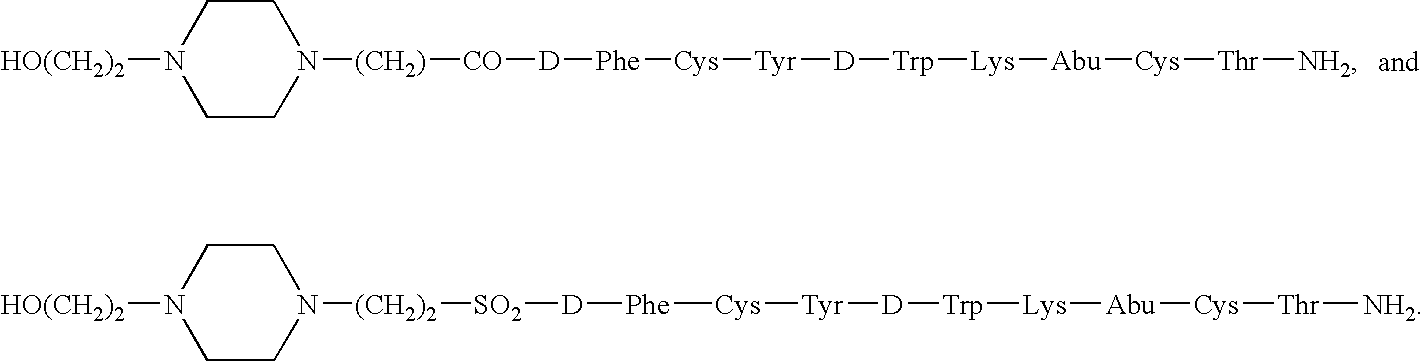Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
119results about "Corticotropin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation of protein microparticles by supercritical fluid precipitation
InactiveUS6063910AUniform and fine particle sizePowder deliveryPeptide/protein ingredientsMicroparticleSolvent
The present invention comprises passing a solution of a soluble material, preferably a protein, in a solvent through a continuum of supercritical antisolvent fluid and precipitating the soluble material. This can be conducted by passing the solution through the continuum of supercrital fluid in the form of droplets, which can be sprayed through the supercritical fluid. The plurality of droplets can be passed cocurrently or countercurrently with respect to a stream of antisolvent fluid. Alternatively, the solution can be passed through the continuum of supercritical antisolvent fluid in the form of a thin film or a plurality of fine streams.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Activatable clostridial toxins
InactiveUS20080032931A1Minimize security riskEnhanced efficiency and rateHydrolasesPeptide/protein ingredientsClostridial toxinSaxitoxin
Owner:ALLERGAN INC
Non-aqueous single phase vehicles and formulations utilizing such vehicles
InactiveUS20050008661A1Increase stickinessReduce occlusionPowder deliveryPeptide/protein ingredientsEngineeringSolvent
The present invention includes materials and methods for providing vehicles useful for providing drug formulations that address the potential drawbacks of known nonaqueous formulations. In particular, the present invention includes nonaqueous vehicles that are formed using a combination of polymer and solvent that results in a vehicle that is miscible in water. The nonaqueous vehicles facilitate the formulation of drug formulations that are stable over time, even when stored at, or exposed to, elevated temperatures. Moreover, the miscible vehicles of the present invention allow the preparation of drug formulations that work to reduce the occurrence of partial or complete occlusions of the delivery conduits included in delivery devices used to administer the drug formulations.
Owner:DURECT CORP
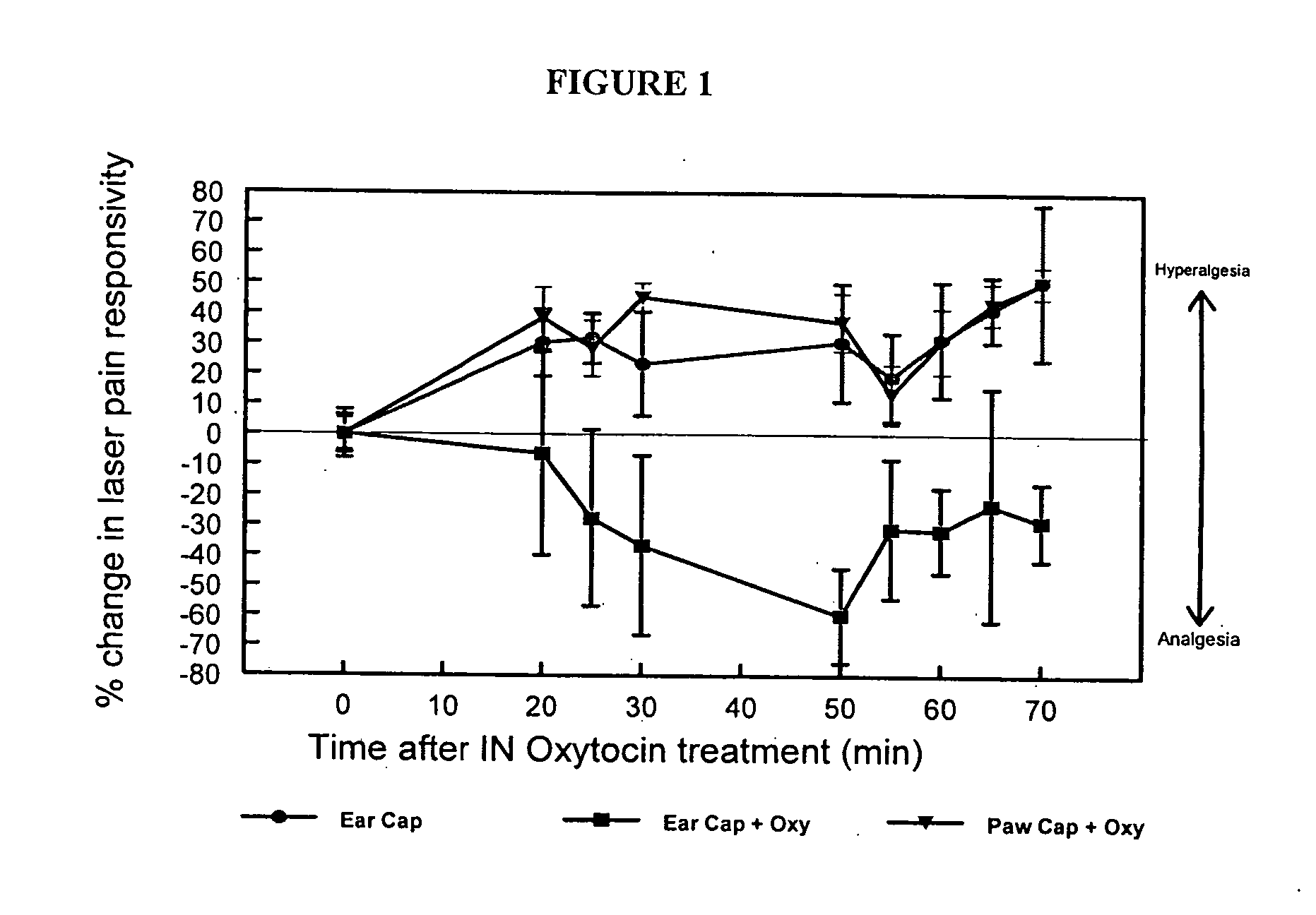
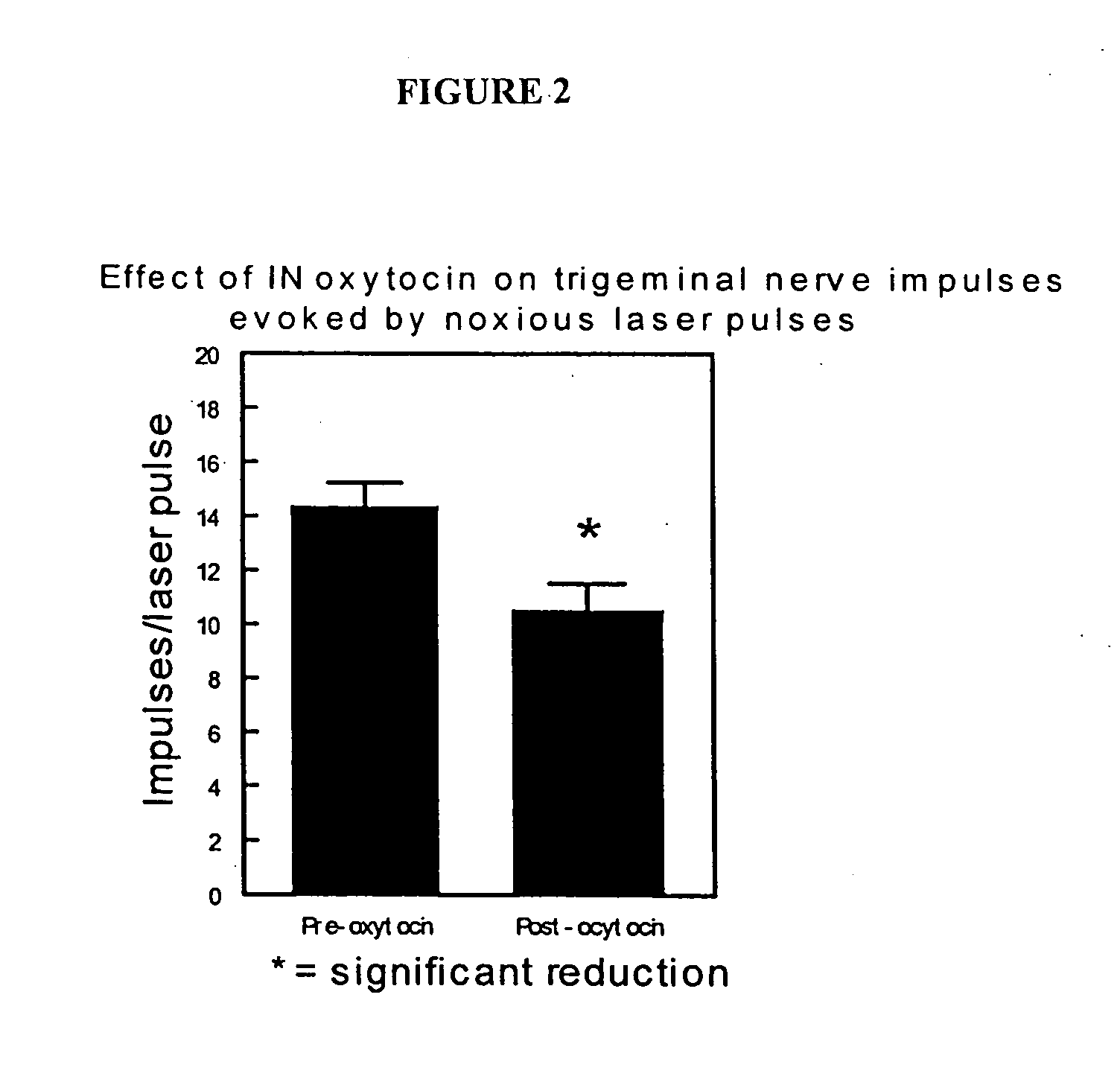
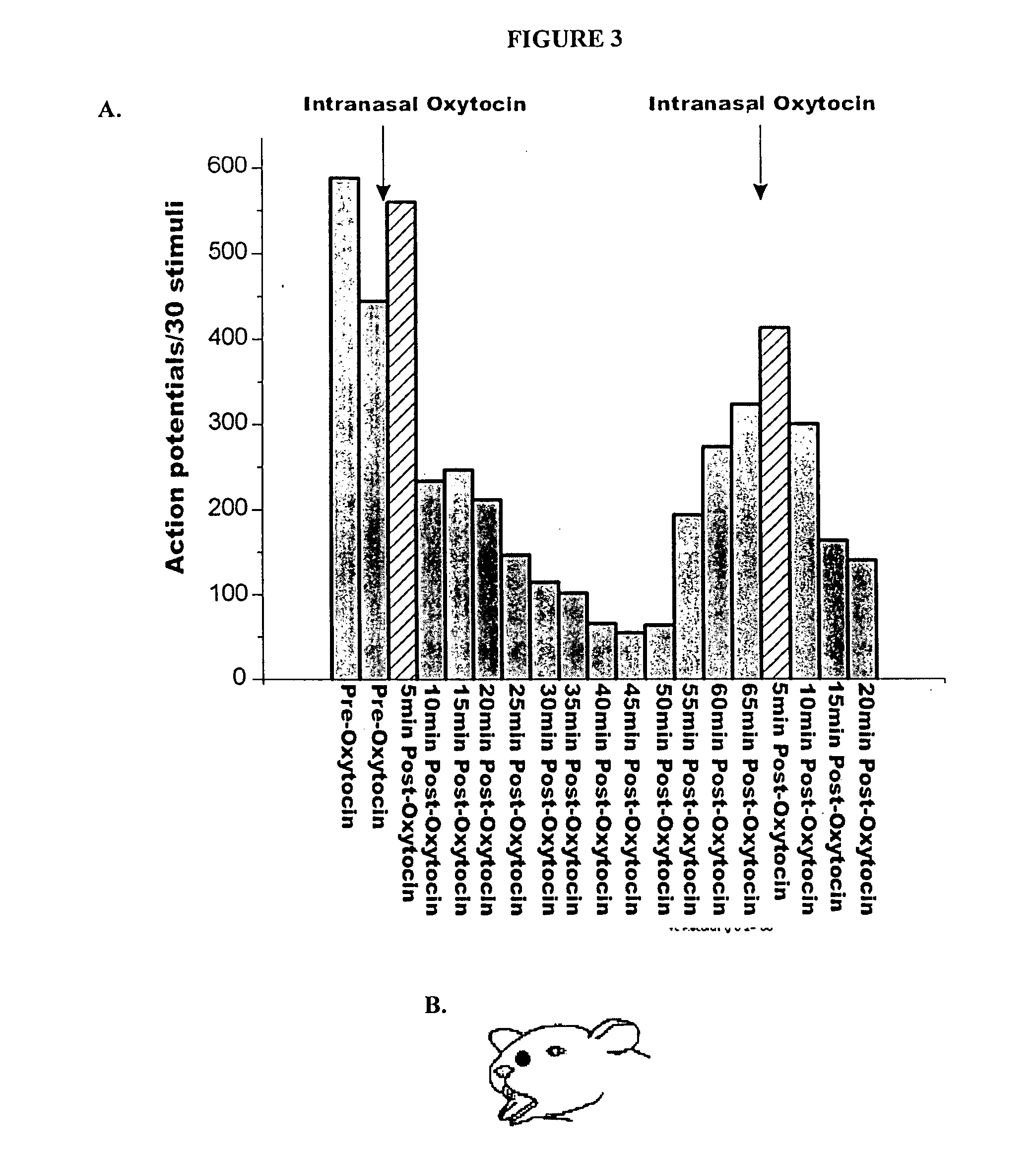
Methods for treatment of headaches by administration of oxytocin
InactiveUS20070054843A1Reduction of pain ratingReduce probabilityOrganic active ingredientsNervous disorderHeadache DisordersTrigeminal neuralgia
The present invention relates to methods for the treatment of headache and headache disorders. The methods comprise administration of an oxytocin peptide for the treatment of primary and secondary headaches or trigeminal neuralgia.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +2
Non-aqueous single phase vehicles and formulations utilizing such vehicles
InactiveUS20050276856A1Decrease in levelLower Level RequirementsPowder deliveryPeptide/protein ingredientsMedicineDelivery vehicle
Owner:DURECT CORP
Polymer-based sustained release device
ActiveUS20050271702A1Improve bioavailabilityMinimizes loss of activityPowder deliveryPeptide/protein ingredientsMedicineSugar
This invention relates to compositions for the sustained release of biologically active polypeptides, and methods of forming and using said compositions, for the sustained release of biologically active polypeptides. The sustained release compositions of this invention comprise a biocompatible polymer having dispersed therein, a biologically active polypeptide and a sugar.
