3D (Three dimensional) printed hydrochlorothiazide tablets and preparation method thereof
A technology for selling hydrochlorothiazide tablets and chlorothiazide tablets, which is applied in the field of 3D printing hydrochlorothiazide tablets and its preparation, can solve the problems of microbial contamination, inaccurate dosage, hyponatremia, etc., and achieve accurate volume, beautiful appearance, and drug combination. with safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
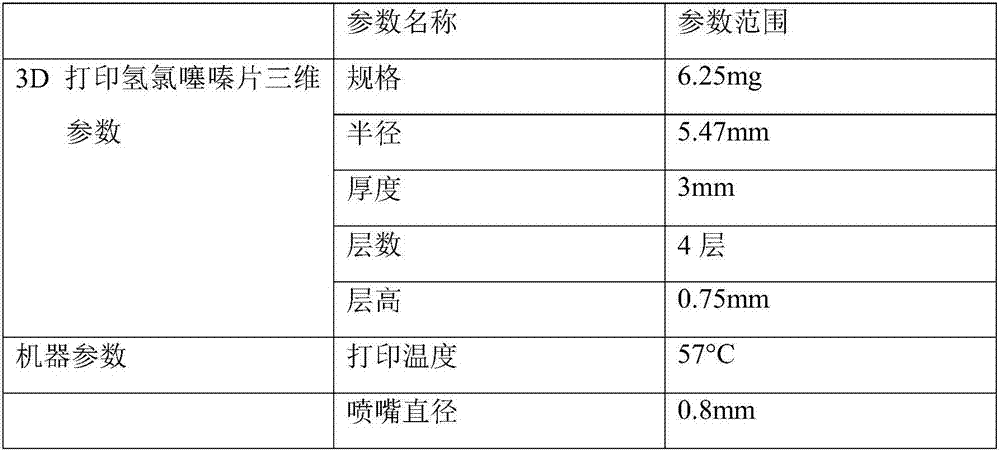
[0039] (1) Preparation of printed drug powder
[0040] Take commercially available hydrochlorothiazide tablets, poloxamer 407, PEG4000, magnesium stearate, crush and grind, pass through a 120-mesh sieve, and accurately weigh according to the following mass ratio:
[0041]
[0042] Put PEG4000 and poloxamer 407 in an evaporating dish, heat and stir in a water bath at 58°C to melt. Add commercially available hydrochlorothiazide tablet powder several times in small amounts, and stir with a glass rod until the drug and excipients are evenly mixed in the evaporating dish. Put the mixed molten printing material into the refrigerator, and store it at 4°C for 4 hours to solidify. Take out the printed material and pulverize it with a pulverizer, pass through an 80-mesh sieve, add 1 / 19 magnesium stearate and mix with it to obtain 3D printed hydrochlorothiazide tablet powder.
[0043] The density of the printed drug powder was measured to be ρ=1.41067g / mL.
[0044] The drug content...
Embodiment 2
[0058] (1) Preparation of printed drug powder
[0059] Take commercially available hydrochlorothiazide tablets, poloxamer 407, PEG4000 (i.e. polyethylene glycol 4000), magnesium stearate, crush and grind, pass through a 120-mesh sieve, and accurately weigh according to the following mass ratio:
[0060]
[0061] Put PEG4000 and poloxamer 407 in an evaporating dish, heat and stir in a water bath at 58°C to melt. Add commercially available hydrochlorothiazide tablet powder several times in small amounts, and stir with a glass rod until the drug and excipients are evenly mixed in the evaporating dish. Put the mixed molten printing material into the refrigerator, and store it at 4°C for 4 hours to solidify. Take out the printed material and pulverize it with a pulverizer, pass through an 80-mesh sieve, add 1 / 19 magnesium stearate and mix with it to obtain 3D printed hydrochlorothiazide tablet powder.
[0062] The density of the 3D printed hydrochlorothiazide tablet powder was...
Embodiment 3
[0078] (1) Preparation of printed drug powder
[0079] Take commercially available hydrochlorothiazide tablets, poloxamer 407, PEG4000, and magnesium stearate with a specification of 25 mg, crush and grind, pass through a 120-mesh sieve, and accurately weigh according to the following mass ratio:
[0080]
[0081] Put PEG4000 and poloxamer 407 in an evaporating dish, heat and stir in a water bath at 58°C to melt. Add commercially available hydrochlorothiazide tablet powder several times in small amounts, and stir with a glass rod until the drug and excipients are evenly mixed in the evaporating dish. Put the mixed molten printing material into the refrigerator, and store it at 4°C for 4 hours to solidify. Take out the printed material and pulverize it with a pulverizer, pass through an 80-mesh sieve, add 1 / 19 magnesium stearate and mix with it to obtain 3D printed hydrochlorothiazide tablet powder.
[0082] The density of the printed drug powder was measured to be ρ=1.410...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density ρ | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com