Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Bovine spongiform encephalopathy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bovine spongiform encephalopathy (BSE), commonly known as mad cow disease, is a neurodegenerative disease of cattle. Symptoms include abnormal behavior, trouble walking, and weight loss. Later in the course of the disease the cow becomes unable to move. The time between infection and onset of symptoms is generally four to five years. Time from onset of symptoms to death is generally weeks to months. Spread to humans is believed to result in variant Creutzfeldt–Jakob disease (vCJD). As of 2018, a total of 231 cases of vCJD have been reported globally.
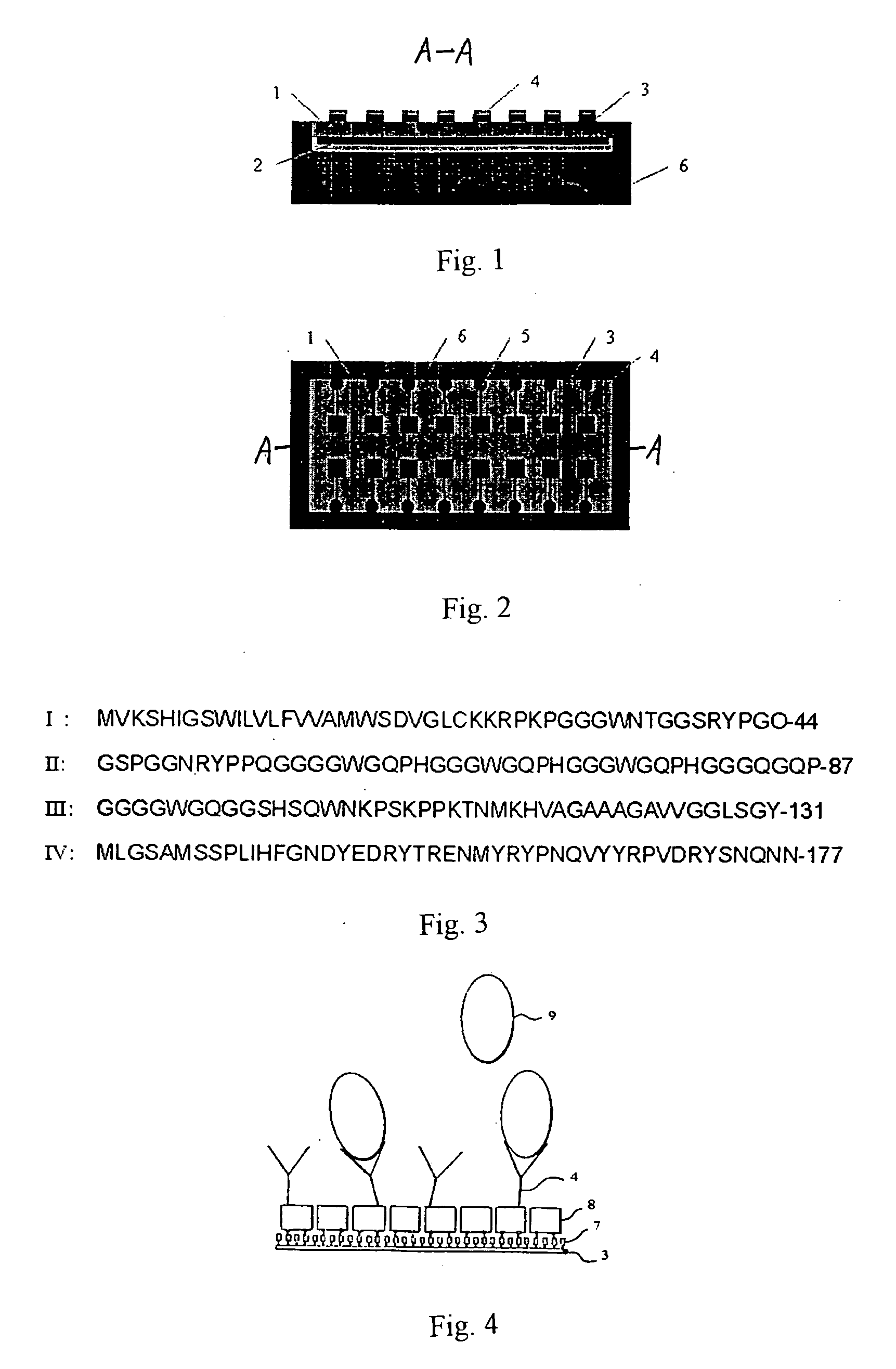
Piezoelectric bio-chip for detecting pathogen of mad cow disease and thereon preparation
InactiveUS20060121531A1High-precision detectionEasy to operateBioreactor/fermenter combinationsBiological substance pretreatmentsQuarantineOrganism
The present invention relates to the device for animal inspection and quarantine and the preparation method thereof. The present invention is especially applicable to the detection of the pathogen of bovine spongiform encephalopathy (BSE) (also known as “mad cow disease”). The present invention employs a piezoelectric chip, a microelectrode array and a common electrode fixed on the lower side surface and upper side surface of the piezoelectric chip, respectively, and a BSE PrP antibody array to constitute the piezoelectric biochip for the detection of the BSE pathogen. The BSE PrP antibodies are immobilized on the electrodes of the microelectrode array in a format corresponding uniquely to the electrodes of the microelectrode array by adsorbing, bonding, cross-linking, embedding or self-assembly process. The combination of the piezoelectric biochip and a detector constitutes the piezoelectric biochip detection system for the BSE pathogen. When the antibodies react with the corresponding PrPs immunochemically, the information about the PrPs can be detected at real time by measuring the resonant frequency, and the PrPs thus can be analyzed qualitatively and quantitatively. The present invention is applicable to the early, effective and rapid detection of the BSE pathogen.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
Transgenic ungulates having reduced prion protein activity and uses thereof
ActiveUS20050097627A1Animal cellsMicrobiological testing/measurementBiotechnologyGenetically engineered
The invention provides cloned transgenic ungulates (e.g., bovines) in which prion protein activity is reduced by one or more genetically engineered mutations. Desirably, these transgenic bovines are also genetically modified to express xenogenous (e.g., human) antibodies. Because of their resistance to prion-related diseases such as bovine spongiform encephalopy (also known as mad cow disease), these bovines are a safer source of human antibodies for pharmaceutical uses and a safer source of agricultural products.
Owner:SAB LLC
Liquid Product of Botulinum Toxin Type A
InactiveUS20120302507A1Easy to useConserves potencyHormone peptidesPeptide/protein ingredientsLiquid productCross infections
Disclosed are a liquid product of botulinum toxin type A and a method for conserving the potency of botulinum toxin type A using a dextrose solution. Free of a stabilizer, such as albumin or gelatin, the liquid product of botulinum toxin type A completely excludes the possibility of cross infections such as AIDS and bovine spongiform encephalopathy. In addition, botulinum toxin type A is preserved as a liquid product in combination with a dextrose solution and can be clinically used as is, without the aid of physiological saline. Therefore, the liquid product enjoys the advantage of being convenient for use and avoiding a decrease in the potency as occurs upon dilution with physiological saline. Serving as a natural preserving and stabilizing agent, the dextrose solution allows botulinum toxin type A to be stored and distributed in the form of liquid phase over a long period of time and conserves the potency of the toxin at a constant level, which in turn guarantees constant clinical results.
