Levodopa/carbidopa/entacapone pharmaceutical preparation
A technology of entacapone and levodopa, applied in the directions of drug combination, drug delivery, oil/fat/wax inactive ingredients, etc., can solve the problem of not describing the oral solid composition containing entacapone, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
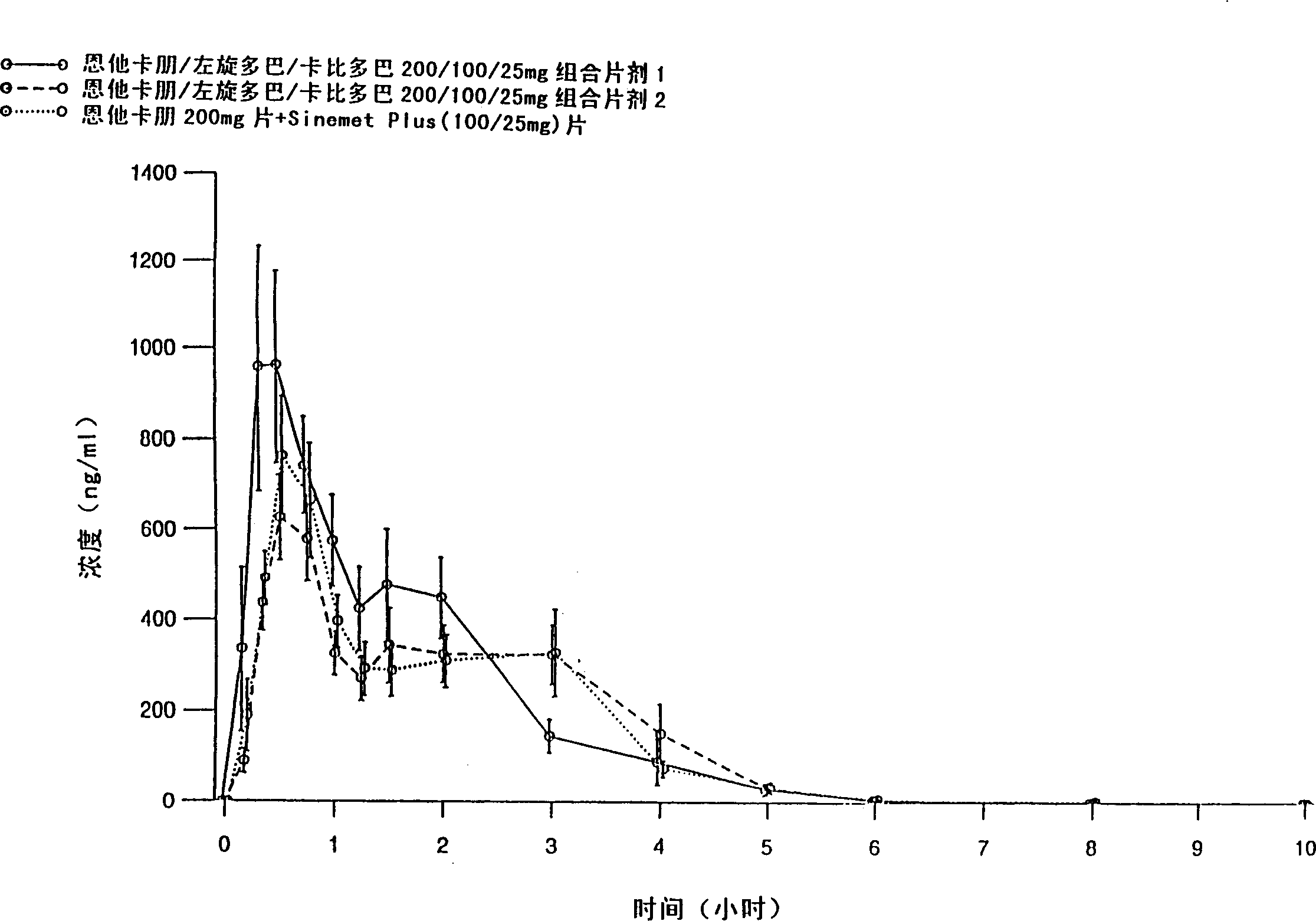
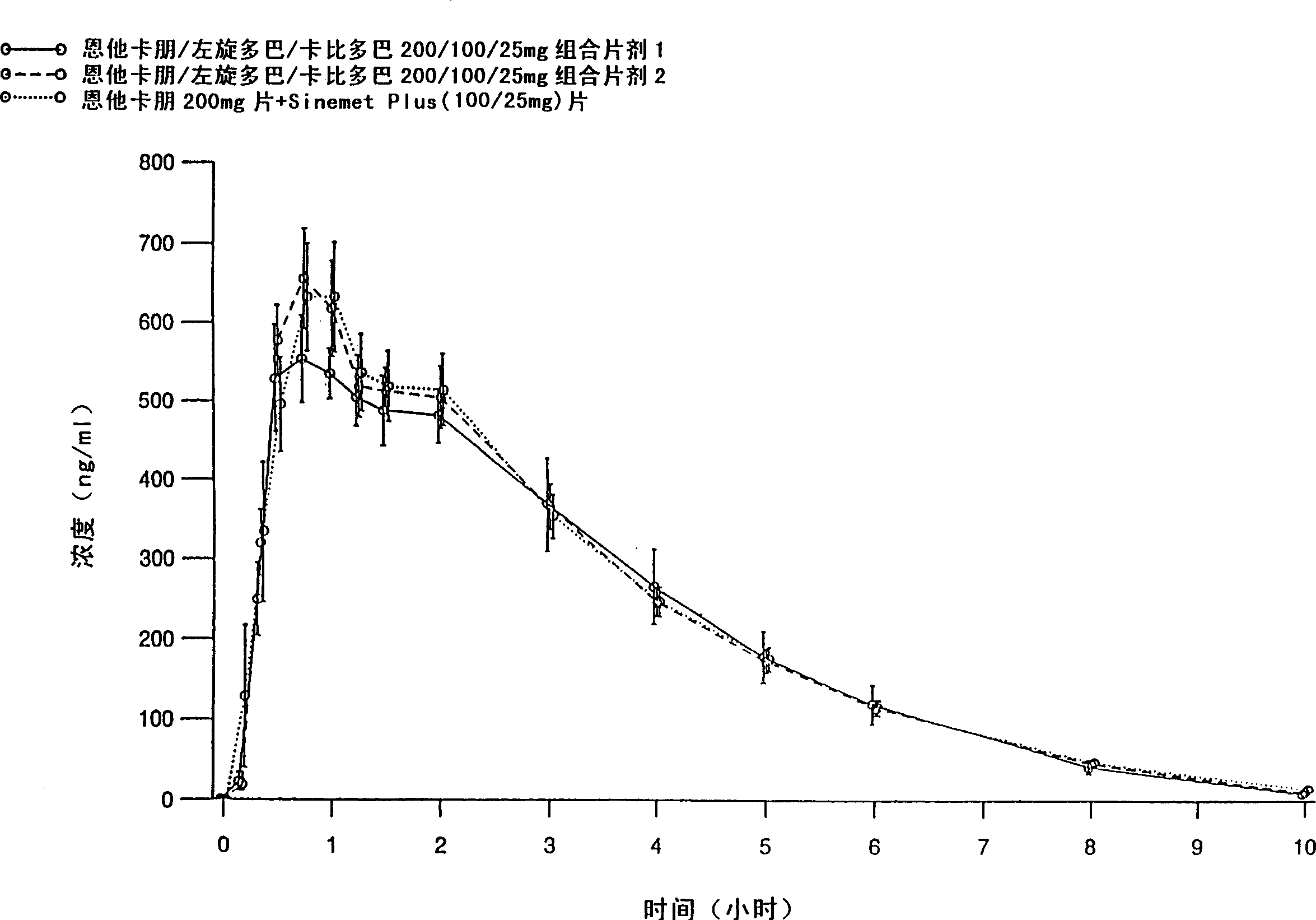
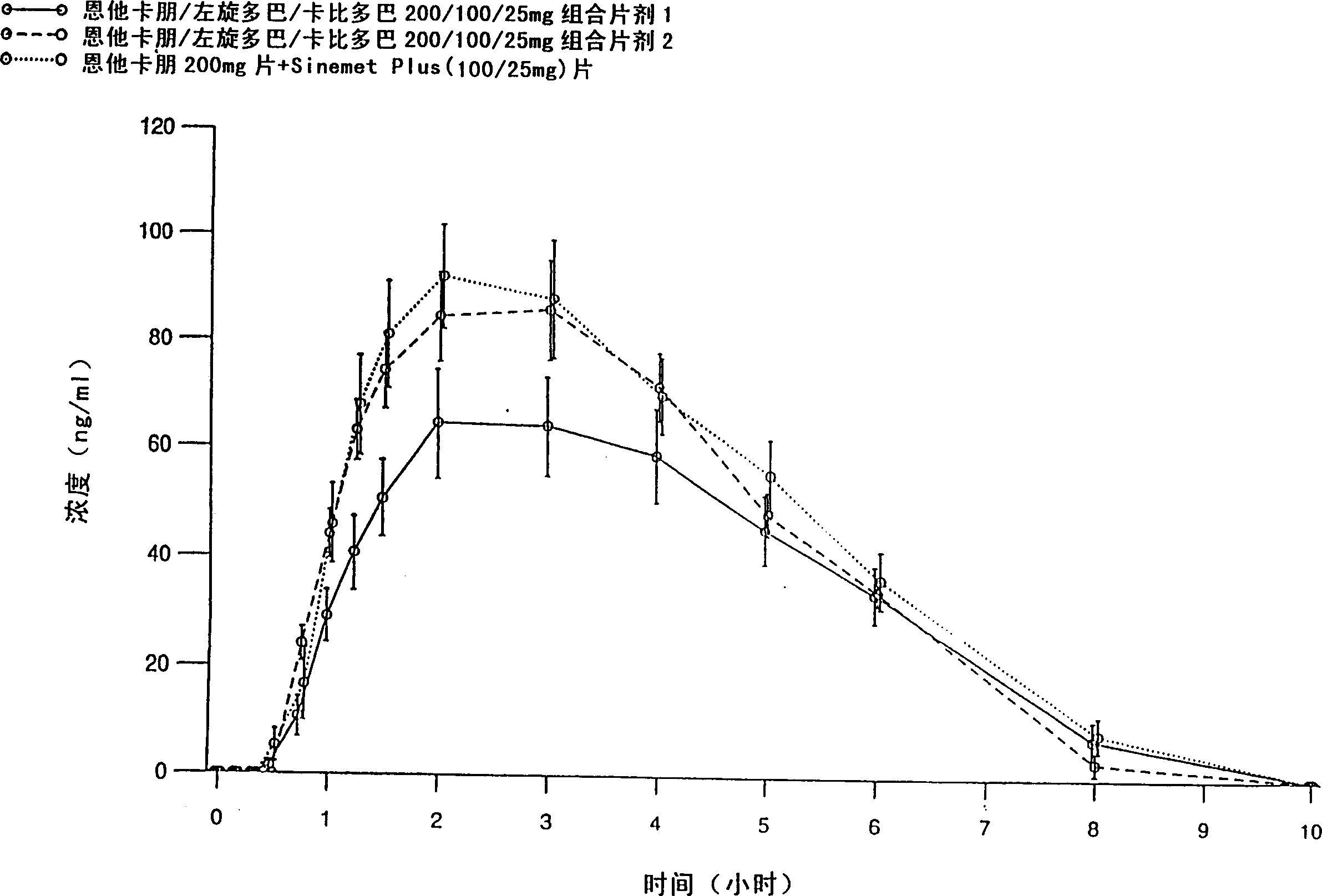
[0083] Entacapone / levodopa / carbidopa 200 / 100 / 25 mg tablets containing different excipients prepared by different methods were tested after a single oral administration to 15 healthy volunteers Peng, levodopa and carbidopa absorption. Tablets were prepared by wet granulation of all active ingredients simultaneously (Formulation 1), and compaction granulation of all active ingredients simultaneously (Formulation 2). The formulations are listed in Table 1.