Owner:ALKERMES PHARMA IRELAND LTD
Method of treating osteoarthritis with an antibody to NGF
Methods are disclosed for treating osteoarthritis in a human subject in need thereof, comprising administering to the subject a therapeutically effective amount of an anti-human NGF antibody, or antigen-binding fragment thereof, wherein at least one symptom associated with osteoarthritis is prevented, ameliorated or improved.
Owner:REGENERON PHARM INC
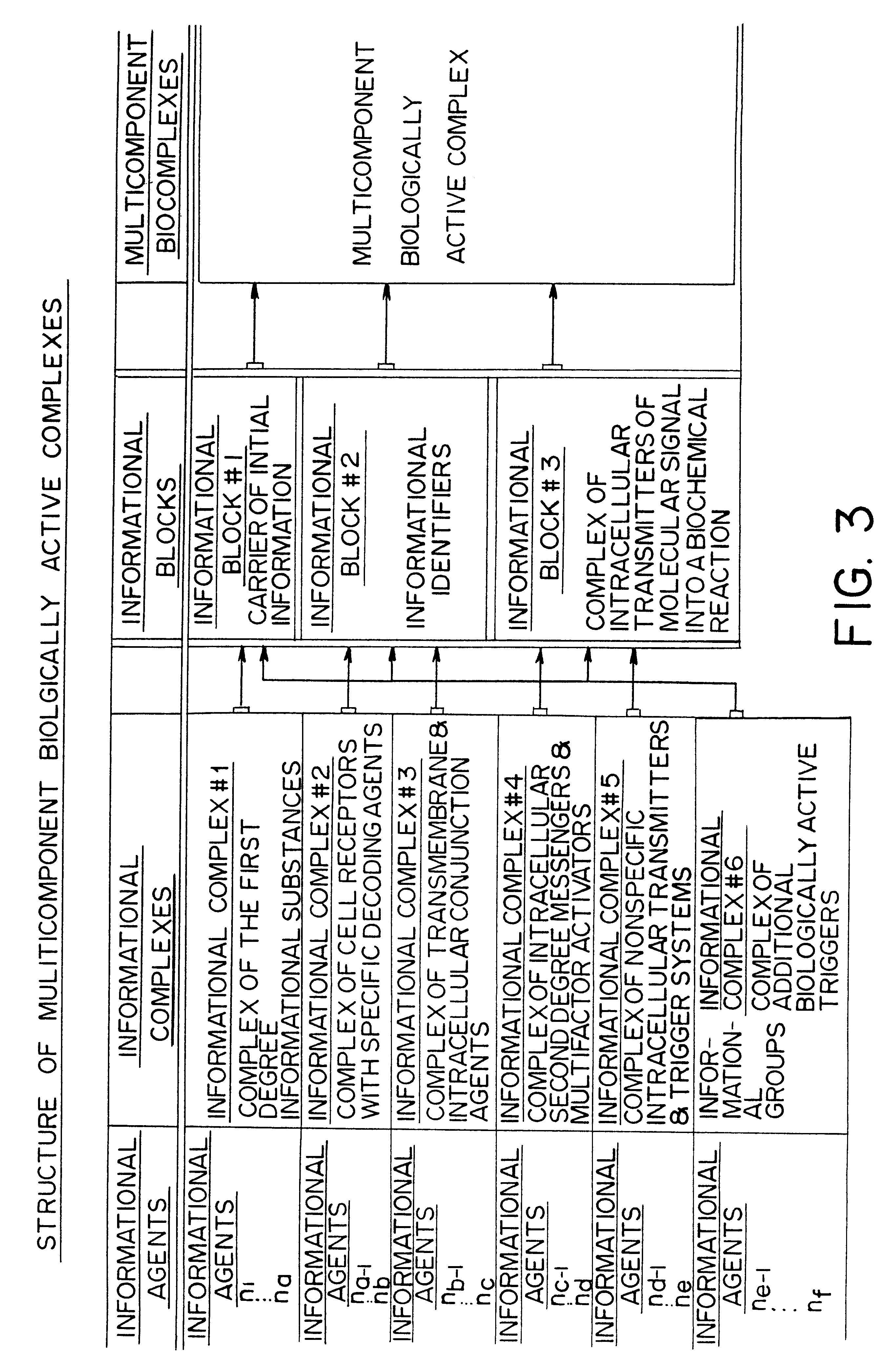
Therapeutic methods utilizing naturally derived bio-active complexes and delivery systems therefor
InactiveUS6303588B1Restore lipid membraneHigh oxygen utilizationCosmetic preparationsBiocideMedicineNormal cell
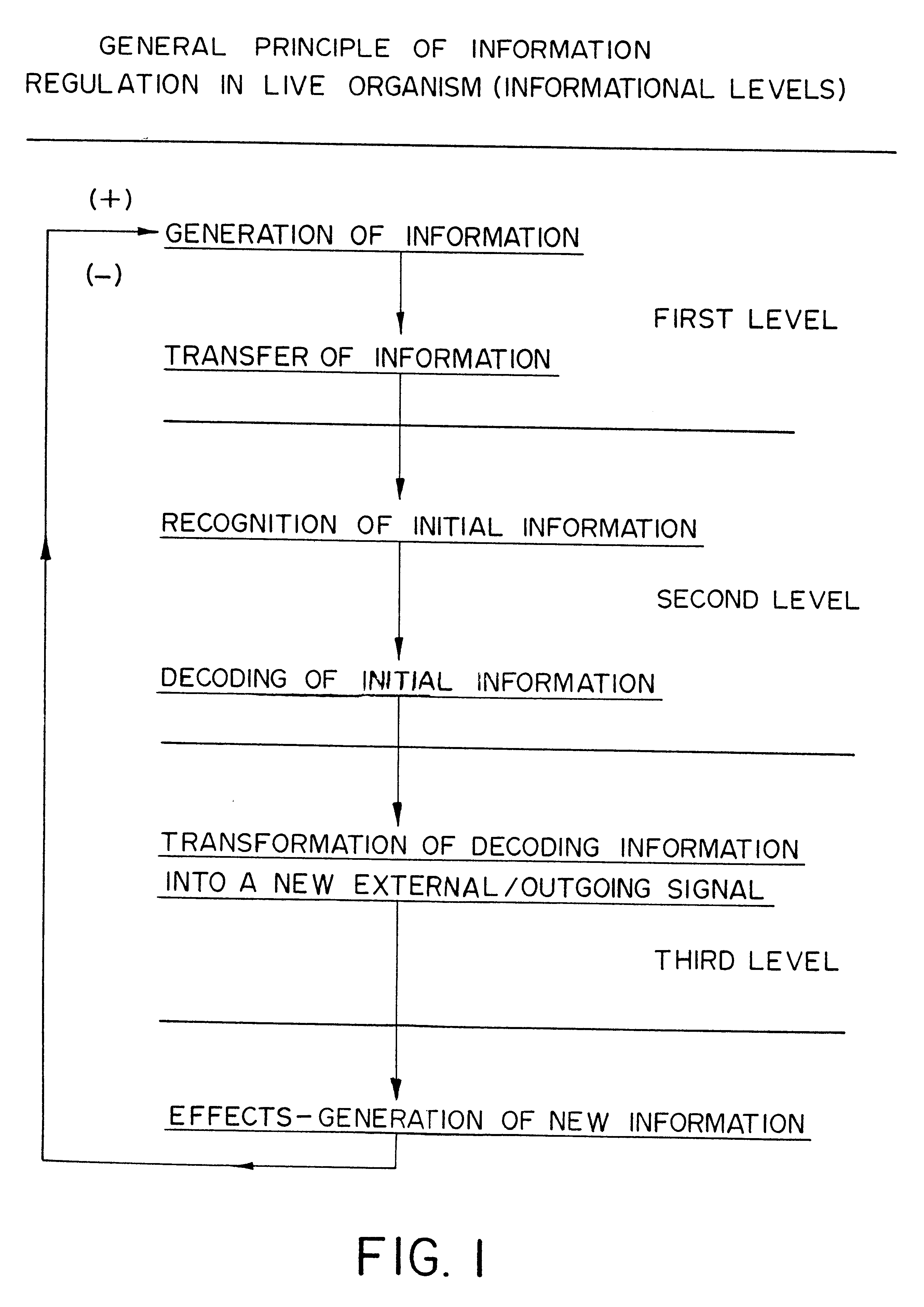
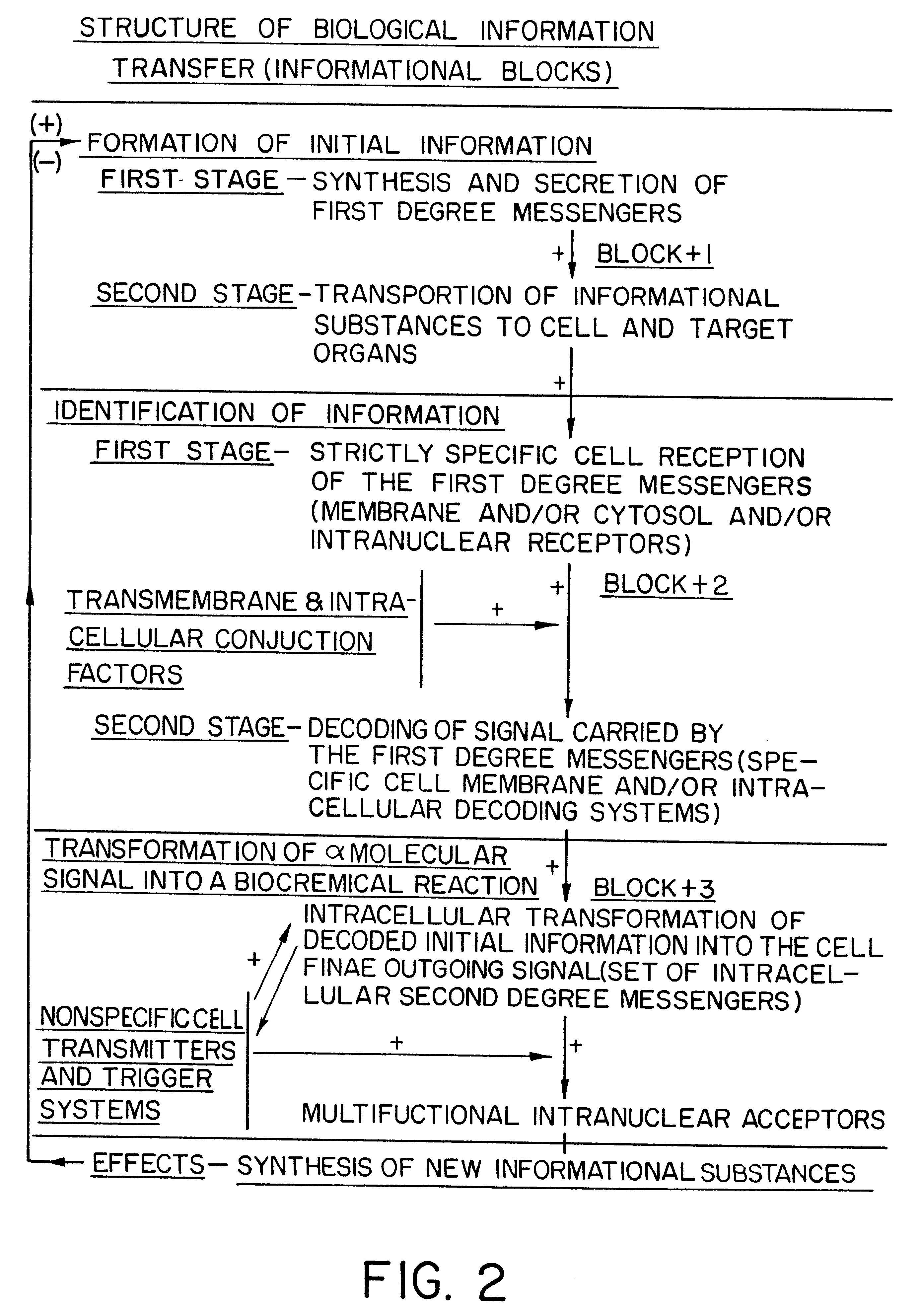
Methods are disclosed for correcting biological information transfer in a patient in need of such therapy which comprise administration to a patient of a composition comprising a therapeutically effective amount of a biocomplex comprising at least one bioactive agent from each of the three informational blocks of biological information transfer, each agent being present in an amount sufficient to correct the biological information transfer of the patient under treatment and resulting in the resumption of normal cell metabolism, said amount being less than the buffering amount of said agent; together with a carrier therefor.
