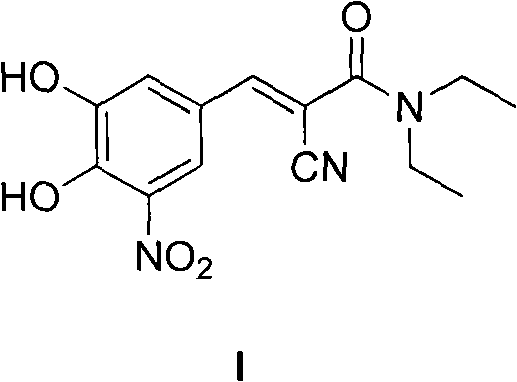
New preparation method of (2E)-2-cyano-3-(3,4-dihydroxy-5-nitrobenzene)-N,N-diethyl-2-acrylamide
A technology of diethyl cyanoacetamide and acrylamide, which is applied in the preparation of carboxylic acid nitriles, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of many steps, low yields, and complicated operations, and meet the reaction conditions. Mild, high yield, short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
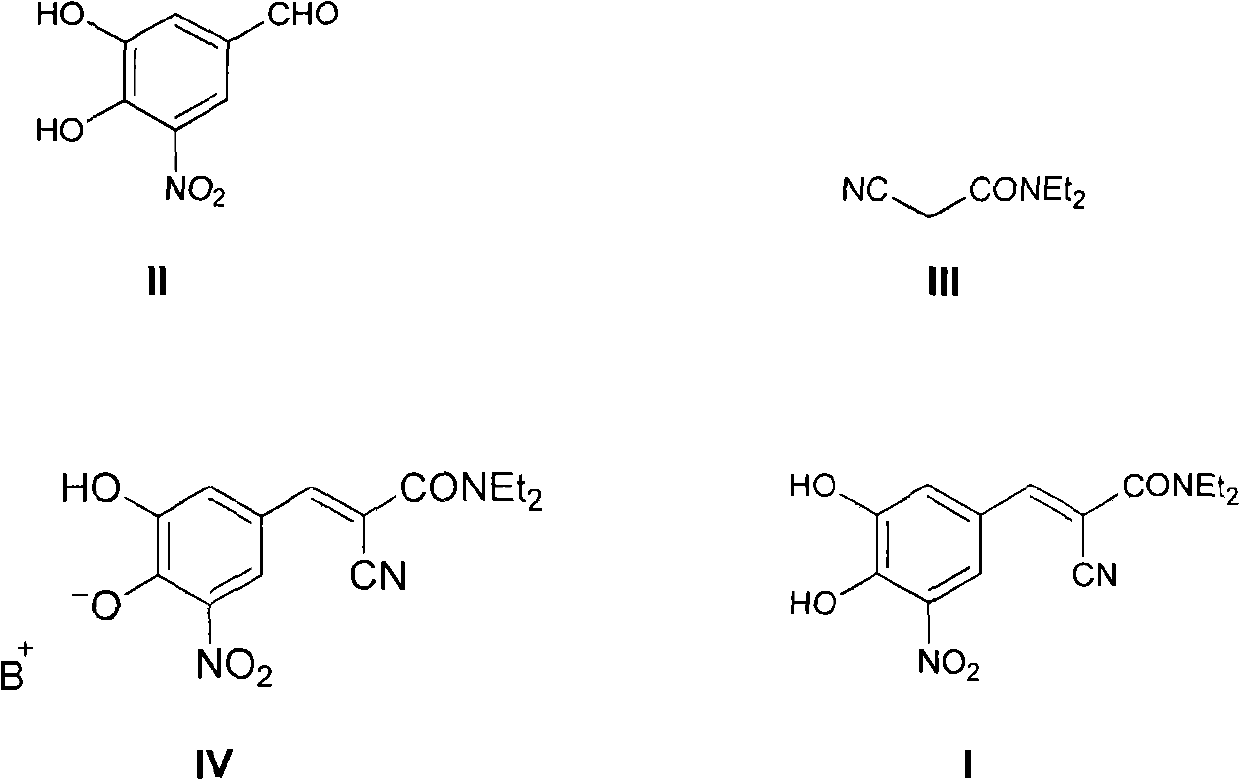
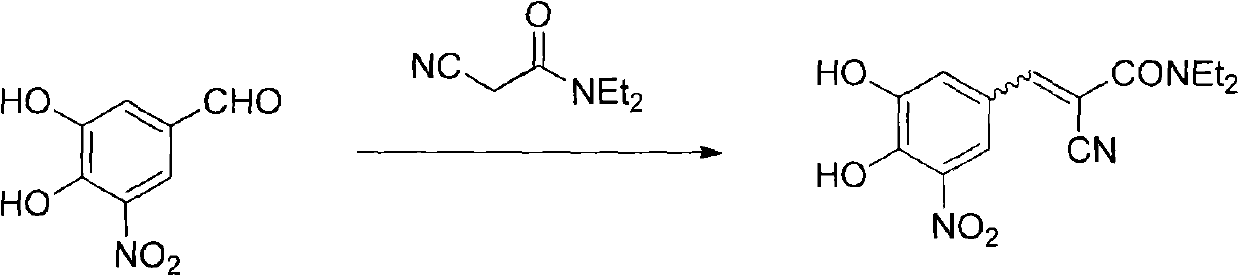
[0033] Add 38.0 grams of compound (II), 34.8 grams of compound (III), 19.4 grams of piperidine, 150 milliliters of ethyl acetate, and 150 milliliters of n-heptane into a 1000 milliliter three-necked reaction flask in sequence, heat to reflux to separate water, and react for 6 hours Afterwards, the raw material reacted completely, cooled to room temperature, stirred for 2 hours, filtered, and the filter cake was washed with a small amount of ethyl acetate / n-heptane=1 / 1 solvent, dried to obtain 75.3 grams of entacapone piperidine salt (IV), Yield 93%.
[0034] HPLC purity 99.2%, Z-isomer 0.1%.
[0035] Suspend the above 75.3 grams of compound (IV) in 400 milliliters of isopropanol, add 23.2 grams of glacial acetic acid in 400 milliliters of distilled aqueous solution dropwise, keep the temperature at 40-50 ° C, after the addition is complete, continue to stir for 2 hours, cool Stir at room temperature for 1 hour, filter, the filter cake is washed successively with 100 millilite...
Embodiment 2
