Application of entacapone to prevention or treatment of obesity and other metabolic syndrome
A technology of entacapone and its composition, which is applied in the field of medicine, can solve the problems that the upstream and downstream action elements and regulatory mechanisms of FTO protein have not been reported, and the mechanism of action of FTO protein is unclear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
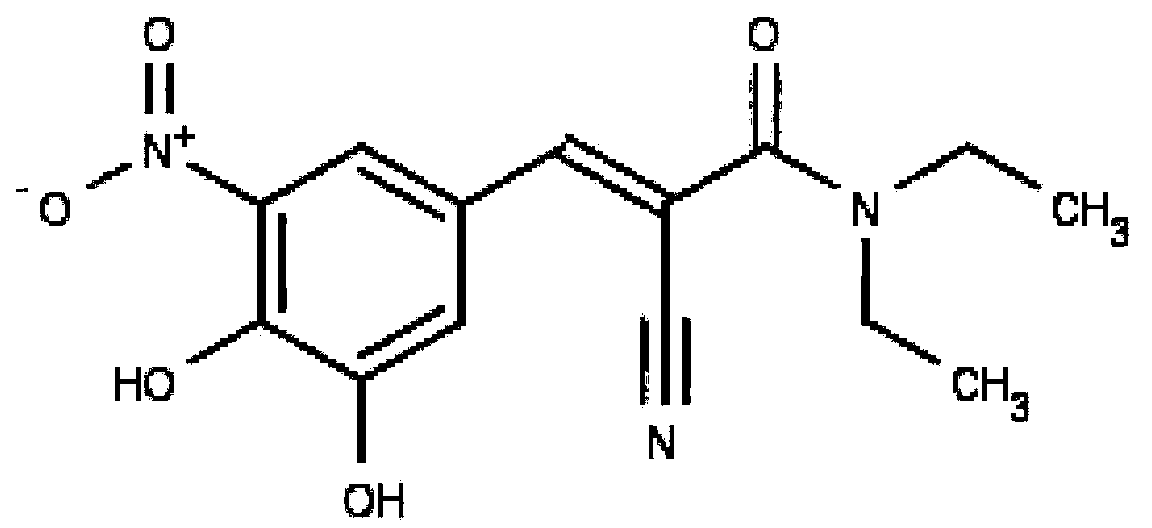
[0083] Structure-Based Virtual Screening . We screened 1323 FDA-approved drugs for the substrate-binding site of FTO protein, and according to some criteria based on physicochemical definitions, such as the number of hydrogen bonds, the number of embedded carbon atoms and hydrophobic interactions, delete Go to some unreasonable conformation. The remaining conformations were subjected to further