Owner:DANIELOV MICHAEL M
Compositions containing satiogens and methods of use
Owner:SATIOGEN PHARMA
Polymer-based sustained release device
ActiveUS20070166352A1Improve bioavailabilityMinimizes loss of activityPowder deliveryOrganic active ingredientsMedicineSugar
This invention relates to compositions for the sustained release of biologically active polypeptides, and methods of forming and using said compositions, for the sustained release of biologically active polypeptides. The sustained release compositions of this invention comprise a biocompatible polymer having dispersed therein, a biologically active polypeptide and a sugar.
Owner:ALKERMES PHARMA IRELAND LTD +1
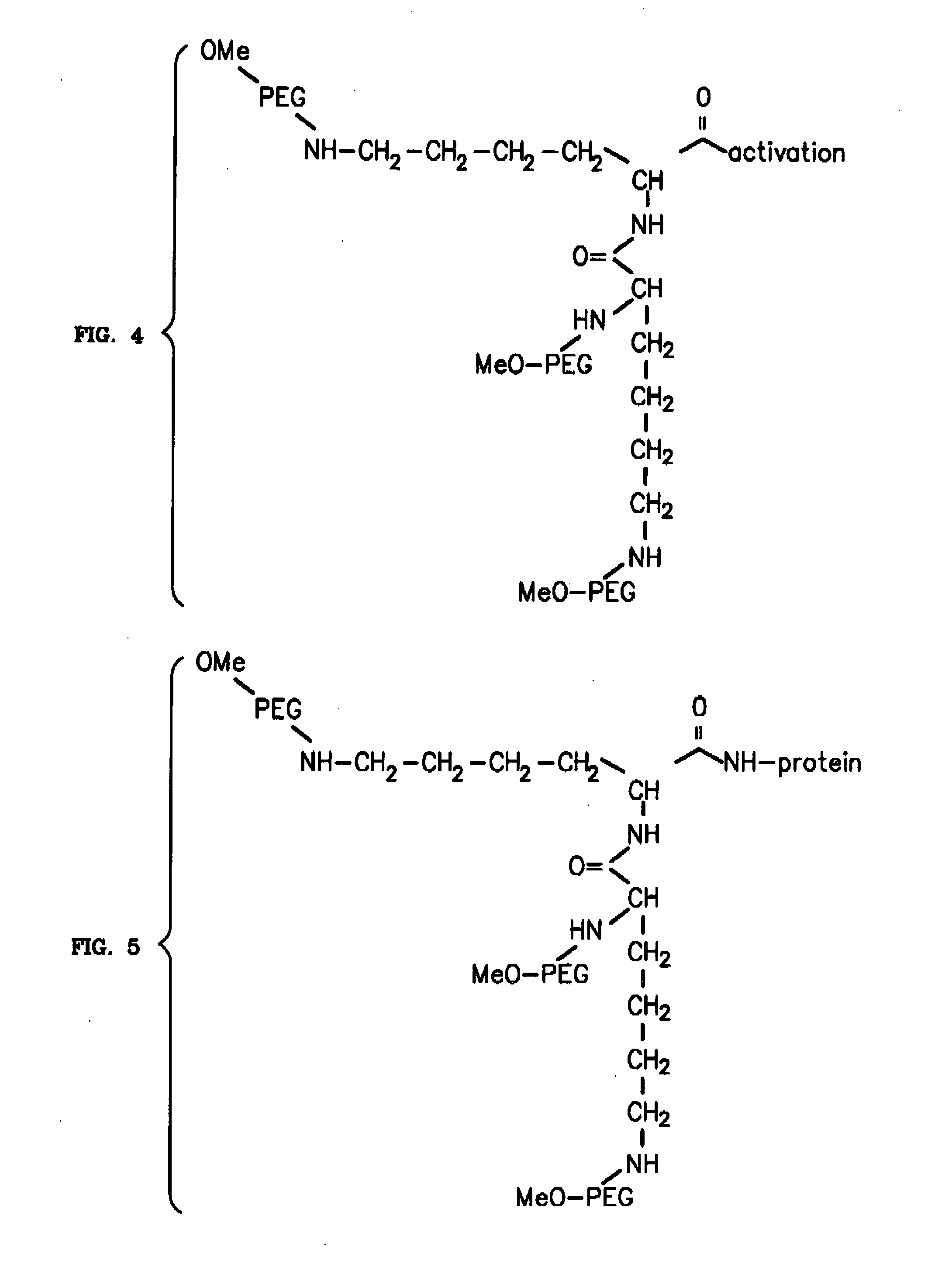
Novel PEGylation agent
InactiveUS20070184015A1Reduce compliancePromote degradationPeptide/protein ingredientsCalcitoninsHalf-lifeBlood plasma
To address the issue of degradation by enzymatic reactions to proteins and peptides, polyethylene glycol (PEGylation) of the proteins and peptides has been established. PEGylated proteins and peptides have increased plasma half-lives and reduced immunogenicity. To further improve and extend the plasma half-life of desired protein or peptide therapeutics, a novel branched molecule of PEG possessing three PEGs with a single point of attachment is designed in this invention disclosure.
Owner:HAHN SOONKAP
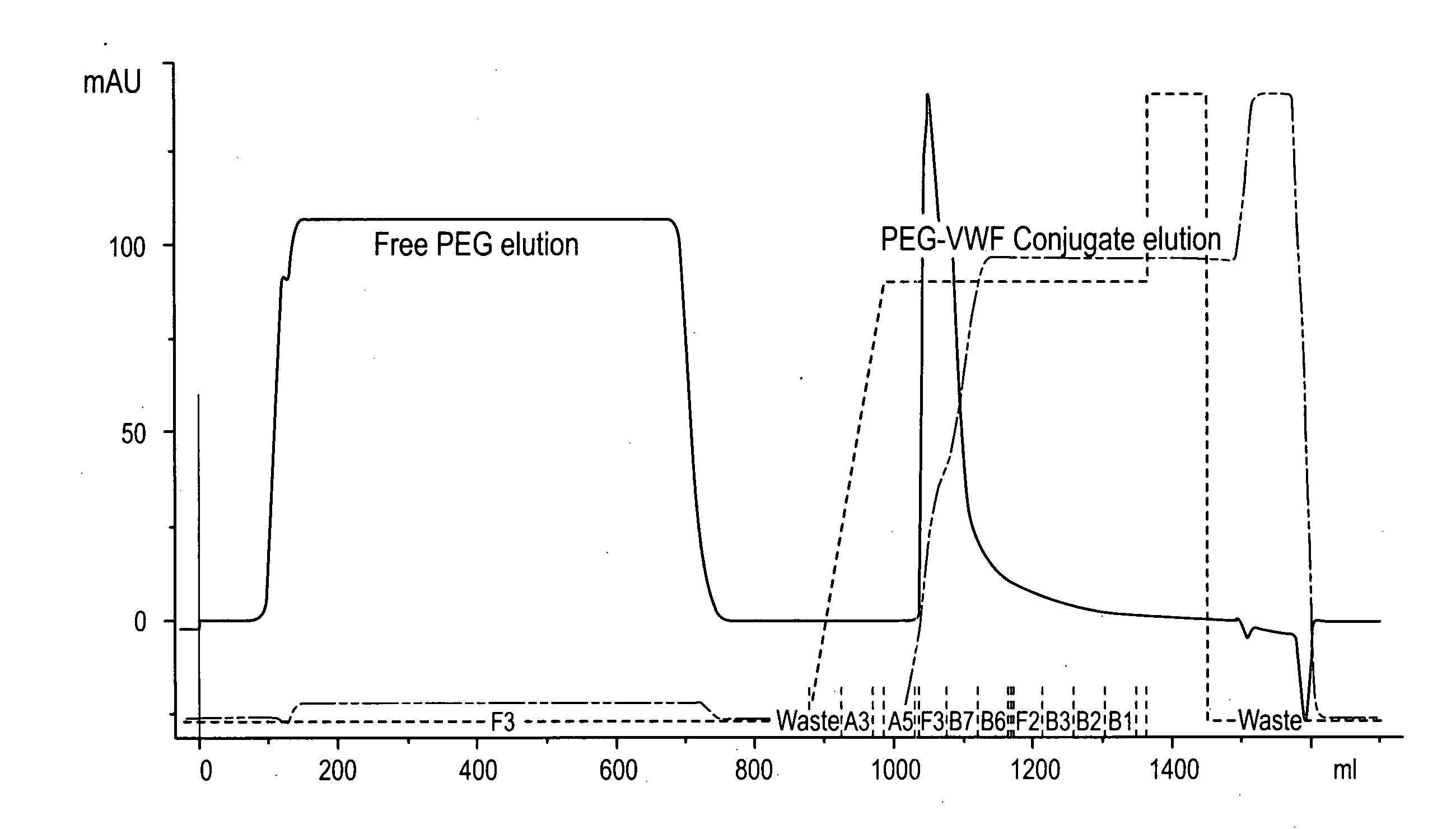
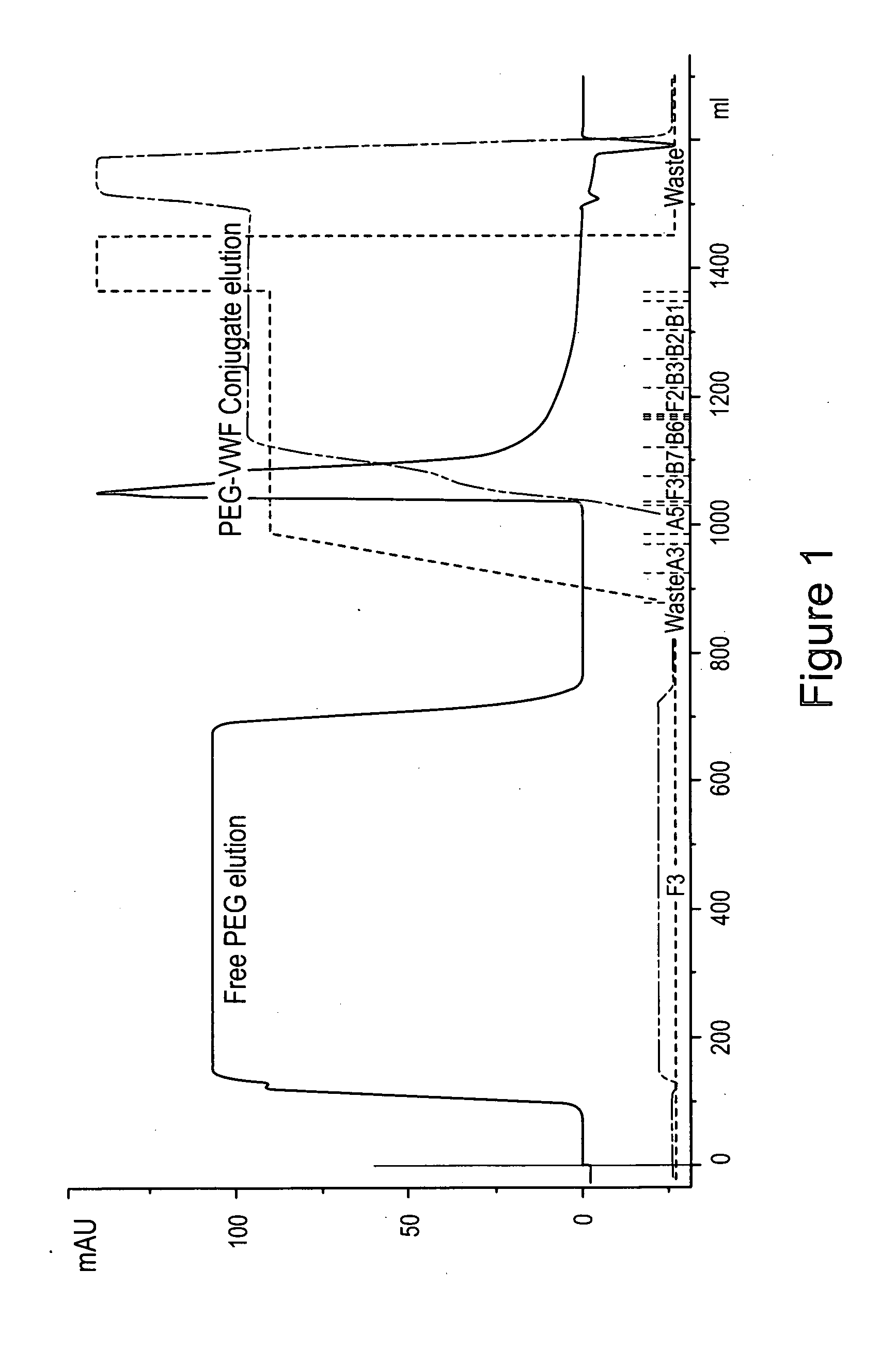
Von Willebrand factor-and factor VIII-polymer conjugates having a releasable linkage
ActiveUS20080234193A1Prolong half-life in vivoFactor VIIPeptide/protein ingredientsFactor VIII vWFFactor ii
The present invention provides von Willebrand Factor-polymer conjugates and Factor VIII-polymer conjugates, each having a releasable linkage. Methods of making conjugates, methods for administering conjugates, are also provided.
