Fully humanized anti-tetanus bispecific antibody as well as construction method and application thereof
A bispecific antibody and fully humanized technology, applied in the biological field, can solve the problems of unreported monoclonal antibody sequences, sequences that have not been cloned and expressed, and have no production and application value, so as to reduce human allergies and overcome the source The effect of limited and significant economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Recombinant expression and purification of tetanus toxoid and mutants.
[0032] Expression sequence plasmid construction
[0033] The tetanus toxoid (Genbank: P04958) protein sequence 864-1315 (amino acid sequence as shown in SEQ ID No.1) is named as TTC, and the corresponding amino acids are mutated to obtain (Y1290A)TTC, (R1226A) respectively ) TTC and (Y1290A / R1226A) TTC mutants. Based on the codon preference characteristics of Escherichia coli, these mutants are optimized to be suitable for expressing codons in Escherichia coli: TTC (nucleotide sequence as shown in SEQ ID No.2, optimized according to the codon preference of Escherichia coli The nucleotide sequence of the tetanus toxin heavy chain C fragment, TGGCCA at the 5-terminal is the MSCI restriction site, and CTCGAG at the 3-terminal is the XhoI restriction site), (Y1290A)TTC, (R1226A) and (Y1290A / R1226A)TTC, Please Shanghai Jierui Biosynthetics synthesized these sequences separately, and then add...
Embodiment 2
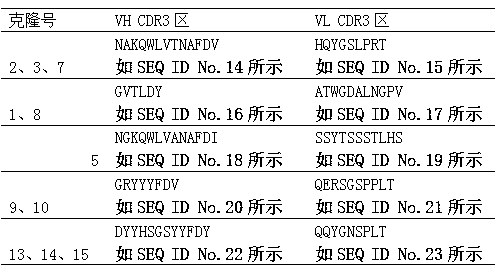
[0045] Example 2 Construction, screening and sequencing of fully humanized phage immune antibody library
[0046] 2.1 Construction of fully humanized phage immune antibody library
[0047] 1) PCR amplification of human tetanus VH and VL genes
[0048] Total RNA was extracted from the peripheral blood lymphocytes of 3 healthy plasma donors with high tetanus antibody titers according to the Invitrogen RNA extraction kit instructions. Using purified RNA as a template, cDNA was synthesized by reverse transcription with OligodT primers. According to the human antibody sequence, the antibody light and heavy chain variable region framework primers (nature biotechnology 14:309-314 (1996)) were used. The primers covered 7 kinds of VH family genes, 7 kinds of Vκ family genes, 8 kinds of Vλ family genes, antibody genes Covering more than 95% of the diversity of human antibody genes. PCR conditions: pre-denaturation at 94°C for 5 min, denaturation at 94°C for 30 s, annealing at 57°C fo...
Embodiment 3
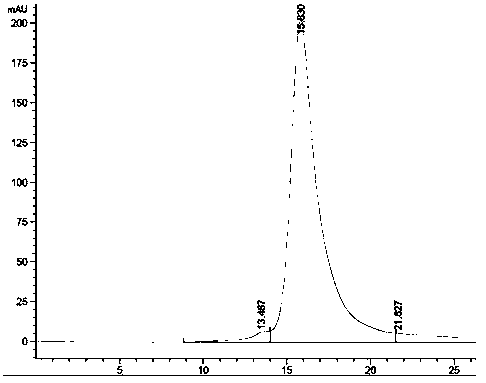
[0067] Example 3 Bispecific antibody expression, purification and identification
[0068] 3.1 Construction of vector pCHO 1.0-hygromycin
[0069] Take a section of nucleotide sequence 2118-3141 of pcDNA3.1 / Hygro (the nucleotide sequence encoding the hygromycin gene, as shown in SEQ ID No.3), and replace the section 8860-9459 of the vector pCHO 1.0 to make it PGK The promoter (phosphoglycerate kinase promoter) expresses hygromycin.
[0070] bispecific expression vector
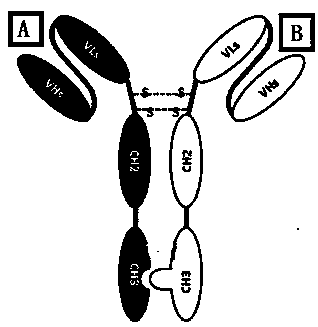
[0071] 3.2.1 Fc + hinge region knob mutant (or hole mutant) sequence
[0072] The amino acid sequence 221-446 (EU coding system) of human IgG1 (IMGT named IGHG1) contains three regions: 221-234 is the Hinge region, and 234-341 is the C H 2 area and 342-446 for C H 3 area. In the present invention, refer to literature (nature biotechnology (1998), 16 (7): 677-681) to C H The amino acids in the 3 regions are mutated, and one chain expresses the knob mutant of S354C and T366W (see SEQ ID No.6 for its polypept...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Potency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com