Owner:HAM JONG WOOK
Ruminant animal-origin feedstuff identification method based on lipid Raman spectrums
InactiveCN105424675AEasy accessIntuitively and significantly reflect the characteristics of lipid composition differencesRaman scatteringRuminant animalFodder
The invention provides a ruminant animal-origin feedstuff identification method based on lipid Raman spectrums. The ruminant animal-origin feedstuff identification method includes the steps that animal-origin feedstuff with known origins and of different species are collected; lipid samples are extracted; Raman spectrum information data of the lipid samples are collected, and the spectrum range is 400-3600 cm<-1>; a distinguishing and analyzing model of the ruminant animal-origin feedstuff is built; the built distinguishing and analyzing model is evaluated; lipid samples of the animal-origin feedstuff to be detected are extracted, and the Raman spectrum information data are collected and input into the distinguishing and analyzing model for species identification. The Raman spectrums of the lipid samples are directly collected, spectrum information and species origin information are correlated, the ruminant animal-origin feedstuff can be identified and analyzed fast and effectively, the analyzing requirement for species identification of the ruminant animal-origin feedstuff of feed quality safety supervision in China is met, and it can be ensured that while 'bovine spongiform encephalopathy' is efficiently prevented, and sustainable development and cyclic utilization of the feed industry are effectively implemented.
Owner:CHINA AGRI UNIV
Preparation method of gelatine-free double-layer hollow capsule
ActiveCN102885799AFit for consumptionAvoid worriesPharmaceutical non-active ingredientsCapsule deliveryPullulanAdditive ingredient
The invention relates to a preparation method of a gelatine-free double-layer hollow capsule. The preparation method is characterized by comprising the following steps of: by taking microbial polysaccharide gum, algal polysaccharide gum and plant polysaccharide gum as raw materials; and mixing, dissolving the gums, degassing, dipping the gums, drying, dipping the gums again, drying again, shelling, cutting and trimming, so as to prepare the gelatine-free double-layer hollow capsule, wherein the inner layer of the capsule contains pullulan and sodium alginate, and the outer layer of the capsule contains hydroxypropyl methyl cellulose and methylcellulose. With the adoption of the double-layer structure, the disintegration performance of the capsule can be greatly improved, and the good mechanical strength of the whole capsule can be kept. The product can be used for filling various medicines and healthcare foods by taking the microbial polysaccharide gum, the algal polysaccharide gum and the plant polysaccharide gum as the raw materials and avoiding the use of any gelatine ingredient; the product is safe, reliable, and is suitably eaten by Buddhist, Moslems and vegetarian; and the worry of people about eating for gelatine products caused by 'bovine spongiform encephalopathy' is avoided.
Owner:JIANGSU QINGYUANKANG BIOLOGY TECH
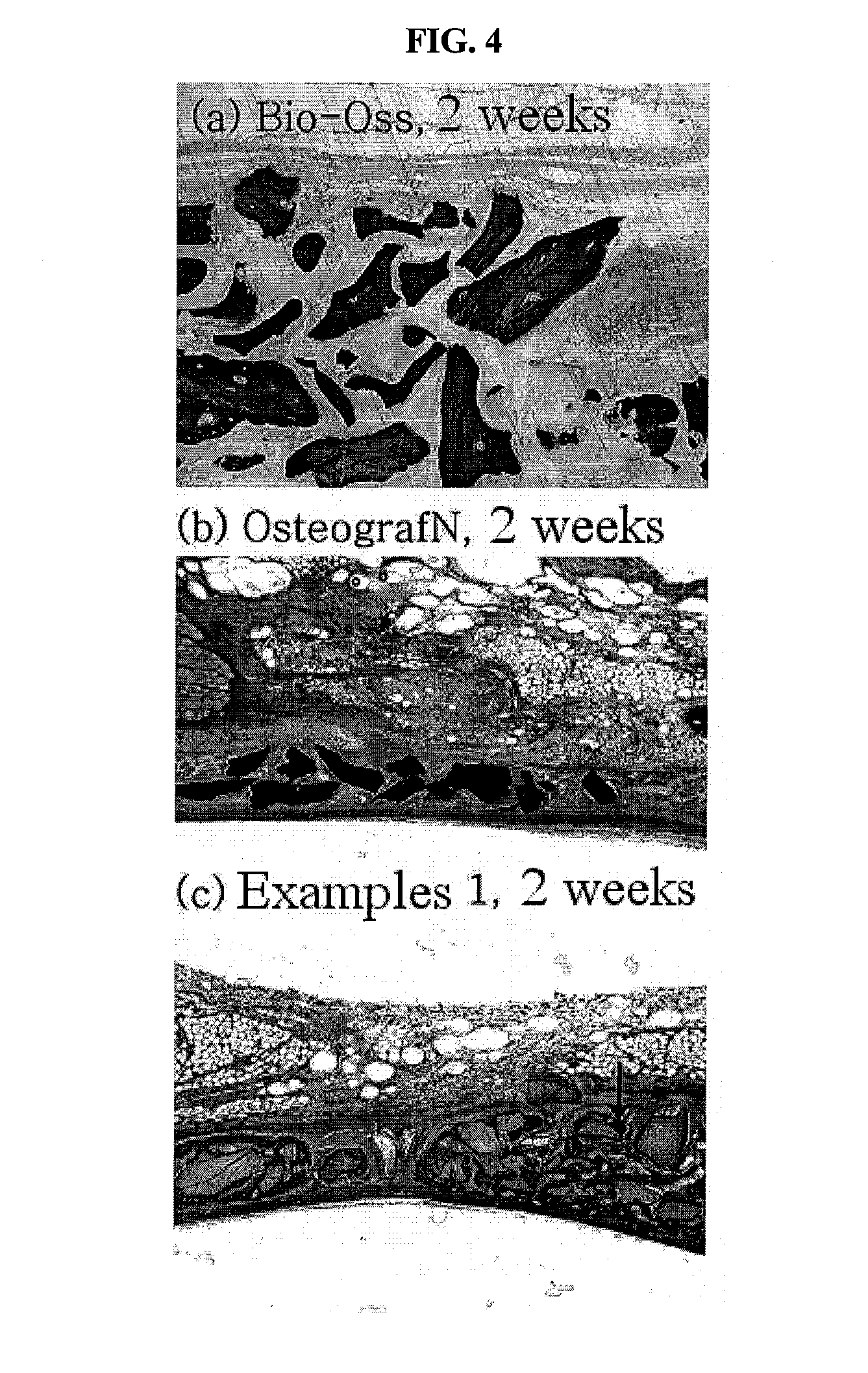
Method for preparing a prion-free bond grafting substitute
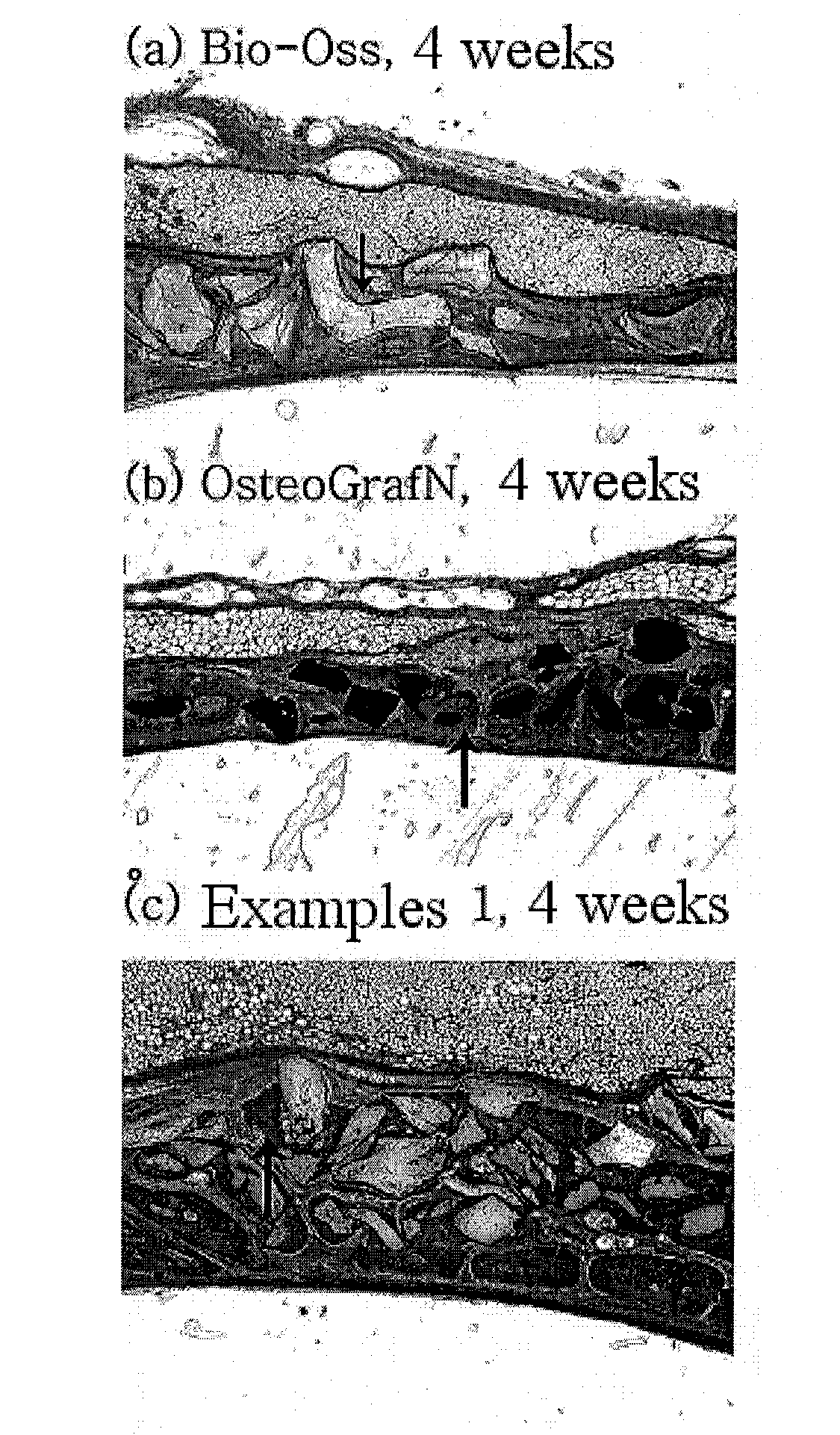