conformational optimization and recalculation of relative binding free energies. According to binding conformation and energy, one of the top-ranked compounds is entacapone ( figure 1) .
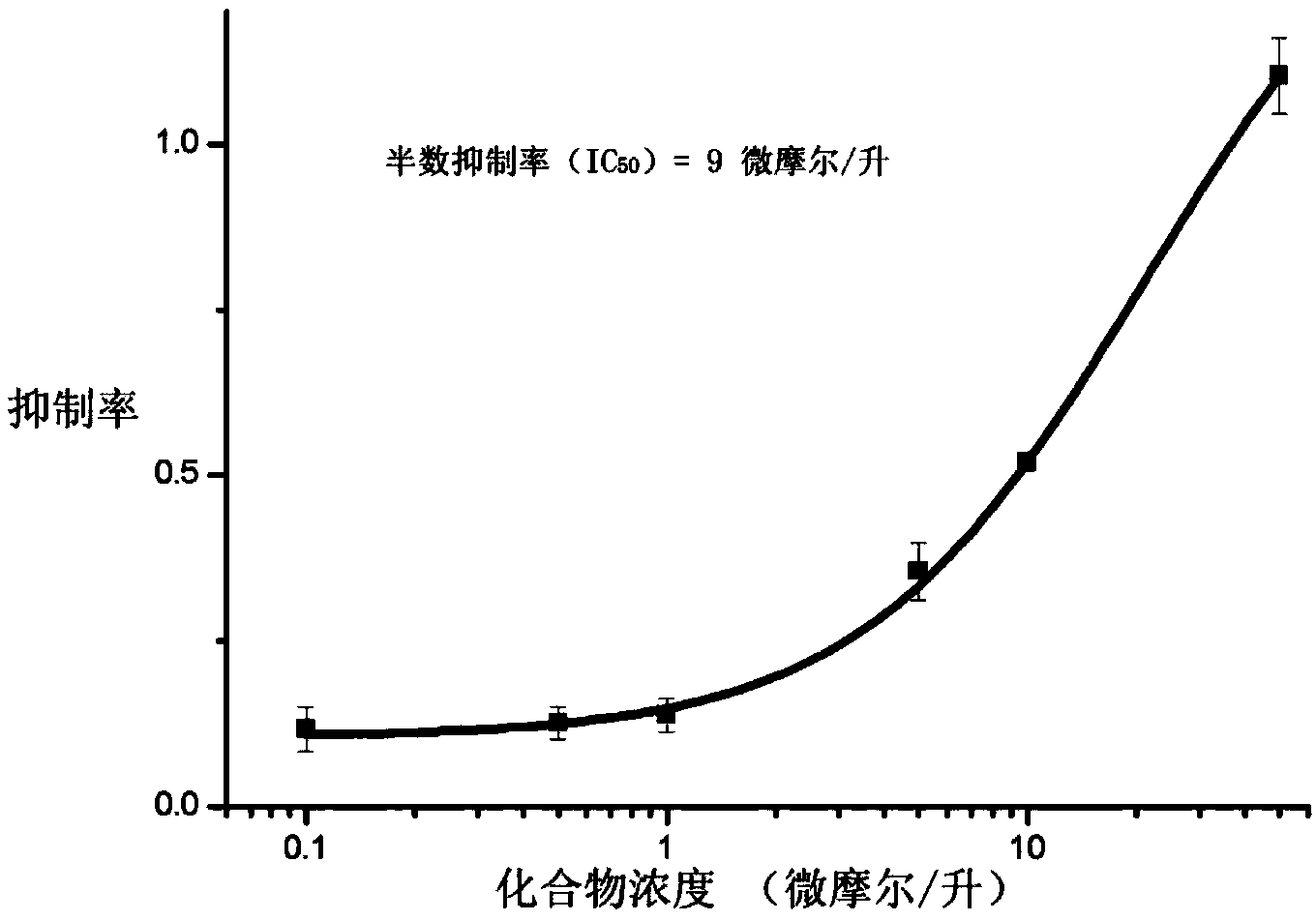
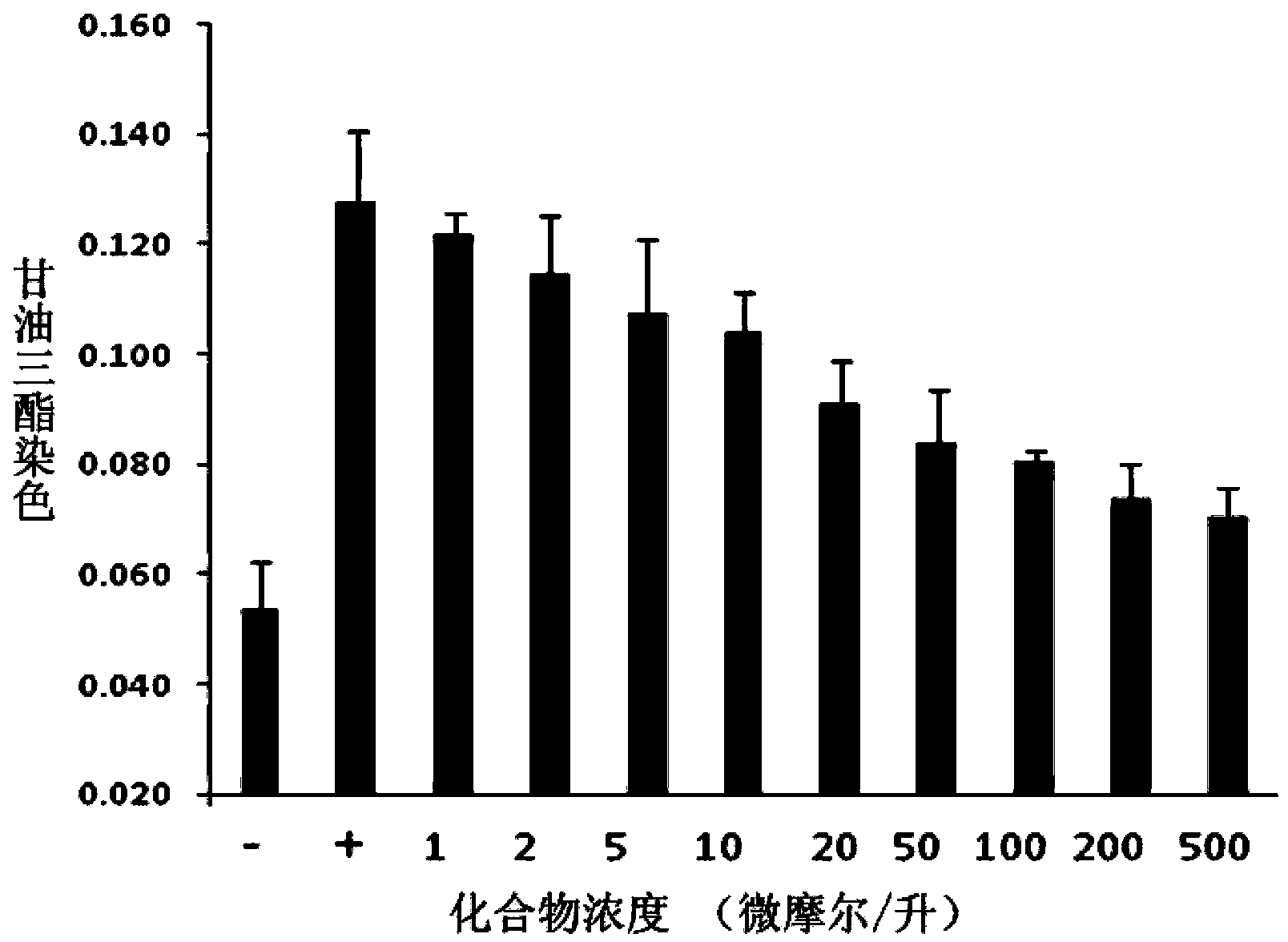
[0084] Enzyme activity inhibition test . We determined the inhibitory activity of entacapone on FTO protein by demethylation assay. We used a 100μl reaction system containing 50mM HEPES buffer (pH=7.0), 100μM a-KG, 100μM (NH 4 ) 2 Fe(SO 4 ) 2 , 1mM L-ascorbic acid, 50μg / ml BSA, 0.5μM ssDNA with N 6 -mA(5'-ATTGTCA(m 6 A) CAGCAGA-3'), and 0.1 μM FTO protein. The reaction system was incub...
Embodiment 2
[0090] Embodiment 2 co-packaging or co-preparation embodiment
[0091] 1. Entacapone / orlistat are jointly made into tablets at 1000mg / 120mg or 2000mg / 120mg.
[0092] 2. Entacapone / sibutramine are jointly made into tablets at 1000mg / 10mg or 2000mg / 10mg.
[0093] 3. Entacapone / Greencaserin are jointly made into tablets at 1000mg / 10mg or 2000mg / 10mg.
[0094] 4. Entacapone / rimonabant is jointly made into tablets at 1000mg / 20mg or 2000mg / 20mg.
[0095] 5. Entacapone / Metformin are jointly made into tablets at 1000mg / 500mg or 2000mg / 500mg.
[0096] 6. Entacapone / Exenatide: 1000mg or 2000mg per tablet / 250mg / mL solution co-packed
[0097] 7. Entacapone / Exenatide: 1000mg or 2000mg per tablet / suspension ER 2mg (Bydureon), co-packaged
[0098] 8. Entacapone / pramlintide: 1000mg or 2000mg per tablet / 600mg / mL), co-packaged.
[0099] 9. Entacapone / phentermine is jointly made into tablets at 1000mg / 10mg or 2000mg / 10mg.
[0100] 10. Entacapone / atorvastatin are jointly made into tablets a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com