Acid-base bifunctional catalyst for synthesis of methyl methacrylate by methyl propionate and formaldehyde
A methyl methacrylate, acid-base bifunctional technology, applied in the field of acid-base bifunctional catalysts, can solve the problems of low catalyst activity, poor selectivity, easy deactivation, etc. short step effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
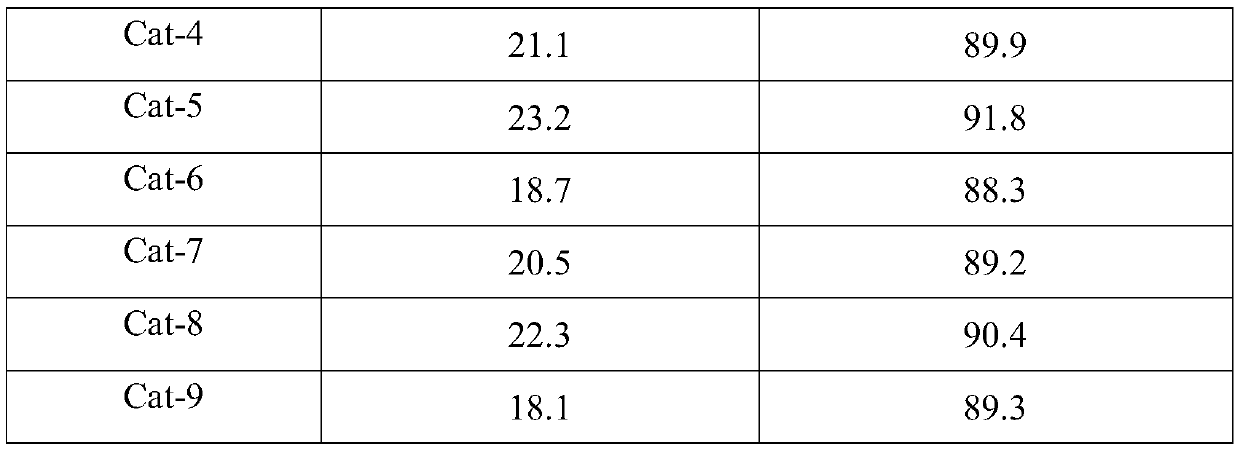
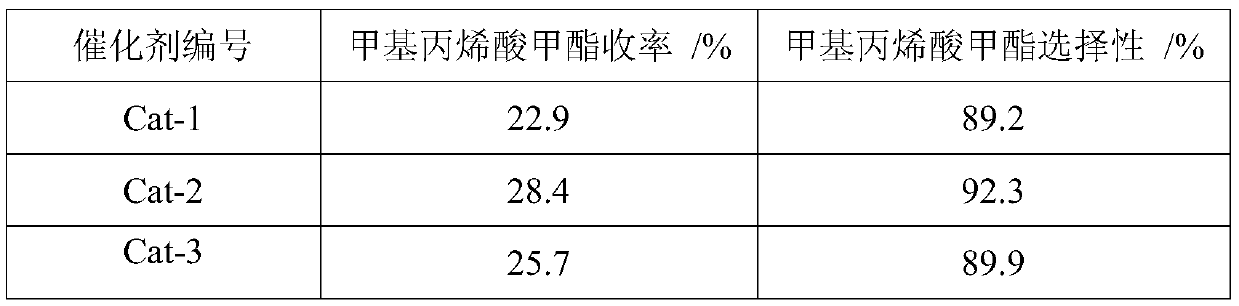
Image
Examples
Embodiment 1
[0031] The catalyst of this embodiment is composed of 5% active component Cs, 5% active component P, 0.1% additive Zr and γ-Al 2 o 3 Vector composition. Wherein, the source of Cs is cesium nitrate, the source of P is ammonium dihydrogen phosphate, and the source of Zr is zirconium nitrate. The specific preparation method is as follows:
[0032] (1) Take by weighing 0.36g cesium nitrate and 0.41g ammonium dihydrogen phosphate and add water to dissolve, be mixed with aqueous solution;
[0033] (2) Weigh 10g γ-Al 2 o 3 For the carrier, add the carrier to the solution prepared in step (1) according to the equal-volume impregnation method, age at room temperature for 10 hours, and then dry at 100° C. for 12 hours;
[0034] (3) Take by weighing 0.035g zirconium nitrate pentahydrate and add water to dissolve, be mixed with aqueous solution;
[0035] (4) Add the solid dried in step (2) to the solution prepared in step (3) by equal volume impregnation, age at room temperature for...
Embodiment 2
[0037] The catalyst of this embodiment consists of 5% active component Cs, 5% active component P, 0.1% additive Fe and β-Al 2 o 3 Vector composition. Among them, the source of Cs is cesium nitrate, the source of P is diammonium hydrogen phosphate, and the source of Fe is iron nitrate. The specific preparation method is as follows:
[0038] (1) Take by weighing 0.36g cesium nitrate and 0.47g diammonium hydrogen phosphate and add water to dissolve, be mixed with aqueous solution;
[0039] (2) Weigh 10g γ-Al 2 o 3 For the carrier, add the carrier to the solution prepared in step (1) according to the equal-volume impregnation method, age at room temperature for 10 hours, and then dry at 100° C. for 12 hours;
[0040] (3) Take by weighing 0.025g ferric nitrate nonahydrate and add water to dissolve, be mixed with aqueous solution;
[0041] (4) Add the solid dried in step (2) to the solution prepared in step (3) by equal volume impregnation, age at room temperature for 10 hours...
Embodiment 3
[0043] The catalyst of this embodiment consists of 5% active component Cs, 10% active component P, 0.3% additive Cu and α-Al 2 o 3 Carrier (after phosphoric acid treatment) composition. Wherein, the Cs source is cesium nitrate, the P source is diammonium hydrogen phosphate, and the Cu source is copper nitrate. The specific preparation method is as follows:
[0044] (1) Weigh 10gα-Al 2 o 3 Carrier, immersed in 4% phosphoric acid aqueous solution for 6h, filtered and washed with deionized water, dried at 100°C for 12h before use;
[0045] (2) Take by weighing 0.36g cesium nitrate and 0.94g diammonium hydrogen phosphate and add water to dissolve, be mixed with aqueous solution;
[0046] (3) Add the carrier obtained in step (1) to the solution prepared in step (2) according to the equal volume impregnation method, age at room temperature for 10 h, and then dry at 100° C. for 12 h;
[0047] (4) Take by weighing 0.091g copper nitrate trihydrate and add water to dissolve, be mixe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com