The present invention relates to a method for preparing a bone graft substitute using bovine bone, and more particularly to a method for preparing a safe bone graft substitute which does not have the risk of infection with bovine spongiform encephalopathy, the method comprising treating bovine bone with sodium hypochlorite and treating the treated bone at a high temperature of more than 600° C. The bone graft substitute does not cause an immune response, because it is prepared by effectively removing lipids and organic substances from bovine bone having a structure very similar to that of the human bone. Also, it has excellent osteoconductivity, and is free of prion, and thus it does not have the risk of infection with bovine spongiform encephalopathy. According to the disclosed invention, the bone graft substitute having such advantages can be prepared in a simple manner.
Owner:SEOUL NAT UNIV R&DB FOUND
Sheep enoxaparin sodium compound preparation method, compound and application of compound
InactiveCN105131153AQuality is easy to controlRaw materials are easy to getOrganic active ingredientsPharmaceutical delivery mechanismSheep farmingMedicine
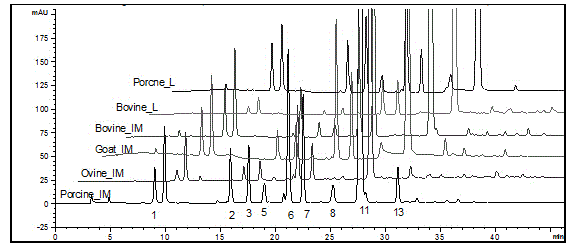
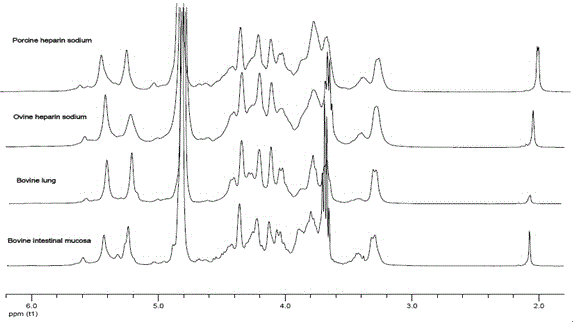
The invention discloses a method for preparing sheep enoxaparin sodium from sheep intestinal mucosa heparin. The method comprises the following steps: 1, preprocessing sheep heparins; 2, preparing a sheep heparin quaternary ammonium salt; 3, preparing sheep heparin benzyl ester; and 4, carrying out alkali depolymerization on the sheep heparin benzyl ester, decoloring, neutralizing by using an acid, carrying out alcohol precipitation, refining, and drying to obtain finished sheep enoxaparin sodium. The simple and efficient method for preparing the sheep enoxaparin sodium from the sheep intestinal mucosa heparin is screened and established, and researches of the systemic physical and chemical properties, the biological activity and the molecule structure are carried out on the prepared sheep enoxaparin sodium. The sheep enoxaparin sodium prepared in the invention completely accords with USP37 and EP8.0 quality release criteria of sheep enoxaparin sodium, and has extremely high practical values and medical application prospect. The sheep enoxaparin sodium has the advantages of simple and easily available raw material, controllable quality, no existence of bovine spongiform encephalopathy virus risk, promotion of the effective utilization of sheep culture and slaughter wastes (intestinal mucosa), and great economy potential.
Owner:SUZHOU RONGXI BIOTECH CO LTD
Assay for species sources
ActiveUS20050112592A1Low costAvoid the needSugar derivativesMicrobiological testing/measurementBiotechnologyMad Cow Diseases
A family of PCR assays is disclosed for determining, both qualitatively and quantitatively, presence of material from a predetermined species source and for quantifying the amount of such material. The assays are based respectively on SINEs uniquely characteristic of pig species, cow species, chicken species, and ruminant sub-order, and having a high copy number. The assays disclosed permit rapid, inexpensive evaluation of meat samples to facilitate elimination from their diet of pork or beef by persons desiring to avoid such food sources; as well as the assay of cattle feed to determine presence therein of ruminant-source proteins, which are a potential source of bovine spongiform encephalopathy (BSE), commonly referred to as “mad cow disease.” The assays amplify the predetermined unique SINEs and the resulting amplified mixture is then evaluated qualitatively by electrophoresis on gel containing ethidium bromide or quantitatively by SYBR Green-based detection or TaqMan chemistry. The invention also extends to kits, primers, and other products used in connection with the assays. The amplicons are selected to be from about 100 to 170 bp long.
