Bivalent vaccine for porcine reproductive and respiratory syndrome and classical swine fever prevention or treatment, and preparation method thereof
A technology for porcine blue-ear disease and dual vaccine, which is applied to medical preparations containing active ingredients, antiviral agents, pharmaceutical formulations, etc., can solve the problems of large immunization workload and high cost, and achieves high titer content and preparation method. The effect of simple, economical immunization procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
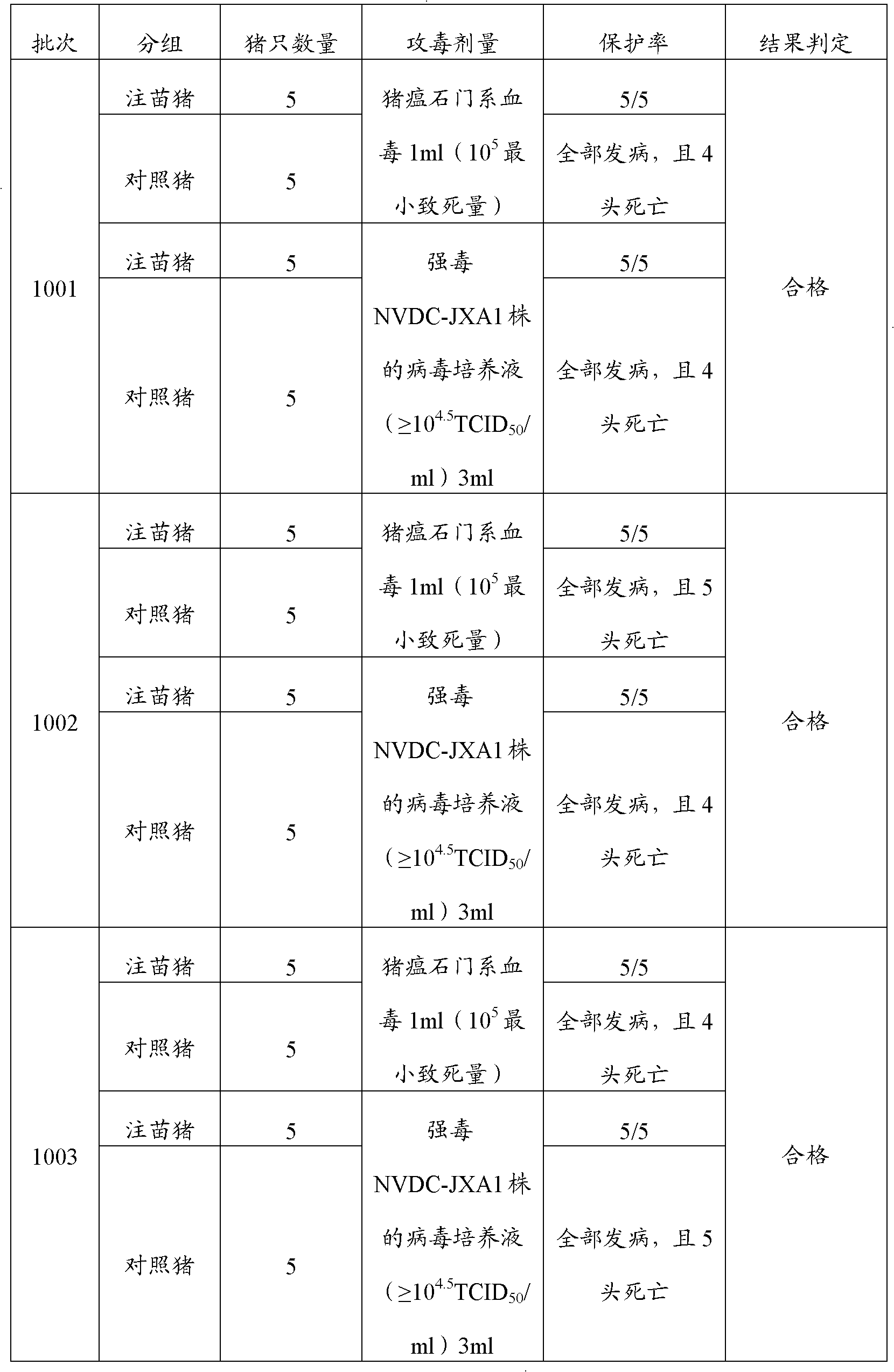
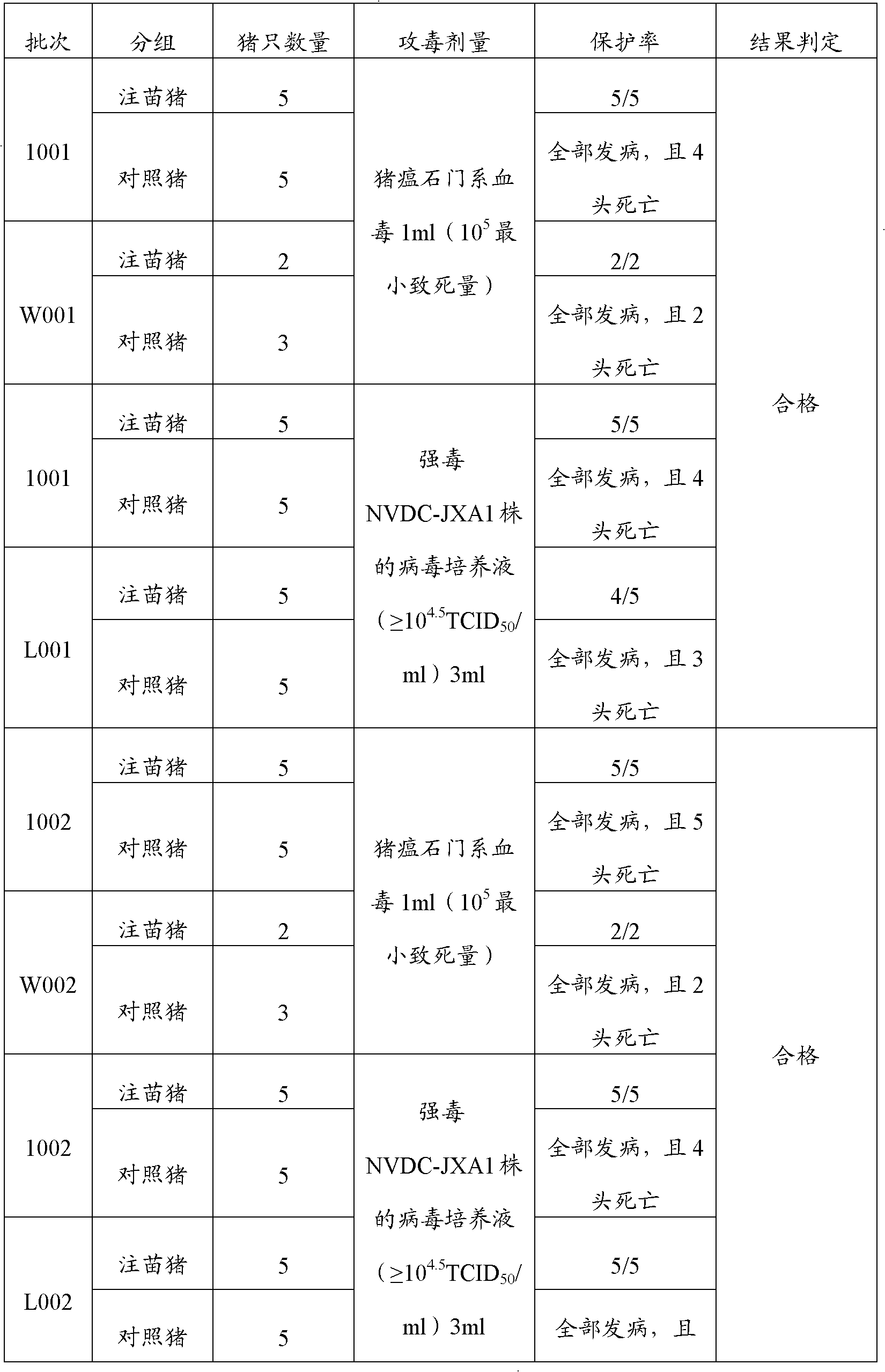
[0023] Embodiment 1, the preparation of porcine blue-ear disease and swine fever dual live vaccine
[0024] 1. Preparation of poisonous seeds for production
[0025] Preparation of porcine blue-ear virus species: get well-grown Marc-145 cells (purchased from China Veterinary Drug Administration), digest with trypsin, and inoculate in cell bottles, and simultaneously use virus species with 1% v / v fetal MEM (manufactured by Invitrogen) maintenance solution of bovine serum (manufactured by PAA) was diluted 10 times, inoculated with virus according to 5% volume inoculum, cultured at 37°C, and observed for cytopathic changes (CPE) every day, when more than 70% of the cells showed CPE Harvest the virus at the same time, freeze and thaw 3 times, harvest the virus, the virus titer is 10 per 1ml virus content 9.5 TCID 50 .
[0026] The preparation of classical swine fever virus kind: use the α-MEM (Invitrogen company production) cell maintenance fluid that contains 1%v / v fetal calf ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com