Owner:NEKTAR THERAPEUTICS INC +1
Method of Treating Osteoarthritis with an Antibody to NGF
Methods are disclosed for treating osteoarthritis in a human subject in need thereof, comprising administering to the subject a therapeutically effective amount of an anti-human NGF antibody, or antigen-binding fragment thereof, wherein at least one symptom associated with osteoarthritis is prevented, ameliorated or improved.
Owner:REGENERON PHARM INC
Dispersant for sustained release preparations
InactiveUS20060088595A1Improve featuresEasy passAntibacterial agentsBiocideImmediate releaseWater soluble
The present invention provides a bilayer dispersing agent for a sustained-release preparation, which contains a non water-soluble solvent and an aqueous solvent, and a sustained-release preparation wherein a microcapsule containing a physiologically active substance is dispersed in the aforementioned dispersing agent. When this preparation is subcutaneously administered, the initial release of a physiologically active substance immediately after administration is strikingly suppressed, a constant amount of a physiologically active substance is released for a long period of time from immediately after administration, superior dispersibility is afforded, and the preparation can readily pass through a needle as an injection.
Owner:TAKEDA PHARMA CO LTD
Polymer-based sustained release device
InactiveUS20060110423A1Improve bioavailabilityMinimizes loss of activityPeptide/protein ingredientsMicrocapsulesMedicineSugar
This invention relates to compositions for the sustained release of biologically active polypeptides, and methods of forming and using said compositions, for the sustained release of biologically active polypeptides. The sustained release compositions of this invention comprise a biocompatible polymer having dispersed therein, a biologically active polypeptide and a sugar.
Owner:WRIGHT STEVEN G +8
Methods of altering the binding affinity of a peptide to its receptor
InactiveUS20040102381A1Improve hydrophilicityEnhance amphipathicAntibacterial agentsPeptide/protein ingredientsOligomerNucleotide
The present invention relates to amphiphilic drug-oligomer conjugates capable of traversing the blood-brain barrier ("BBB") and to methods of making and using such conjugates. An amphiphilic drug-oligomer conjugate comprises a therapeutic compound conjugated to an oligomer, wherein the oligomer comprises a lipophilic moiety coupled to a hydrophilic moiety. The conjugates of the invention further comprise therapeutic agents such as proteins, peptides, nucleosides, nucleotides, antiviral agents, antineoplastic agents, antibiotics, etc., and prodrugs, precursors, derivatives and intermediates thereof, chemically coupled to amphiphilic oligomers.
Owner:BIOCON LTD
Prolonged release microspheres for injectable administration
The present invention provides microspheres intended to be administered by injection comprising a protein active ingredient and an agent coating the active ingredient intended to prolong its release, wherein they are free of any trace of organic solvent and they can be obtained according to a coating method involving bringing the active ingredient and the coating agent into contact, with stirring, in a supercritical fluid, said coating agent being soluble in this supercritical fluid.
Owner:ETHYPHARM SA
Compositions for nasal administration of pharmaceuticals
Compositions for nasal administration, which comprise a pharmaceutical, a physiologically active peptide, or a peptide-related compound, and as the carrier thereof, crystalline cellulose with a specific particle diameter and / or partially pregelatinized starch are provided. Such compositions improve the in vivo absorption efficiency of pharmaceuticals.
Owner:SHIN NIPPON BIOMEDICAL LAB
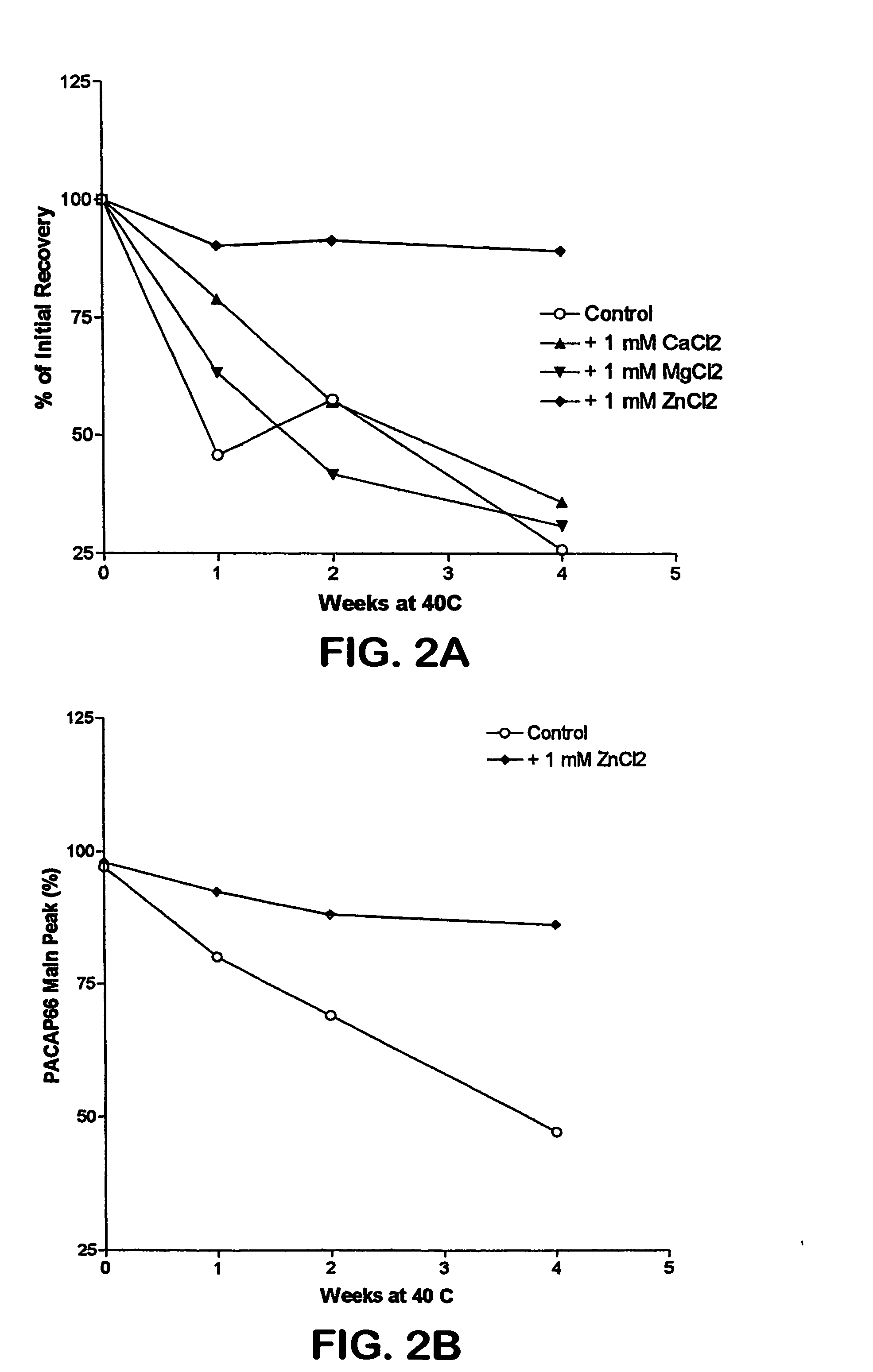
Formulation strategies in stabilizing peptides in organic solvents and in dried states
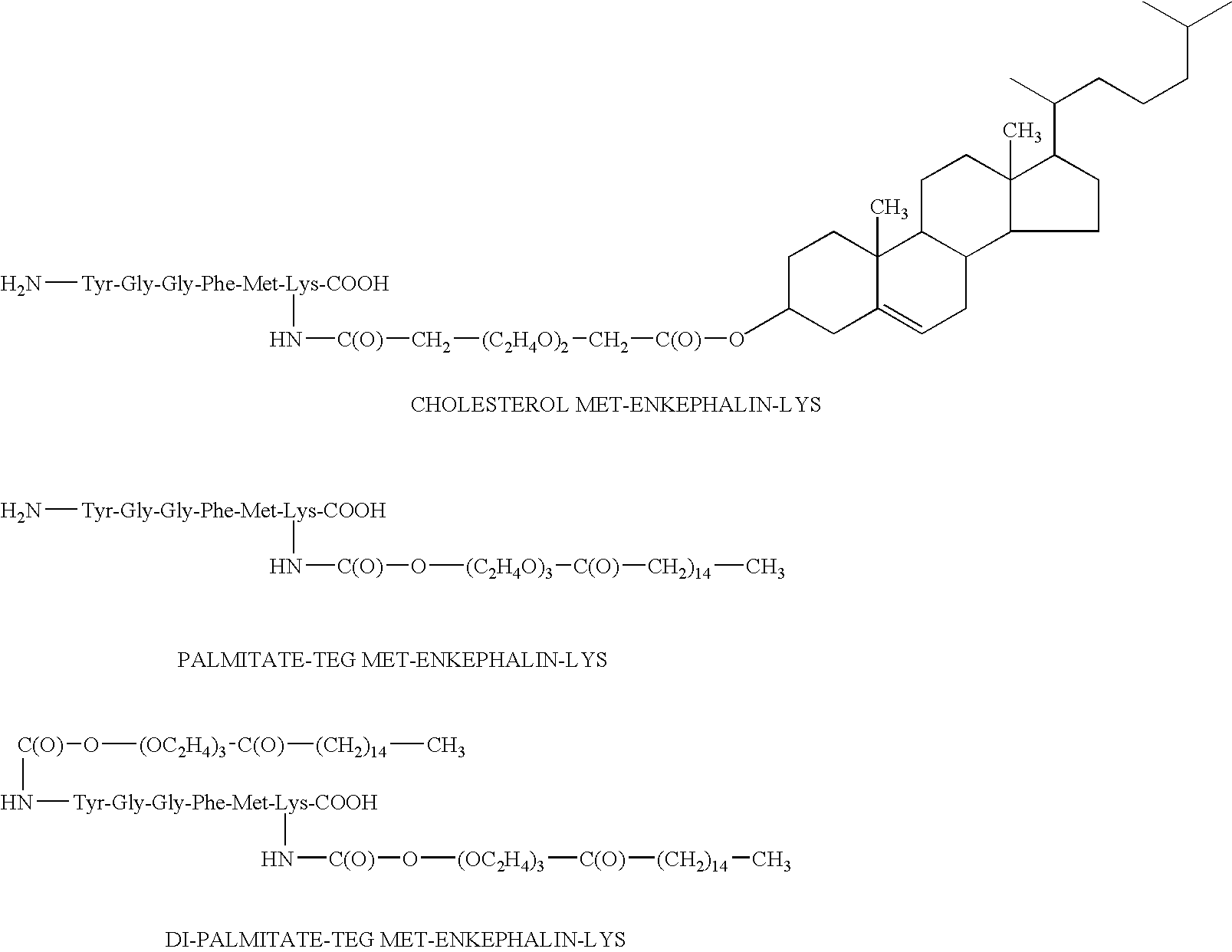
The invention relates to stabilized formulations of therapeutically active peptides, particularly PACAP 66. Formulations of the invention include a peptide containing at least one histidine residue, a transition metal salt and an organic solvent. The above formulations may contain peptides that have at least one asparagine residue and are acidified and dried (such as spray-dried or freeze-dried) before formulation preparation. Other formulations of the invention relate to stabilized formulations of PACAP 66 or peptides containing an asparagine residue, which are acidified and dried (such as spray-died or freeze-dried) with or without a transition metal salt.