Owner:RELIAGENE TECH +2
Immunological assay for spongiform encephalopathies
InactiveUS20010010918A1Quick checkMethod is fastChemiluminescene/bioluminescenceImmunoglobulins against animals/humansAntibodySpongiform encephalopathy
Owner:OCONNOR MICHAEL
Orally disintegrating preparation
InactiveCN104606683AEasy to takeNo side effectsOrganic active ingredientsNervous disorderOlder peopleAlcohol sugars
The invention provides an orally disintegrating preparation. The orally disintegrating preparation comprises treatment medicines and auxiliary materials, wherein the auxiliary materials comprise collagen peptide and sugar alcohol, the treatment medicines account for 3%-20% of the total weight, the collagen peptide accounts for 2%-18% of the total weight, and the sugar alcohol accounts for 15%-80% of the total weight. The orally disintegrating preparation aims to solve the problem that medicines are difficult to swallow by ALS patients, children, old people and the like, meanwhile, a conventional auxiliary material, namely, gelatin, of the orally disintegrating preparation is abandoned, and the BSE (bovine spongiform encephalopathy) virus danger and the heavy metal chromium problem caused by the gelatin arouse wide concern.
Owner:BEIJING FUYUAN GLOBAL BIOTECH
Method for the detection of proteins of animal origin in complex mixtures
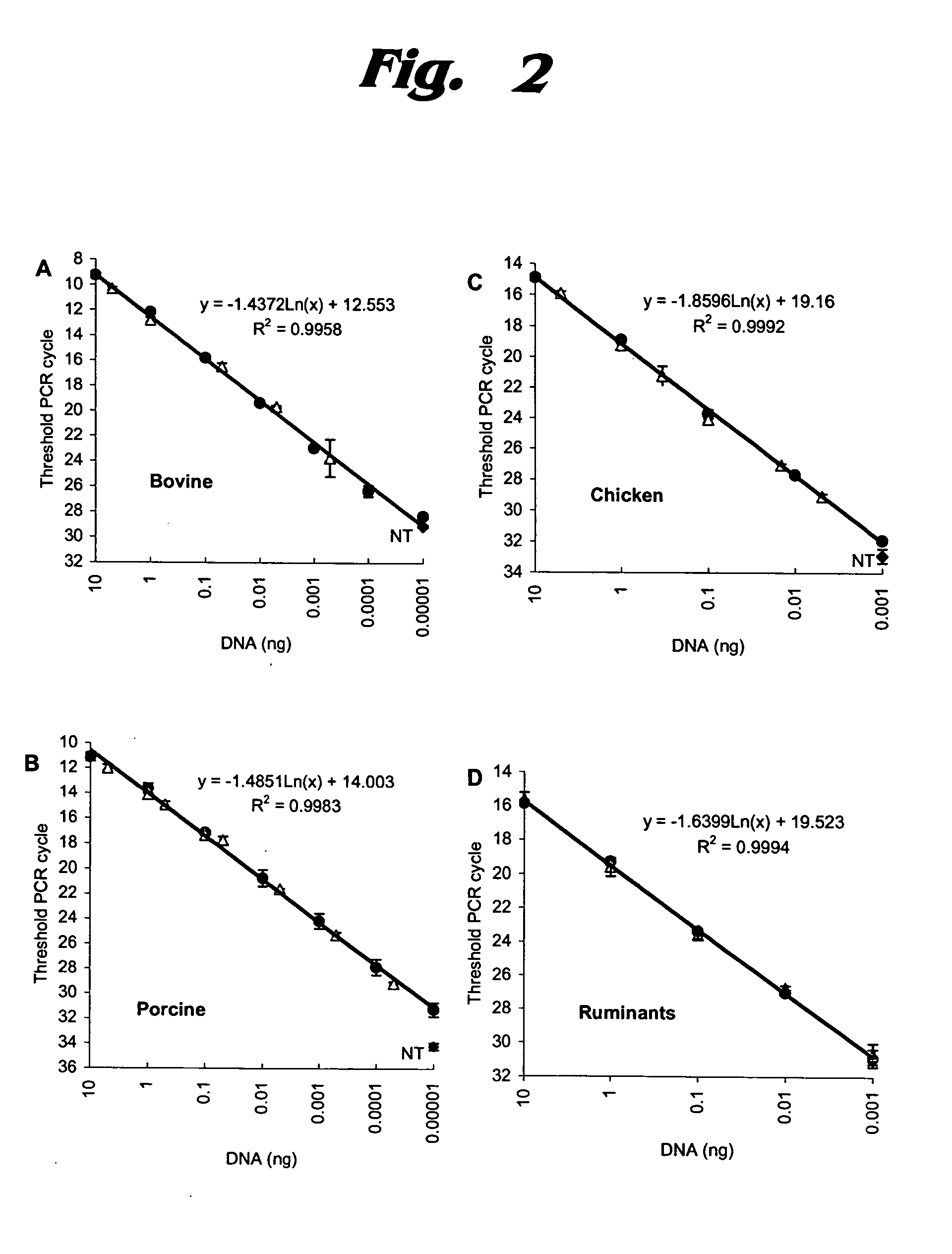
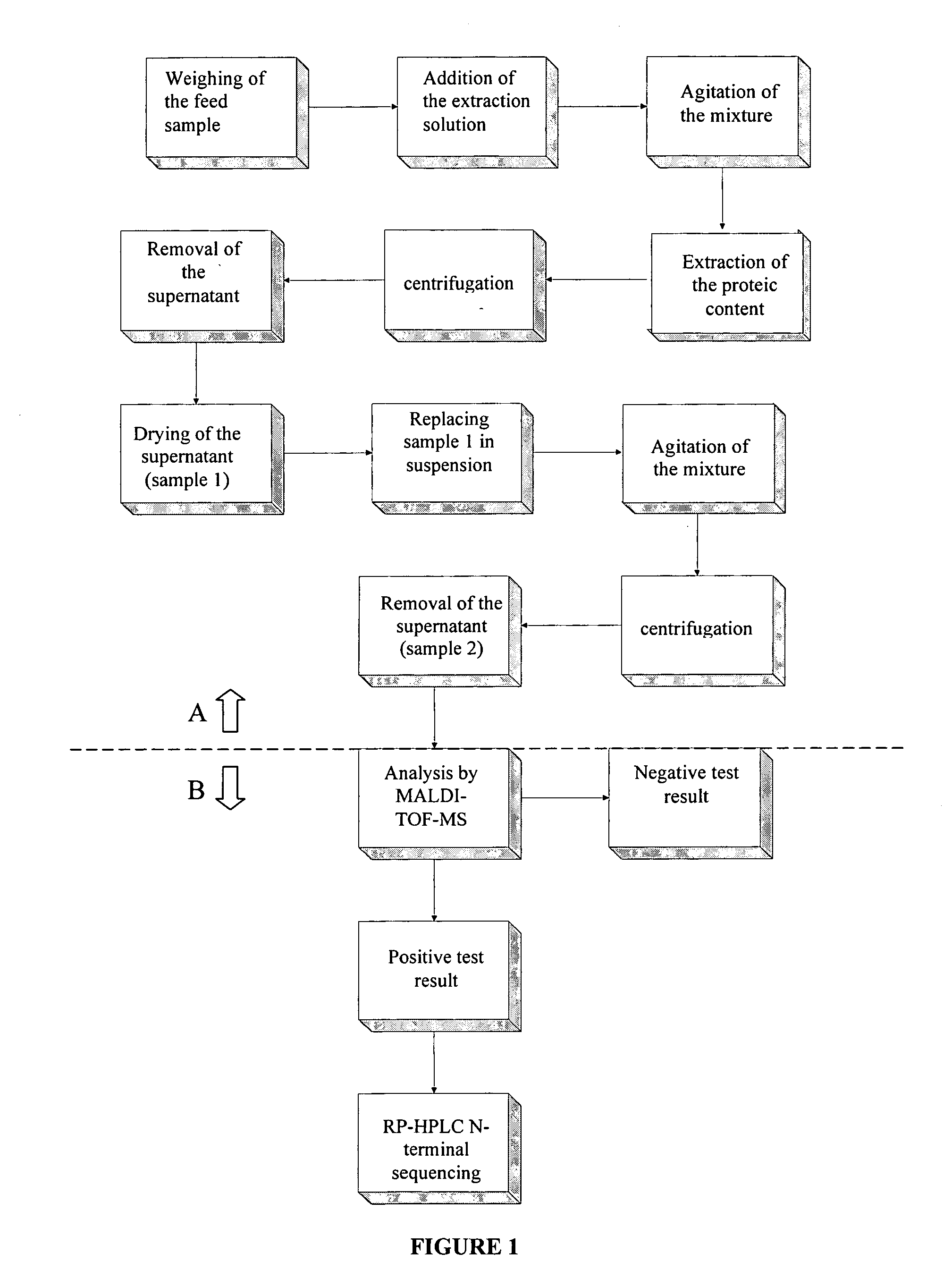
InactiveUS20050118720A1Avoid spreadingLow impurity contentNervous disorderComponent separationHigh concentrationLiquid chromatography mass spectroscopy
The purpose of the present invention is to evaluate the quality of feed for ruminants and consequently, avoid the transmission of TSEs through the detection of animal proteins in these foods. This purpose is embodied in the form of a method for detecting proteins of animal origin in complex mixtures comprising the stages of: (i) extraction of the proteic matter in high concentration from a sample of the initial complex mixture in a manner as to substantially remove all interferents; (ii) preparation of the matrix-analyte in a manner as to maintain low levels of impurities and an adequate matrix-analyte molar rate; (iii) analysis of the material obtained in the prior stage by MALDI-TOF