin levels.
Owner:NANODX HEALTHCARE PVT LTD
Method for removing endotoxin in trypsinase
InactiveCN104017792AReduce operating volumeConducive to dialysisPeptidasesUltrafiltrationEndotoxin removal
The invention discloses a method for removing endotoxin in trypsinase. The method comprises the following steps: dissolving a trypsinase filter cake in water, carrying out ultrafiltration concentration to obtain a concentrated solution, sequentially adding a phosphate water solution and a calcium salt water solution, regulating the pH value to 8-9, and centrifugating. On the premise of not influencing the activity and yield of the trypsinase, the method for removing endotoxin in the trypsinase production process effectively removes the endotoxin pollutant contained in the trypsinase production process, and has been applied to trypsinase raw drug production at present; and the endotoxin content conforms to the related requirement.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Hemostatic material and wound dressing material containing same
InactiveCN110997018APromote healingEasy to useSurgical adhesivesPharmaceutical delivery mechanismCellulosePolymer science
The purpose of the present invention is to provide: a hemostatic material which eliminates the risks characteristic of conventional chitosan-derived products such as endotoxin contamination and triggering of shellfish allergy, can be safely used on more people, and has antibiotic properties and hemostatic effects that widely-used hydrogels do not have; and a wound dressing material containing thesame. Provided are a hemostatic agent containing a cationized cellulose and a wound dressing material containing the same. At least one hydroxyl group of the cationized cellulose is modified with -R2-N+(R3)(R4)(R5).X-, and the other hydroxyl groups of the cationized cellulose have -H or -(CH2CH2O)m-H, where R2 is C1-6 alkylene, C2-6 hydroxyalkylene, -(CH2CH2O)l- or a combination thereof, and l is1 or 2, m is 1 or 2, and X- may be an anionic group.
Owner:医疗发明株式会社 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com