Owner:BAYER HEALTHCARE LLC
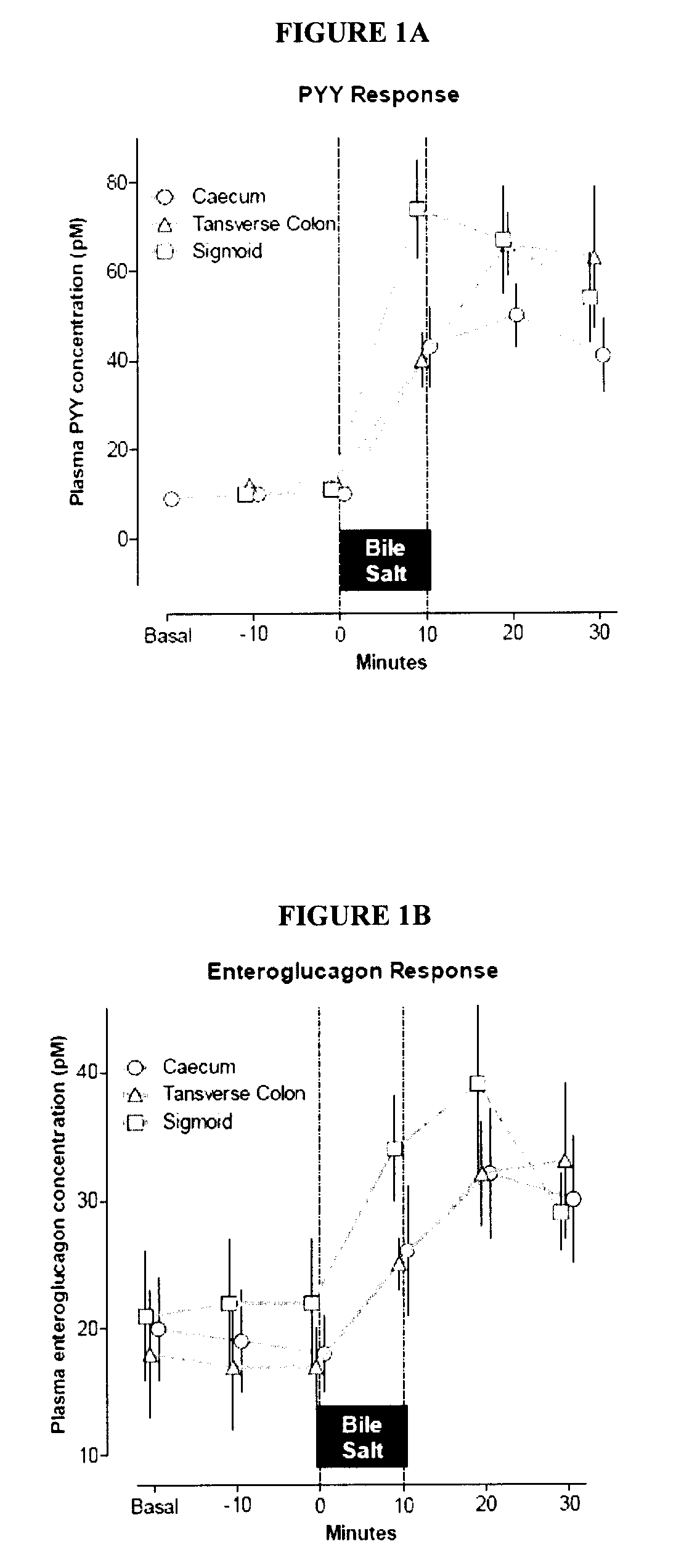
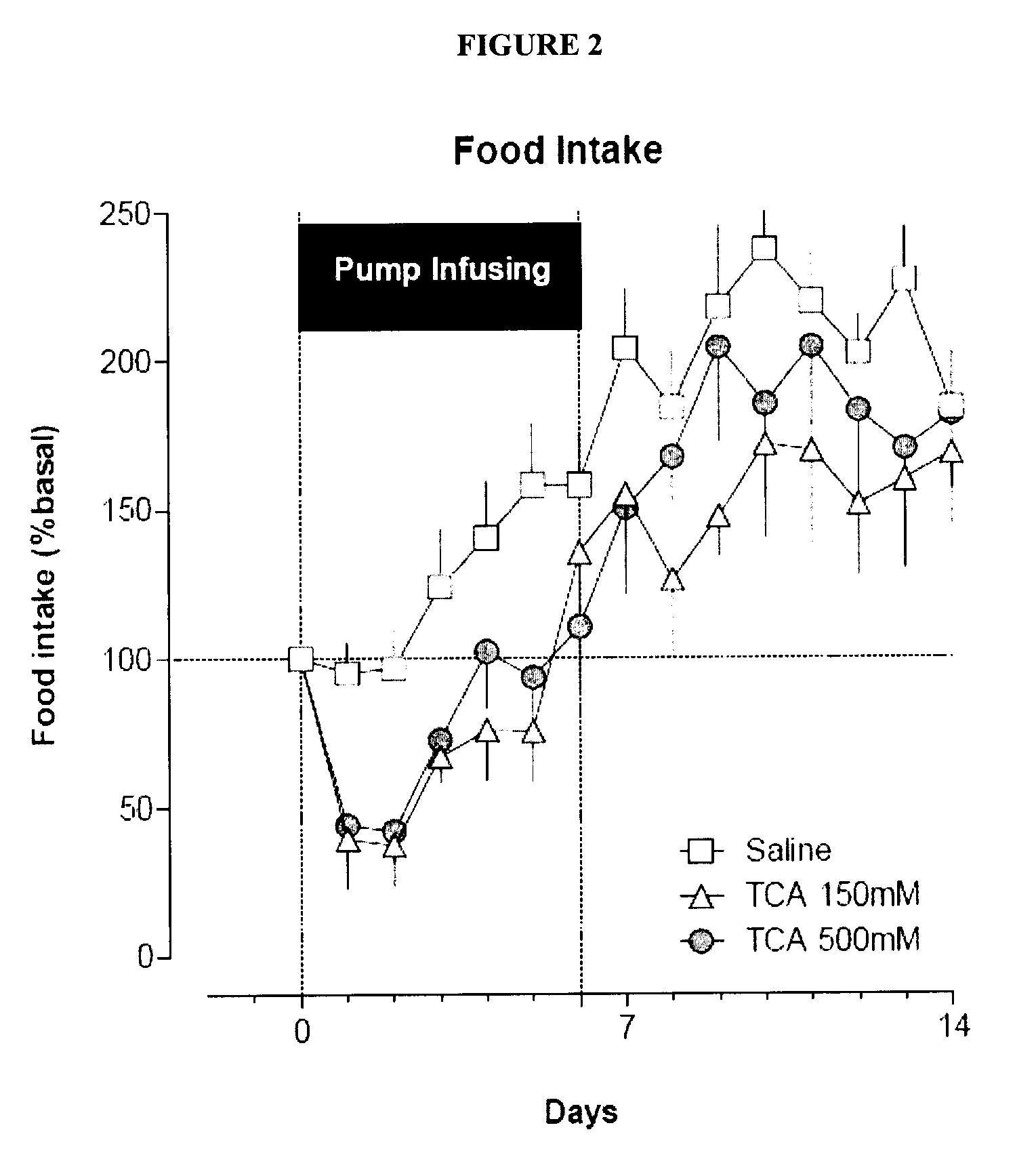
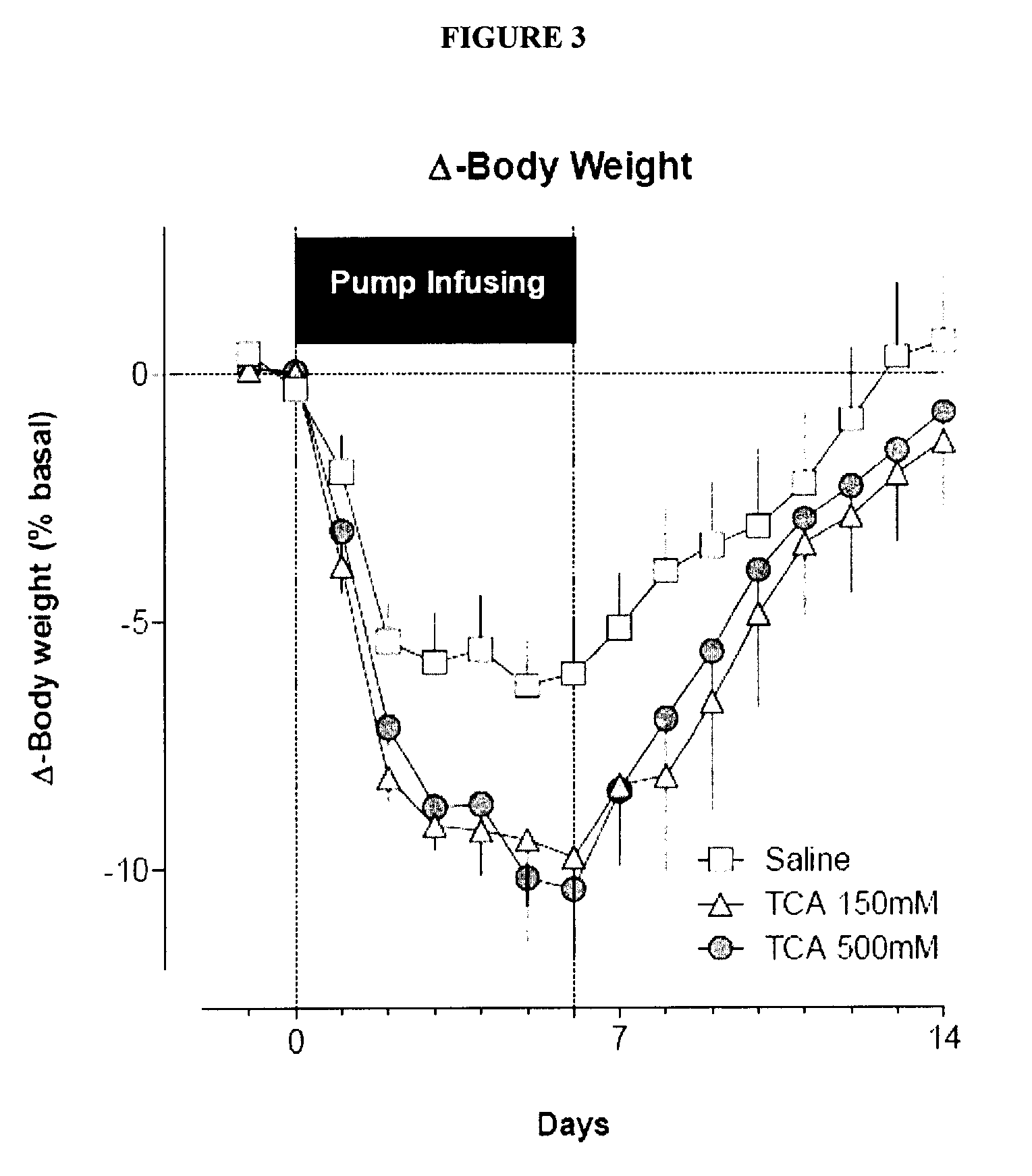
Targeted Therapeutic Agent Release for Weight Loss Therapy
A method for treating obesity comprises anchoring at a first target site within a body a first therapeutic agent delivery device including a first therapeutic agent reservoir coupled to a first outlet which, when the first device is anchored at the first target site, is positioned adjacent to a first target treatment location within a GI tract of a patient and releasing a first therapeutic agent from the first reservoir via the first outlet.