mass spectrometry; (iv) optionally, fractionation of the samples or isolation of the components by RP-HPLC and identification of the components by means of automatic sequencing of the N-terminal region and sequencing of its peptidic fragments by liquid chromatography coupled to mass spectrometry (LC / MS / MS). The present invention also contemplates the use of this method in the detection of proteins of animal origin in feed for ruminants, which permits the interruption of transmission of Transmittable Spongiform Encephalopathies, and more particularly Bovine Spongiform Encephalopathies (BSE).
Owner:EMPRESA BRASILEIRA DE PESQUISA AGROPECUARIA EMBRAPA
Nucleotide sequence for assessing transmissible spongiform encephalopathies
The present invention is directed to the assessment of transmissible spongiform encephalopathy (TSE) using a sample from a living individual. The assessment is based on the use of an RNA marker molecule in a sample of whole blood. The RNA encodes a hypothetical cystein protease. The invention is further directed to the detection of the marker molecule by means of real-time PCR. The invention provides the use of the nucleotide sequence as a marker in the assessment of TSE, a method for assessing bovine spongiform encephalopathy (BSE) in a bovine animal as well as kits to practice the invention.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Detection of nucleic acids to assess risk for bovine spongiform encephalopathy
ActiveUS20060068419A1Sugar derivativesMicrobiological testing/measurementBiologyTransmissible spongiform encephalopathy
The present invention provides a method of detecting abnormal serum nucleic acid profiles to assess the risk of a transmissible spongiform encephalopathy, e.g., BSE.
Owner:CHRONIX BIOMEDICAL
Ultrasensitive detection of prions by automated protein misfolding cyclic amplification
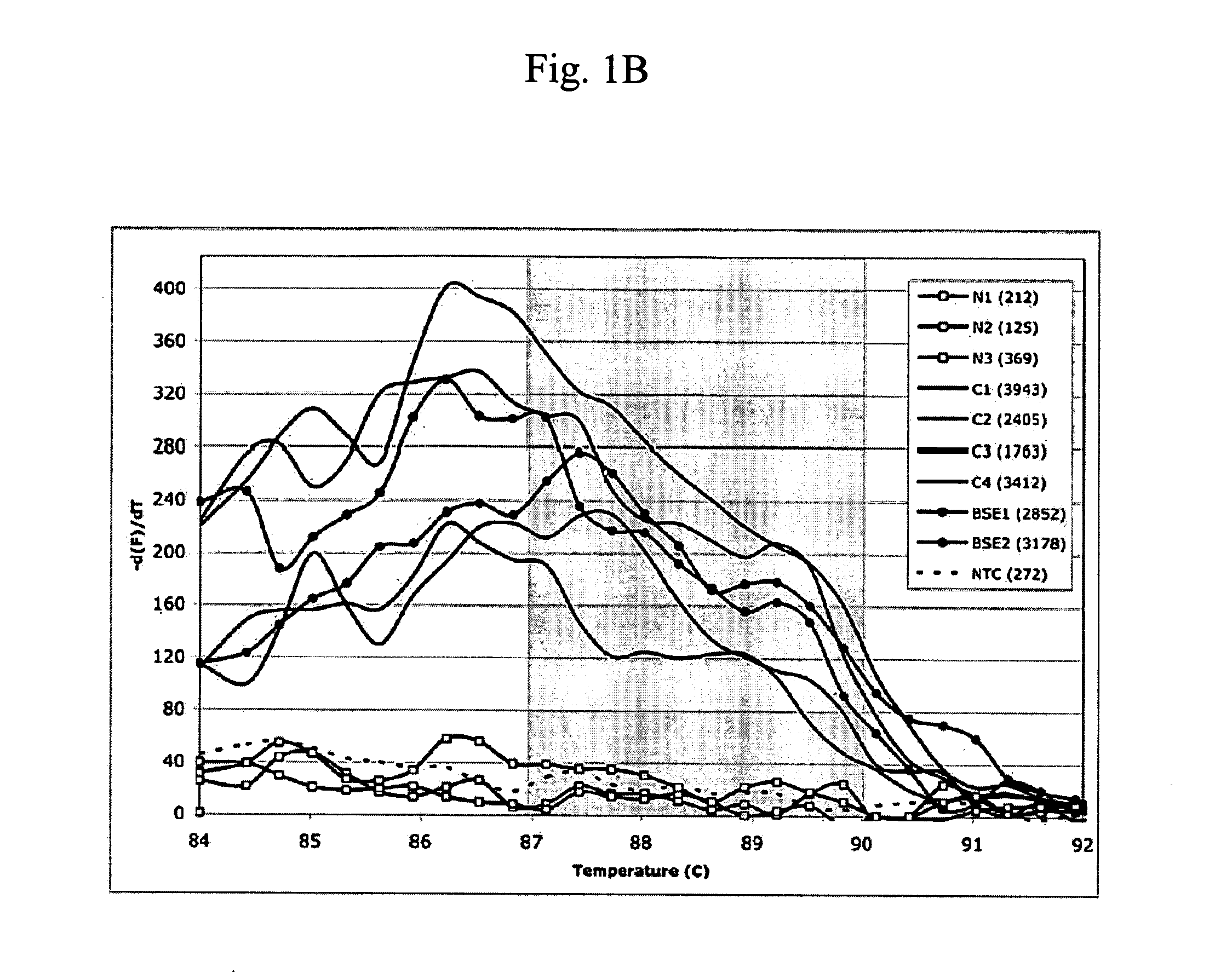
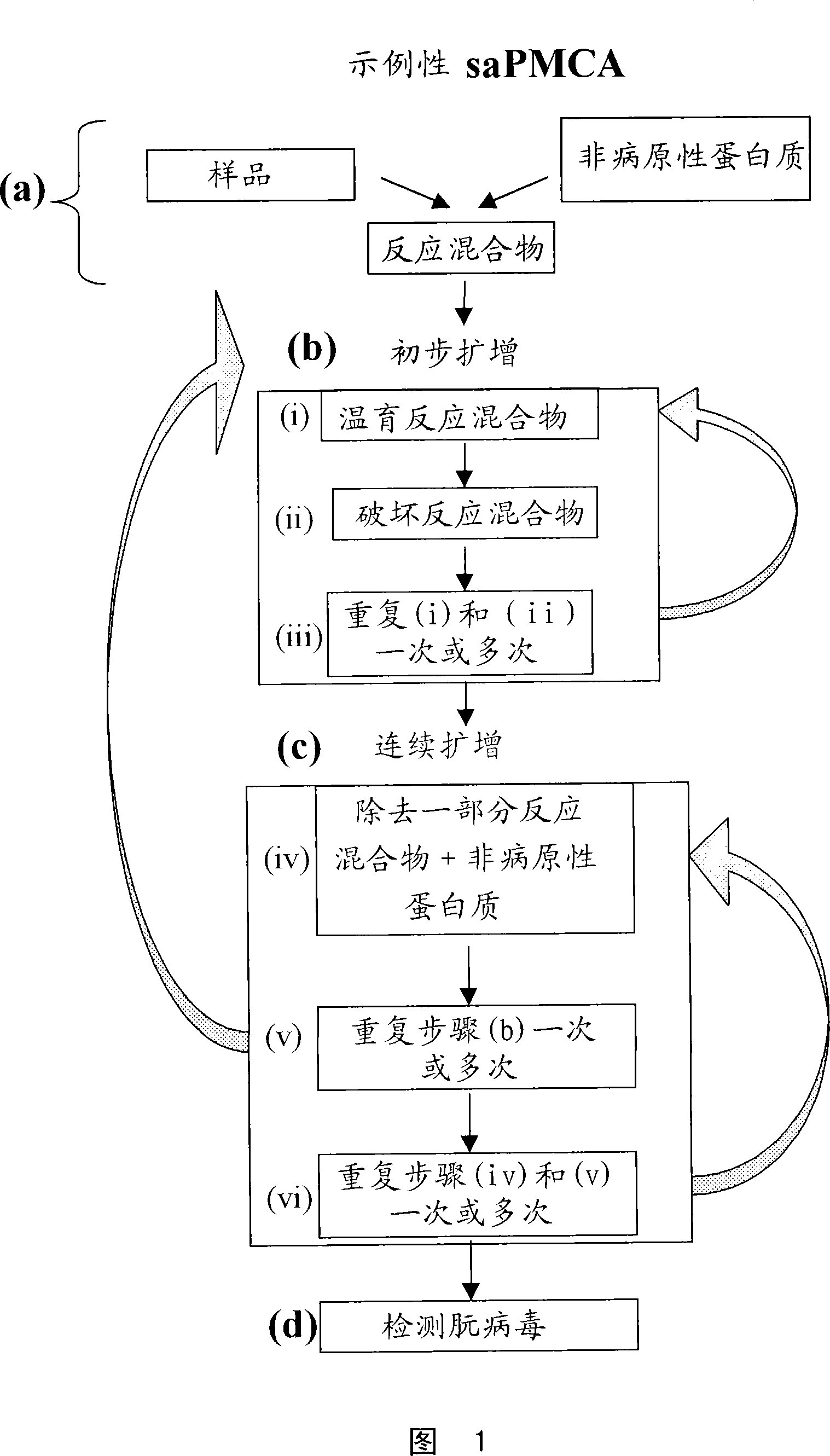
A highly sensitive method for detecting prions in a sample is provided. These methods can be used to diagnose prion-mediated transmissible spongiform encephalopathy, such as bovine spongiform encephalopathy, Creutzfeldt-Jakob disease, scrapie or chronic wasting disease. In particular, methods for continuous automated cyclic expansion of prions are disclosed. The method is fast and highly sensitive, making it ideal for high-throughput testing.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