[0038] Add 38.0 grams of compound (II), 34.8 grams of compound (III), 19.4 grams of piperidine, 150 milliliters of isopropyl acetate, and 150 milliliters of n-hexane into a 1000 milliliter three-necked reaction flask in sequence, heat to reflux to separate water, and react for 6 hours Afterwards, the raw material reacted completely, cooled to room temperature, stirred for 2 hours, filtered, and the filter cake was washed with a small amount of isopropyl acetate / n-hexane=1 / 1 solvent, dried to obtain 72.8 grams of entacapone piperidine salt (IV), Yield 90%.
[0039] HPLC purity 98.9%, Z-isomer 0.2%.
[0040] Suspend the above 72.8 grams of compound (IV) in 400 milliliters of isopropanol, add 22.4 grams of glacial acetic acid in 400 milliliters of distilled aqueous solution dropwise to the system, keep the temperature at 40-50 ° C, after the addition, continue to stir for 2 hours, cool Stir at room temperature for 1 hour, filter, the filter cake is washed successively with 100 m...
Embodiment 3
[0043] Add 38.0 grams of compound (II), 34.8 grams of compound (III), 19.4 grams of piperidine, 100 milliliters of ethyl acetate, 100 milliliters of n-heptane, and 100 milliliters of isopropanol into a 1000 milliliter three-necked reaction flask, and heat to reflux Water was separated, and after 8 hours of reaction, the raw materials were completely reacted, cooled to room temperature, stirred for 2 hours, filtered, and the filter cake was washed with a small amount of ethyl acetate / n-heptane / isopropanol=1 / 1 / 1 solvent, dried to obtain 72.1 Keentacarbone piperidine salt (IV), yield 89%.
[0044] HPLC purity 99.0%, Z-isomer 0.1%.
[0045] Suspend the above 72.1 grams of compound (IV) in 400 milliliters of isopropanol, add 22.2 grams of glacial acetic acid in 400 milliliters of distilled aqueous solution dropwise, keep the temperature at 40-50 °C, after the addition is complete, continue to stir for 2 hours, cool Stir at room temperature for 1 hour, filter, the filter cake is wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com