Owner:BOSTON SCI SCIMED INC
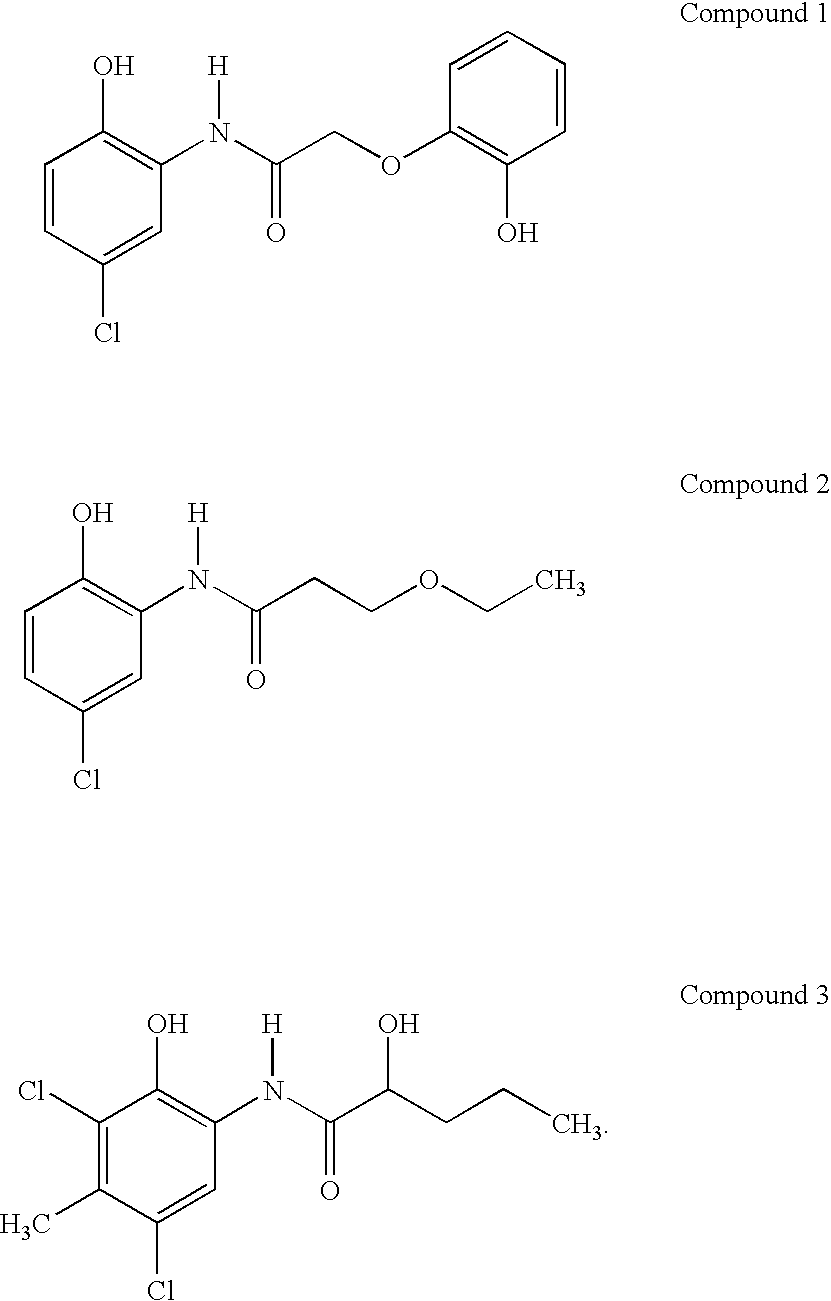
Compounds and compositions for delivering active agents
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
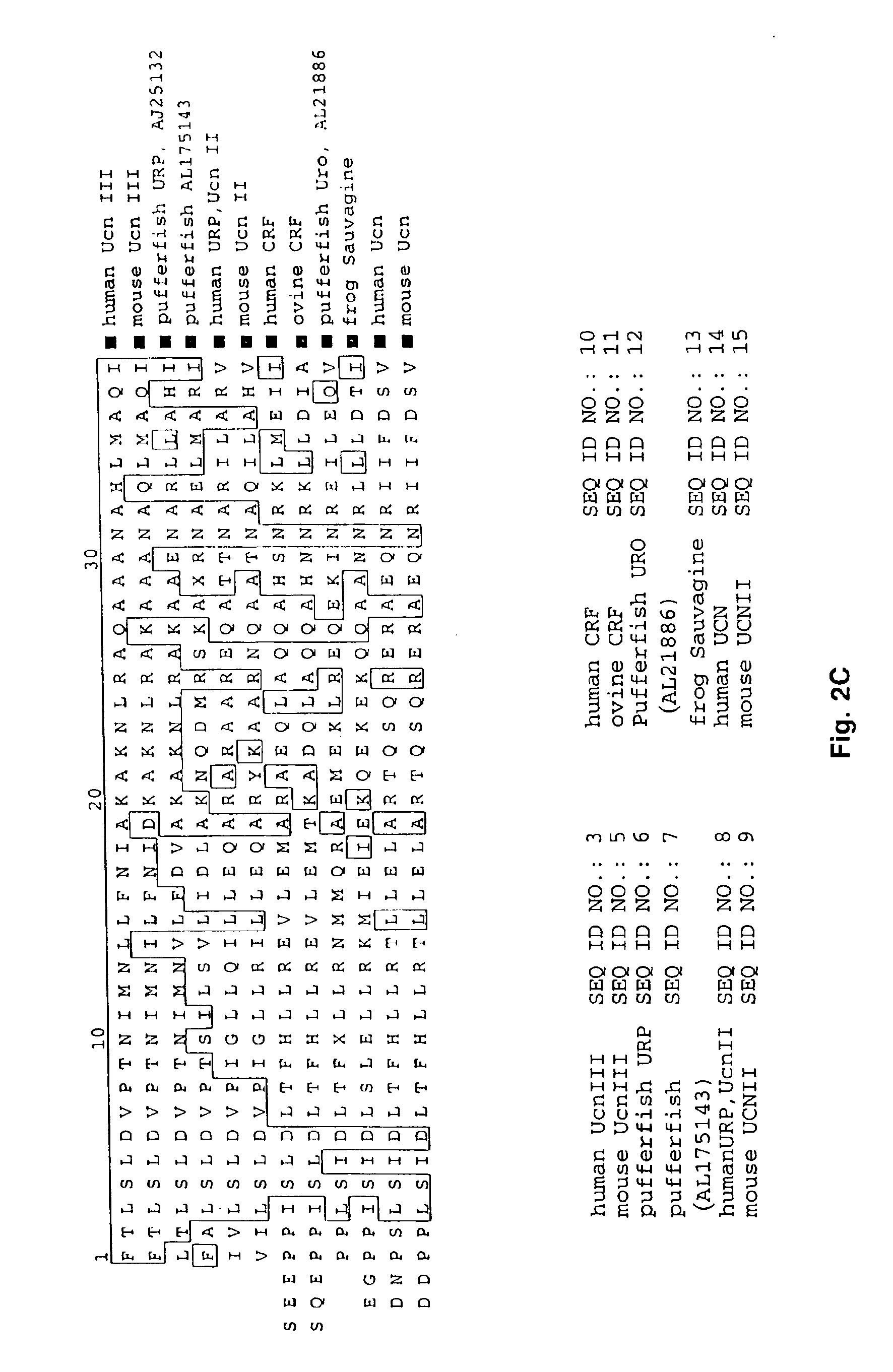
Urocortin-III and uses thereof
A search of the public human genome database identified a human EST, GenBank accession number AW293249, which has high homology to known pufferfish urocortin sequences. The full length sequence was amplified from human genomic DNA and sequenced. Sequence homology comparisons of the novel sequence with human urocortin I and urocortin II revealed that the sequence encoded a novel human urocortin, which was designated urocortin III (UcnIII). While urocortin III does not have high affinity for either CRF-R1 or CRF-R2, the affinity for CRF-R2 is greater than the affinity for CRF-R1. Urocortin III is capable stimulating cyclic AMP production in cells expressing CRF-R2α or β. Thus, the affinity is high enough that urocortin III could act as a native agonist of CRF-R2. However, it is also likely that urocortin III is a stronger agonist of a yet to be identified receptor.
Owner:RES DEVMENT FOUND
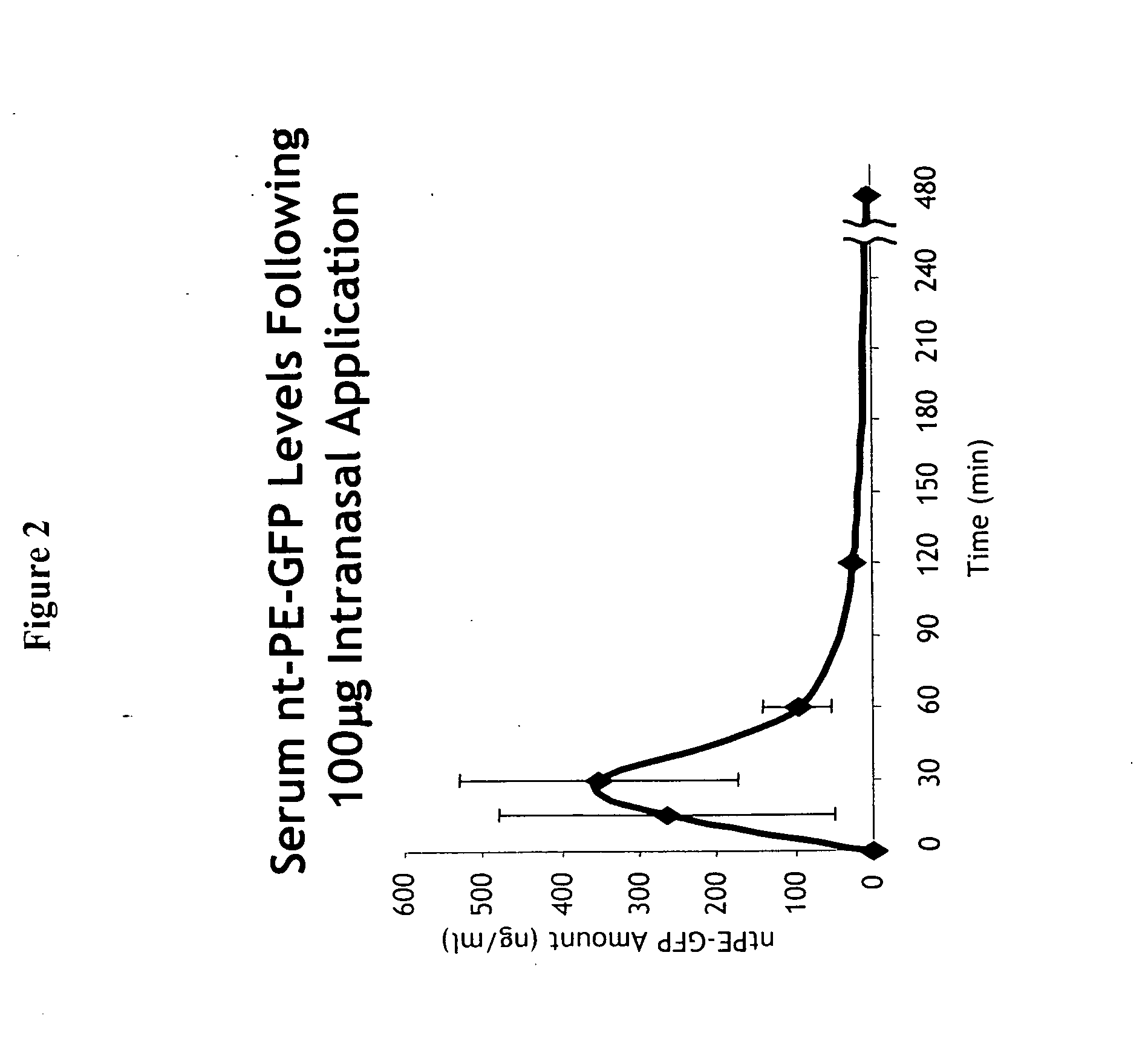
Nasal peptide pharmaceutical formulation
InactiveUS20050158247A1Optimal therapeutic dose levelConstant ratePeptide/protein ingredientsAerosol deliveryEpitheliumDiluent
The invention provides a pharmaceutical formulation comprising: (1) THAM, which is tris(hydroxymethyl)aminomethane, as a selective absorbefacient to enhance through the nasal mucosa-lined epithelium the absorption of substances of peptide nature; and (2) a therapeutically effective amount of active nasal peptide, its pharmaceutically acceptable salt or its peptidic fragment; in a pharmaceutically acceptable, aqueous liquid diluent or carrier, said formulation being in a form suitable for nasal administration.