"prionins", highly specific markers for noninvasive pre-symptomatic detection of tse diseases, and targets for therapeutic reagents to prevent and control tse diseases in animals and humans
InactiveUS20020155552A1Nervous disorderMicrobiological testing/measurementWhole blood productVirus Protein
Proteins expressed from within the prion protein genes of all animals and humans, "prionins", against which reagents can be prepared for accurate pre-symptomatic diagnosis, for detecting latent TSE, for detecting TSE contamination of food, blood and blood products and for therapeutic treatment of Bovine spongiform encephalopathy (BSE) in cows, Scrapie disease in sheep and Creutzfeldt-Jakob syndrome in humans, are revealed.
Owner:BERGMANN JOHANNA +1
Method for efficiently amplifying abnormal prion protein derived from bse
InactiveUS20110124843A1Effective sensitivityHigh sensitivityDepsipeptidesPeptide preparation methodsSulfatePrion protein
Owner:NAT AGRI & FOOD RES ORG
Polymorphism in bovine prion protein gene sequence
A specific, non-synonymous SNP in the Prnp gene encoding the bovine prion protein affects the susceptibility of bovine animals to bovine spongiform encephalopathy (BSE). Depending on the number of octapeptide repeat units present in the Prnp gene, the position of the SNP is either nucleotide 631 of exon 3 (codon 211) when the Prnp gene comprises six octapeptide repeat region sequences, nucleotide 607 of exon 3 (codon 203) when the Prnp gene comprises five octapeptide repeat region sequences, or nucleotide 655 of exon 3 (codon 219) when the Prnp gene comprises seven octapeptide repeat region sequences. Alleles of the bovine Prnp wherein the SNP at these positions is lysine (K) at the corresponding amino acids (i.e., 211, 203 or 219) in the bovine prion protein are all indicative of increased susceptibility to BSE in comparison to alleles which encode glutamic acid (E) at the same position. This SNP may be used as a marker for selecting bovines susceptible to BSE for disposal and / or removal from breeding, the human food and animal feed supplies.