[0084] The purpose of the absorption study was to evaluate the absorption of the active substance between two fixed-dose combination tablets and entacapone 200mg tablets administered together with levodopa / carbidopa 100 / 25mg tablets , the latter being SINEMET PLUS(R), marketed in Europe by DuPont Pharmaceuticals Ltd. The study was conducted according to an open randomized crossover design. Plasma entacapone, levodopa, and carbidopa concentrations were determined using two separate reversed-phase HPLC methods, that is, enta...
Embodiment 2
[0088] Examples of suitable entacapone / levodopa / carbidopa 200 / 100 / 25 mg tablets are described in Table 2. Tablets were prepared by adding carbidopa separately in granulated form (formulation 3) and in powder form itself (formulation 4) to the formulation. Therefore, to prepare formulation 3, entacapone and levodopa were wet granulated with corn starch, mannitol, croscarmellose sodium and povidone in a conventional high shear mixer . Carbidopa was wet granulated with corn starch, mannitol, croscarmellose sodium and povidone in a conventional high shear mixer. Mix the dry entacapone / levodopa granules, dry carbidopa granules, croscarmellose sodium, mannitol and magnesium stearate together, compress the resultant into oval tablets, and use Pigment-containing HPMC-coating agent coating. Formulation 4 was prepared similarly to Formulation 3, but with the addition of carbidopa itself in powder form.
[0089] Formulations 3 and 4 were tested for absorption in 15 healthy volunteers...
Embodiment 3
[0096] Prepare formulations 5 and 6 shown in Table 3 below according to formulation 3, but use 200mg / 50mg / 12.5mg (preparation 5) and 200mg / 150mg / 37.5mg (preparation 6) of entacapone / levodopa / carba Bidoba. Correspondingly, 200 mg / 100 mg / 10 mg of entacapone / levodopa / carbidopa were also prepared.
[0097] ingredient name
[0098] The tablet cores are coated with a colored HPMC-coating agent with a weight gain of 2-3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com