Owner:THERAPICON SRL
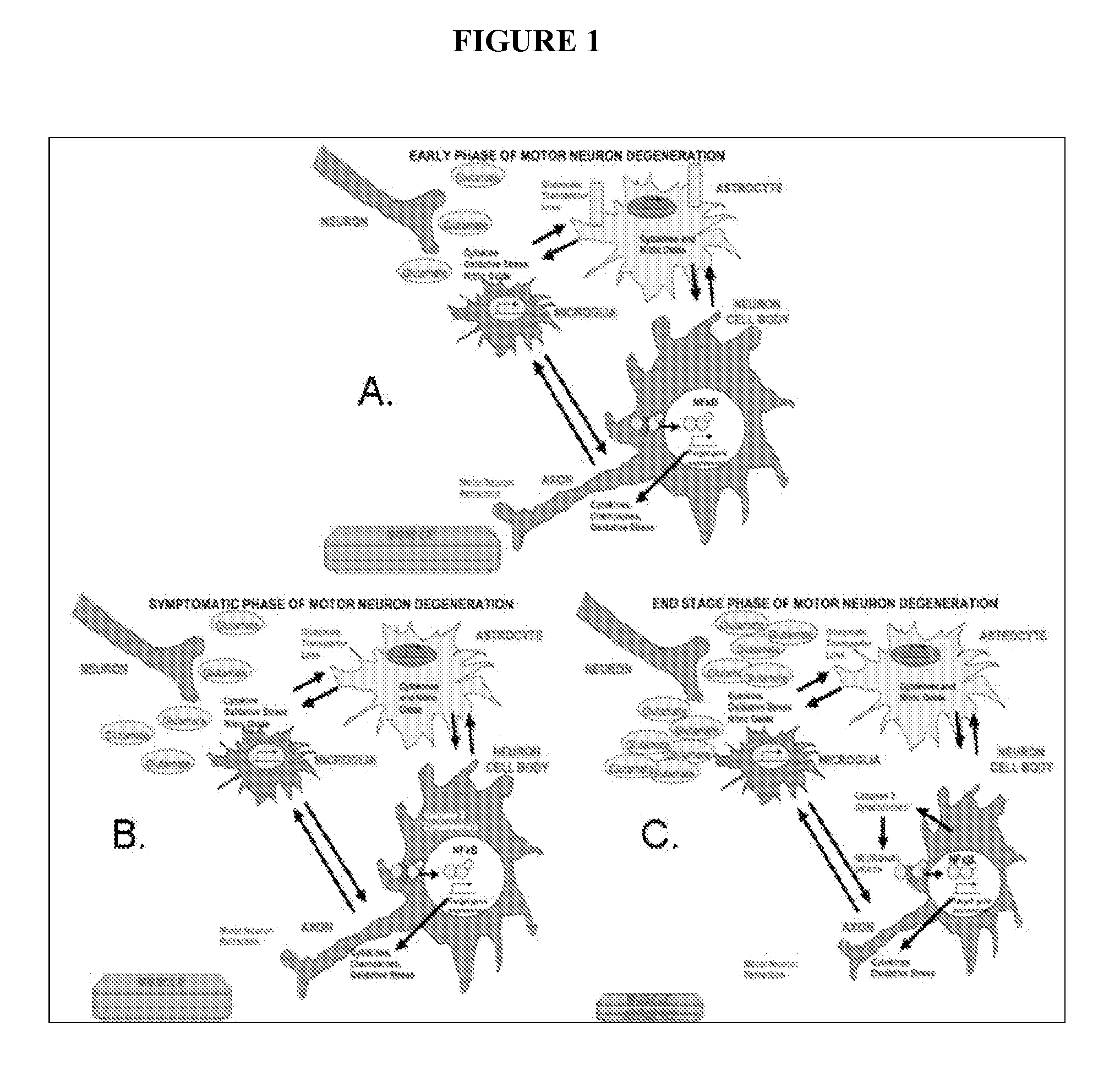
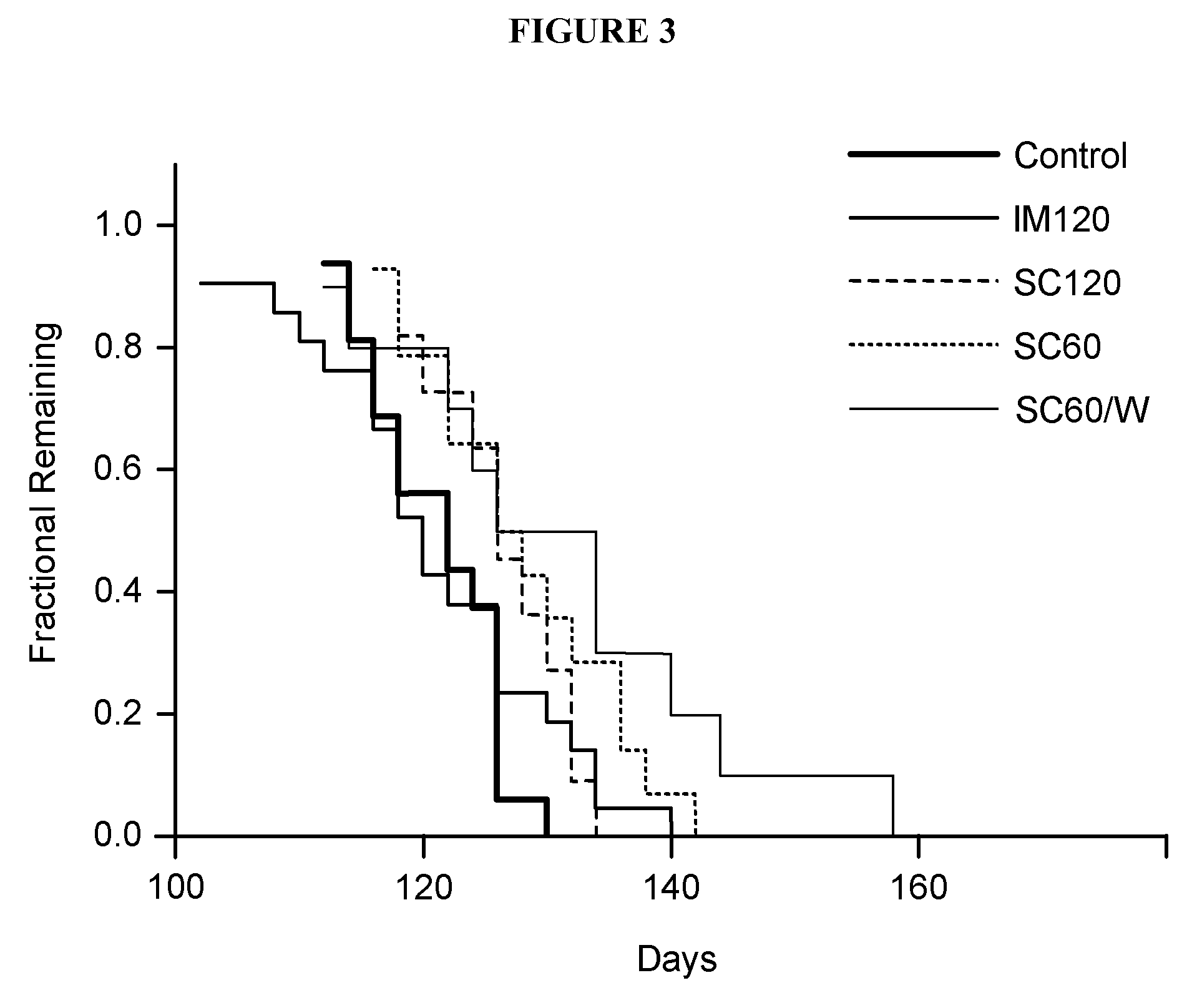
Acth for treatment of amyotrophic lateral sclerosis
InactiveUS20130259875A1Prevent degradationReduce releaseBiocidePeptide/protein ingredientsMedicineAmyotrophic lateral sclerosis
Provided herein are methods of treatment of Amyotrophic Lateral Sclerosis comprising administration of adrenocorticotropic hormone (ACTH), or fragment, analog, complex or aggregate thereof, or any combination thereof, to an individual in need thereof.