Owner:US SEC AGRI
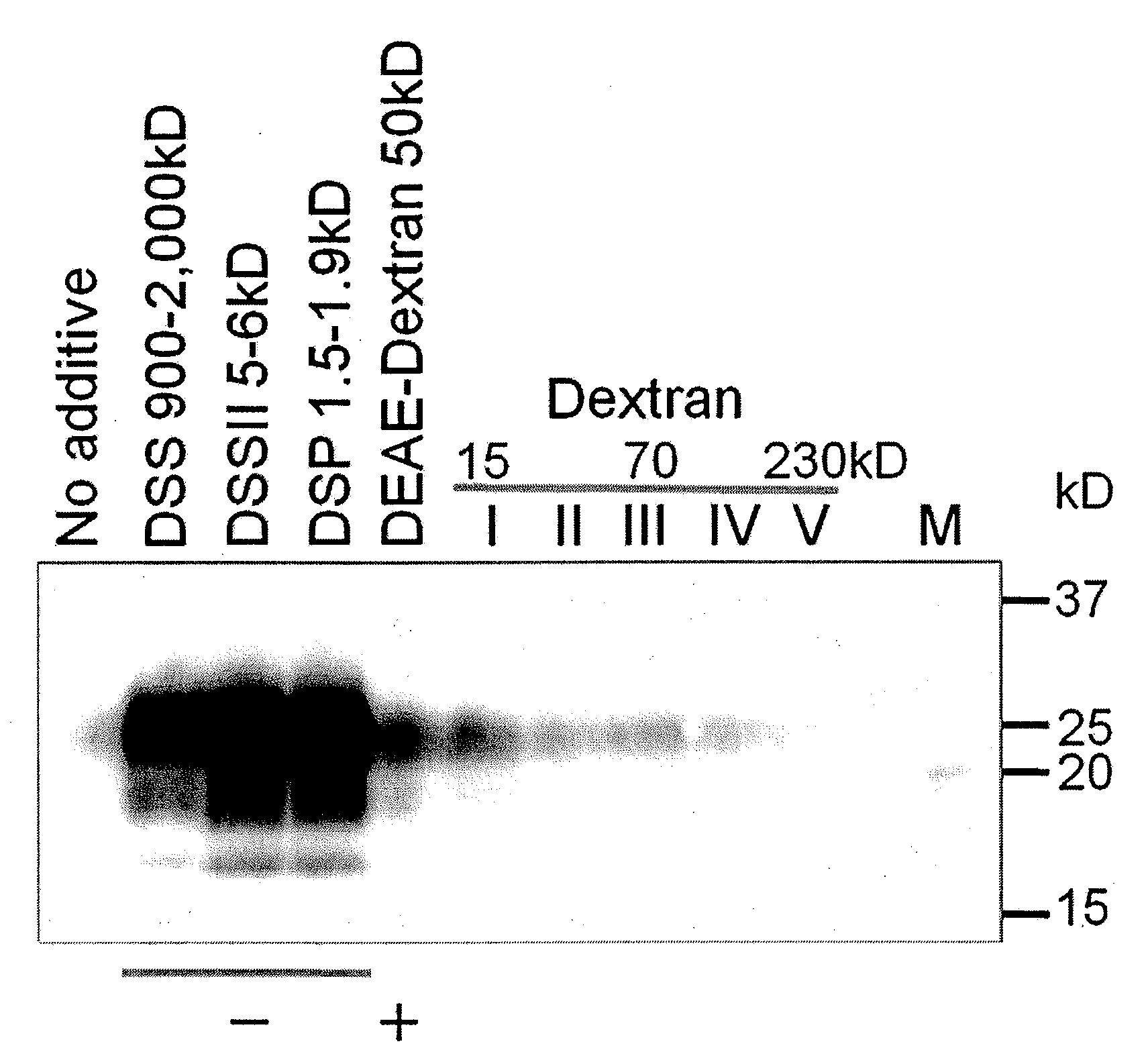
Method for chromatographic removal of prions
A method for removing a prion from a solution comprising the prion and at least one additional biomolecule, comprising directing the solution through an anion-exchange chromatography column under conditions that cause a gradient elution, whereby the prion is separated from at least one of the biomolecules, thereby causing said biomolecule to be collected in an eluate fraction that is distinct from an eluate fraction that includes the prion. In one embodiment, the gradient is a pH gradient, for example, a step gradient. The prion can be a causal agent for a spongiform encephalopathy, such as Creutzfeldt-Jakob disease, Gerstmann-Straussler-Scheinken syndrome, scrapie, or bovine spongiform encephalopathy.
Owner:HEMOGLOBIN OXYGEN THERAPEUTICS
Method for the detection of prion diseases
InactiveUS6972177B1Reliable and fast diagnosticSnake antigen ingredientsPreparing sample for investigationNematodeScrapie Virus
The invention provides methods for the detection of prion diseases, such as scrapie of sheep, bovine spongiform encephalopathy of cattle, Creutzfeld-Jacob disease of man, whereby aberrant proteins or prion proteins are detected in tissues which can be sampled from line animals.
Owner:STICHTING DIENST LANDOUWKUNDIG ONDERZOEK
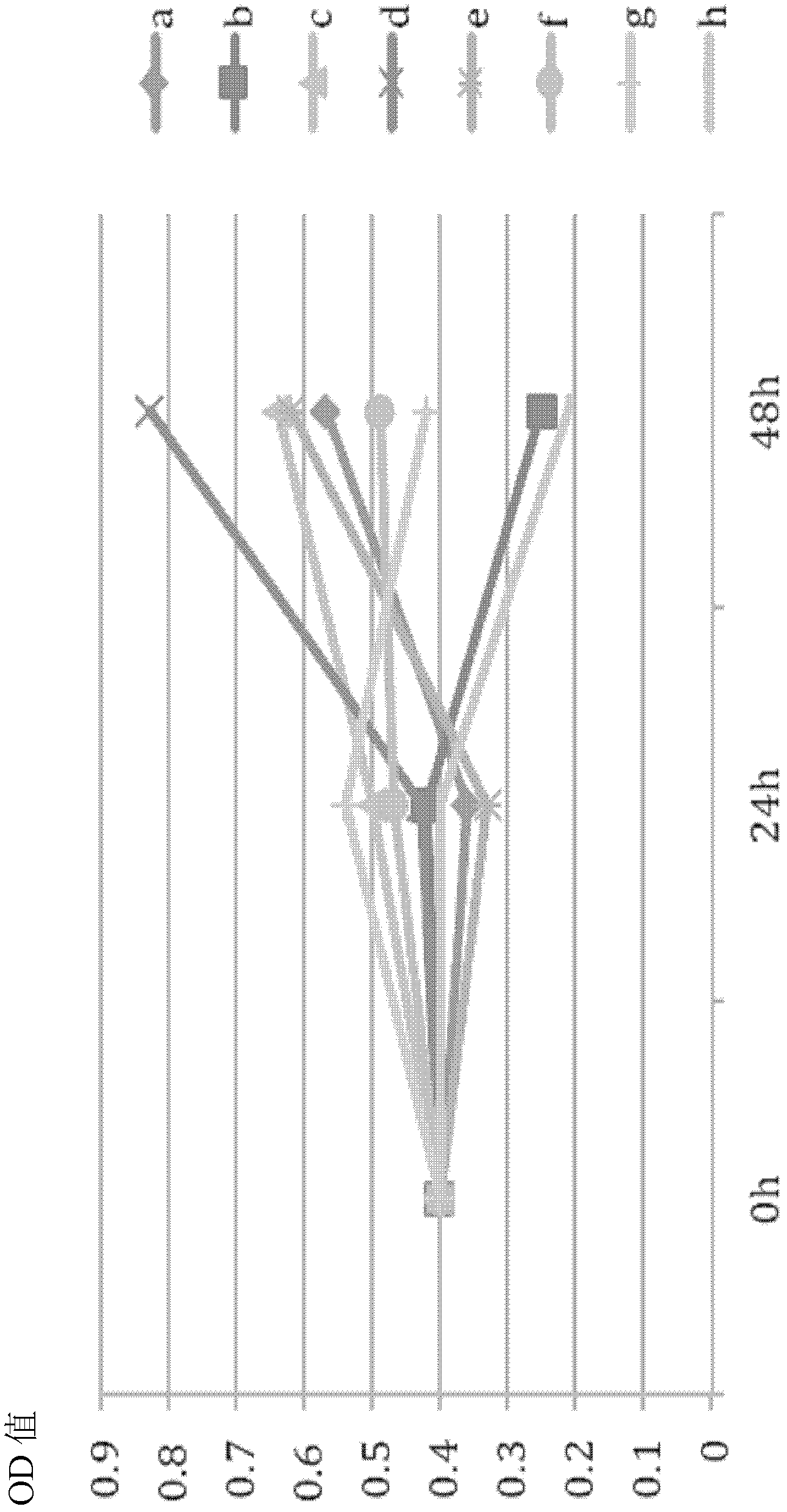
Cell culture medium for substituting serum by sericin
Owner:嘉兴欣贝莱生物科技有限公司
"Prionins", highly specific markers for noninvasive presymptomatic detection of TSE diseases, and targets for therapeutic reagents to prevent and control TSE diseases in animals and humans.