Owner:QUESTCOR PHARMA
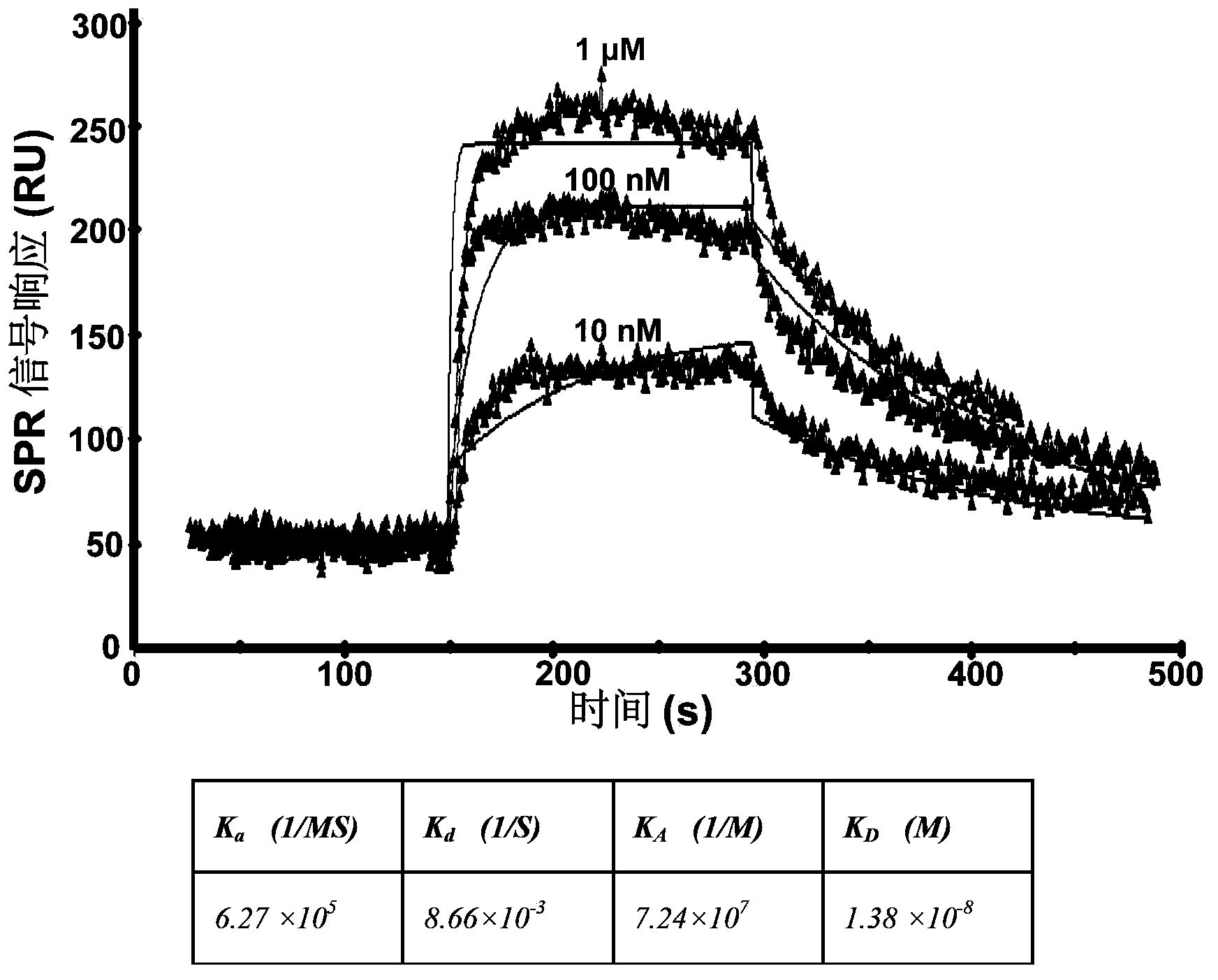
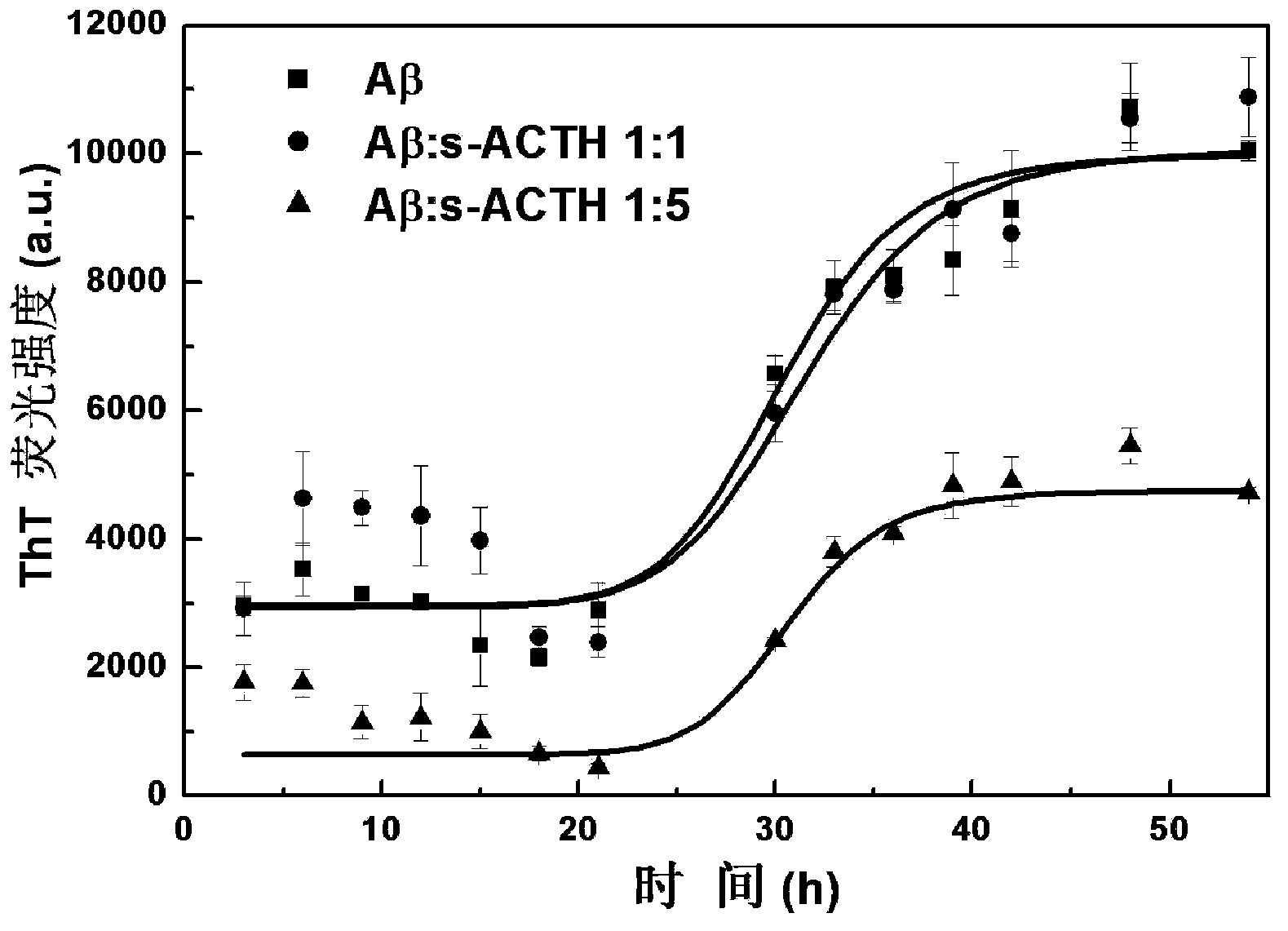
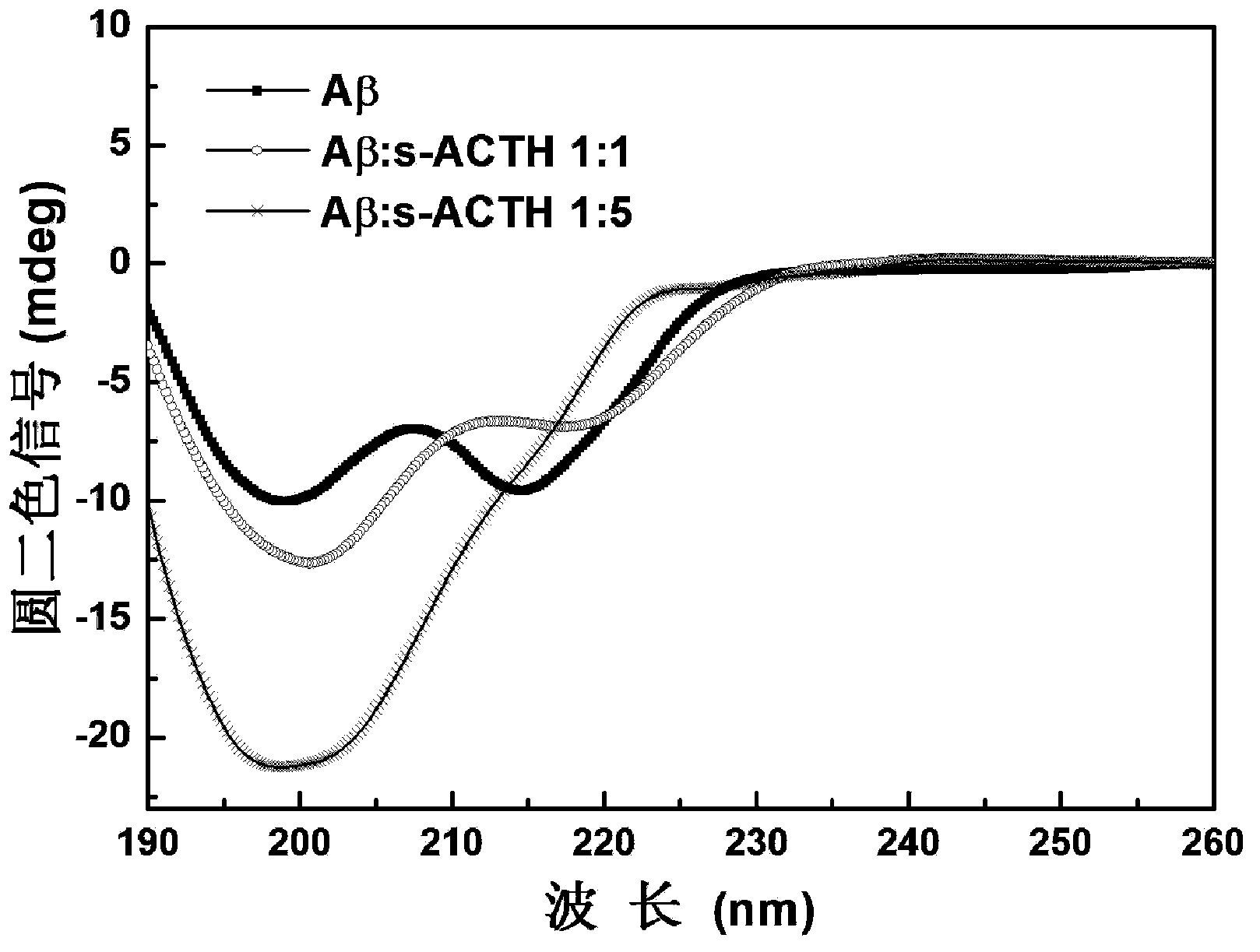
Polypeptide inhibitor for inhibiting aggregation and toxicity of beta amyloid protein and application of polypeptide inhibitor
ActiveCN104277105AImprove bindingReduce the effective contentNervous disorderPeptide/protein ingredientsDiseaseCrystallography
The invention discloses a polypeptide for inhibiting aggregation and toxicity of beta amyloid protein (A beta) or a variant of the polypeptide with polypeptide functions. The polypeptide has a strong binding force with the A beta protein, so that the effective content of a beta structure can be greatly reduced when A beta achieves an aggregation balanced state, the secondary structure of A beta in a solution environment can be changed, the content of the beta structure can be greatly reduced, even the beta structure disappears, the toxicity of A beta to SH-SY5Y cells can be remarkably reduced when the concentration of the polypeptide is extremely low, and generation of A beta-induced active oxygen can be greatly inhibited. Therefore, a feasible method is provided to treatment of Alzheimer disease caused by beta amyloid protein, and thought and reference standard can be provided to discovery of polypeptide pilot compounds for treatment of amyloid protein related diseases.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Glycosylphosphatidylinositol Glycan Signalling Via Integrins Functioning as Glycan Specific Receptors
InactiveUS20080044428A1Treatment and/or prophylaxisOrganic active ingredientsNervous disorderGlycanIntegrin
The present invention relates generally to a method of modulating integrin-mediated cellular activity and to agents useful for same. More particularly, the present invention contemplates a method of modulating ab integrin-mediated cellular activity by modulating GPI-related signalling. The method of the present invention is useful, inter alia, in the treatment and / or prophylaxis of conditions characterised by aberrant, unwanted or otherwise inappropriate integrin-mediated cellular activity. The present invention is further directed to methods for identifying and / or designing agents capable of modulating the subject integrin dependent signalling mechanism.
Owner:WALTER & ELIZA HALL INST OF MEDICAL RES
Methods and compositions for needleless delivery of macromolecules
InactiveUS20060153798A1Organic active ingredientsPeptide/protein ingredientsHigh concentrationBinding domain
Methods and compositions for needleless delivery of macromolecules to the bloodstream of a subject are provided herein. In one aspect, the invention provides a delivery construct, comprising a receptor binding domain, a transcytosis domain, a macromolecule to be delivered to a subject, and a cleavable linker. In certain aspects, the cleavable linker can be cleavable by an enzyme present in higher concentration at or near the basal-lateral membrane of a polarized epithelial cell or in the plasma than elsewhere in the body, for example, at the apical side of the polarized epithelial cell. In other aspects, the invention provides nucleic acids encoding delivery constructs of the invention, kits comprising delivery constructs of the invention, cells expressing delivery constructs of the invention, and methods of using delivery constructs of the invention.
Owner:TRINITY ASSIGNMENT FOR THE BENEFIT OF CREDITORS
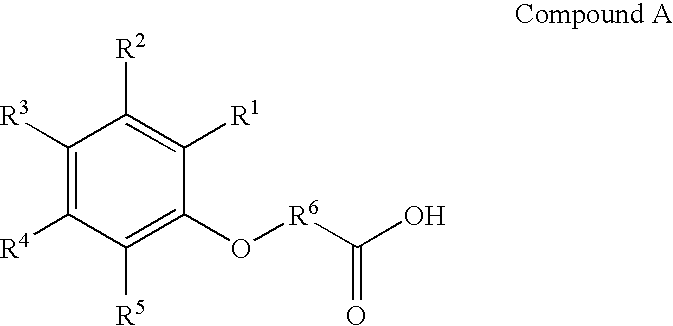
Cyanophenoxy carboxylic acid compounds and compositions for delivering active agents
InactiveUS7115663B2Increased and improved bioavailabilityBiocideCalcitoninsActive agentCarboxylic acid
Cyanophenoxy carboxylic acid compounds and compositions for the delivery of active agents are provided. Methods of administration, treatment of disease and preparation are provided as well.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Encapsulation of water soluble peptides
InactiveUS20070009605A1Peptide/protein ingredientsCalcitoninsControlled releaseBiodegradable microsphere
This invention relates to a process for preparing biodegradable microspheres and or nanospheres using an oil-in-water process for the controlled release of bioactive peptides.
Owner:SOC DE CONSEILS DE RECH & DAPPLICATIONS SCI SAS
Methods and compositions for needleless delivery of binding partners
InactiveUS20070148131A1Extended half-lifeImprove welfarePeptide/protein ingredientsCalcitoninsNeedle freeReceptor
The present invention relates, in part, to methods and compositions for needleless delivery of macromolecules to a subject. In one aspect, the methods and compositions involve administering to the subject a delivery construct comprising a carrier construct non-covalently bound to a binding partner, wherein the carrier construct comprises a receptor-binding domain, a transcytosis domain, and a macromolecule to which the binding partner non-covalently binds, wherein the binding partner binds to the macromolecule with a Ka that is at least about 104 M−1.
Owner:TRINITY ASSIGNMENT FOR THE BENEFIT OF CREDITORS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com