InactiveUS20060147908A1Nervous disorderMicrobiological testing/measurementWhole blood productTherapeutic treatment
Owner:ALTEGEN
Bovine spongiform encephalopathy detection flying mini-robot
InactiveCN106737704AOvercoming timely controlOvercome monitoringDiagnostic recording/measuringSensorsCommunication unitEngineering
The invention discloses a bovine spongiform encephalopathy detection flying mini-robot. The bovine spongiform encephalopathy detection flying mini-robot comprises a data collection unit, a data transmission unit and a data storage unit which are connected with a controller, and further comprises a wireless communication unit and a flying unit. The controller analyzes and processes physiological information and specifically conducts the steps that the collected physiological information is compared with prestored bovine spongiform encephalopathy parameter indexes one to one, and sends the physiological information exceeding bovine spongiform encephalopathy parameter values to a client side. Thus, the problems that in the prior art, a cattle herd cannot be monitored automatically in real time and cattle having the bovine spongiform encephalopathy cannot be controlled in time are solved, cattle herd state information is monitored automatically in real time, mad cattle and infected cattle are found in time, and measures are taken in time.
Owner:HECHI UNIV
Microglia Facilitated Amyloidogenesis Assay
InactiveUS20080167387A1Reduce severityImprove severityBiocideMicrobiological testing/measurementMedicineAmyloid
The present invention describes methods to identify compounds that prevents or treat amyloid accumulation in the brain, as mediated by microglia or cells of macrophage lineage. The present invention further describes compositions containing such compounds, methods of preparing such compositions and methods of using such compositions. The compositions are useful for treating or preventing diseases caused by or associated with cell-mediated amyloid formation, and are particularly useful in treating or preventing neurodegenerative diseases, such as Alzheimer's Disease and bovine spongiform encephalopathy.
Owner:PFIZER INC
Method for preparing a prion-free bond grafting substitute
The present invention relates to a method for preparing a bone graft substitute using bovine bone, and more particularly to a method for preparing a safe bone graft substitute which does not have the risk of infection with bovine spongiform encephalopathy, the method comprising treating bovine bone with sodium hypochlorite and treating the treated bone at a high temperature of more than 600° C. The bone graft substitute does not cause an immune response, because it is prepared by effectively removing lipids and organic substances from bovine bone having a structure very similar to that of the human bone. Also, it has excellent osteoconductivity, and is free of prion, and thus it does not have the risk of infection with bovine spongiform encephalopathy. According to the disclosed invention, the bone graft substitute having such advantages can be prepared in a simple manner.
Owner:SEOUL NAT UNIV R&DB FOUND
Process for preparing prion protein gene-free domestic animals
InactiveCN1970749BSignificant application valueUnderstanding Normal Physiological FunctionClimate change adaptationVector-based foreign material introductionScrapieSomatic cell
The present invention discloses a preparation method for prion protein gene knockout domestic animals. The process is characterized by: knocking off the prion protein gene of domestic animals on a cellular level; and preparing prion protein gene knockout domestic animals through somatic cell cloning method. The function of the prion protein of domestic animals is inactivated. The functional prion protein is not expressed, thus the domestic animals can resist to relative disease of prion protein, such as scrapie, bovine spongiform encephalopathy (mad cow disease) and the like.
Owner:SHANGHAI GENON BIOENG
An orally disintegrating preparation
InactiveCN104606683BEasy to takeNo side effectsOrganic active ingredientsNervous disorderOlder peopleAlcohol sugars
The invention provides an orally disintegrating preparation. The orally disintegrating preparation comprises treatment medicines and auxiliary materials, wherein the auxiliary materials comprise collagen peptide and sugar alcohol, the treatment medicines account for 3%-20% of the total weight, the collagen peptide accounts for 2%-18% of the total weight, and the sugar alcohol accounts for 15%-80% of the total weight. The orally disintegrating preparation aims to solve the problem that medicines are difficult to swallow by ALS patients, children, old people and the like, meanwhile, a conventional auxiliary material, namely, gelatin, of the orally disintegrating preparation is abandoned, and the BSE (bovine spongiform encephalopathy) virus danger and the heavy metal chromium problem caused by the gelatin arouse wide concern.
Owner:BEIJING FUYUAN GLOBAL BIOTECH
Detection of nucleic acids to assess risk for bovine spongiform encephalopathy
ActiveUS7368243B2Sugar derivativesMicrobiological testing/measurementBiologyTransmissible spongiform encephalopathy
The present invention provides a method of detecting abnormal serum nucleic acid profiles to assess the risk of a transmissible spongiform encephalopathy, e.g., BSE.
Owner:CHRONIX BIOMEDICAL
Diagnostic assay for spongiform encephalopathies
InactiveUS20090197270A1Increased proteolytic activityMicrobiological testing/measurementDisease diagnosisControl subjectsBiology
The invention provides a method of diagnosis of a spongiform encephalopathy in a diagnostic sample of a valid body tissue taken from a subject, which comprises detecting an increased proteolytic activity in the diagnostic sample, compared with a sample from a control subject.
Owner:ELECTROPHORETICS LTD
Method of producing pharmaceutical grade sodium hyaluronate from phytone
The invention discloses a method of producing pharmaceutical grade sodium hyaluronate from phytone and relates to the technical field of biological fermentation. The method is characterized by comprising the steps of a) preparing a fermentation medium by taking a vegetable protein as a raw material, and b) preparing sodium hyaluronate from the prepared fermentation medium by a microbial fermentation method, wherein a preparation method of the fermentation medium comprises the steps of dissolution, enzymolysis, enzyme deactivation, filtration, carbon absorption, decarbonization, fermentation and sterilization. The peptone is prepared from the vegetable protein creatively and used for the fermentation production of pharmaceutical grade hyaluronic acid; the risk that sodium hyaluronate prepared by fermentation of bovine bone peptone carried a bovine spongiform encephalopathy virus in the prior art is avoided; the method is high in safety; raw materials are easy to obtain; the raw materialcost is greatly lowered; and the fermentation productivity is improved.
Owner:SHANDONG FOCUSFREDA BIOTECH CO LTD
Transgenic ungulates having reduced prion protein activity and uses thereof
InactiveCN100526460CEasy to produceFused cellsGenetic engineeringAntiendomysial antibodiesTransgenesis
The present invention provides cloned transgenic ungulates (eg, cattle) in which the activity of a prion protein is reduced by one or more genetically engineered mutations. Desirably, these transgenic cattle are also genetically modified to express exogenous (eg, human) antibodies. These cattle are a safer source of human antibodies for medicinal purposes and a safer source of agricultural products due to their protection against prion-related diseases such as bovine spongiform encephalopathy (also known as mad cow disease).
Owner:KYOWA HAKKO KIRIN CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com