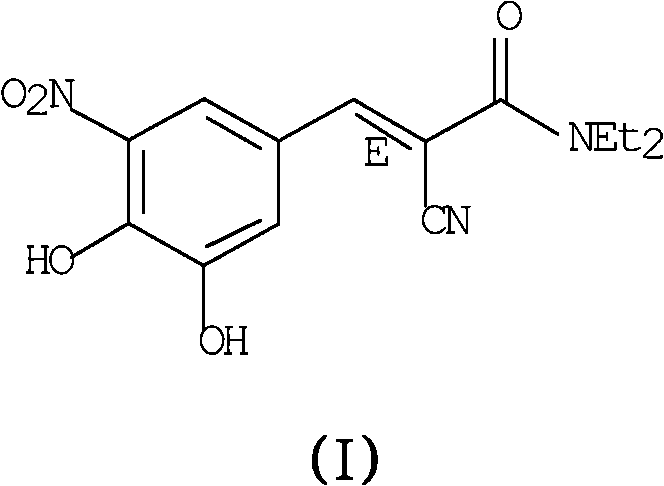
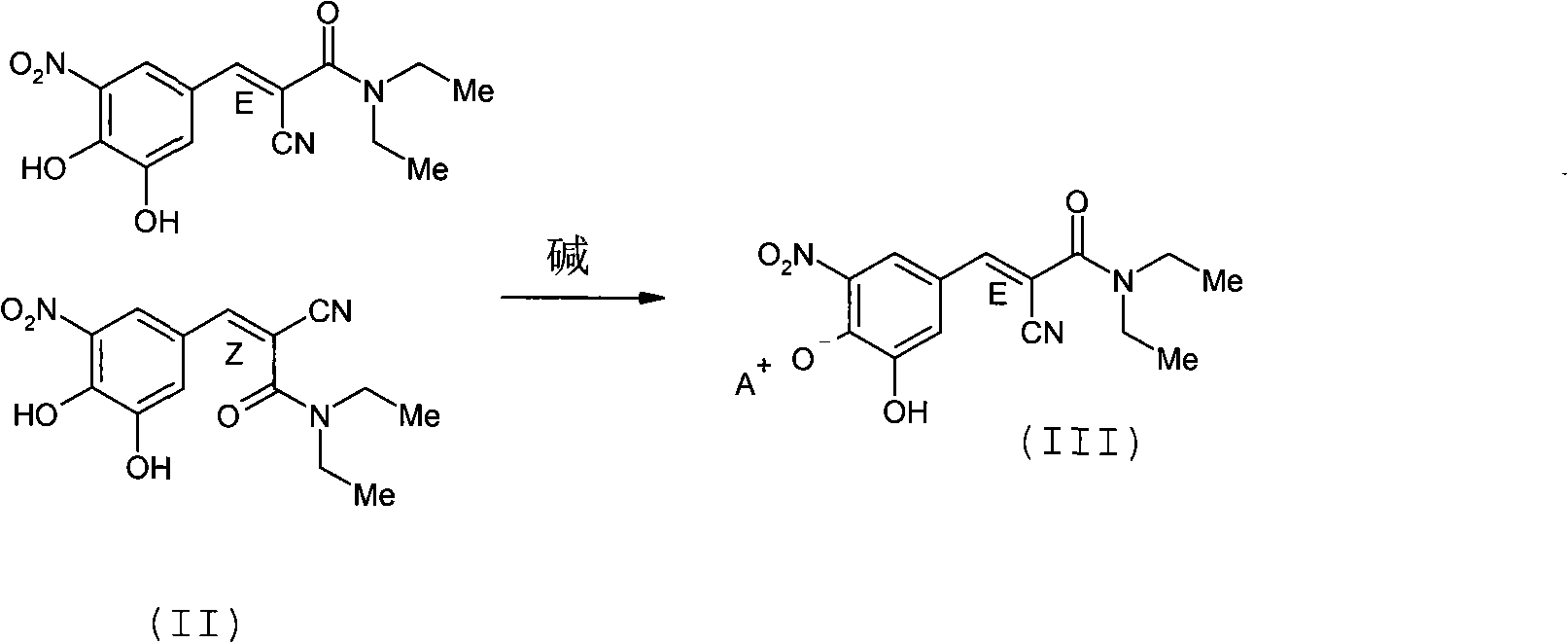
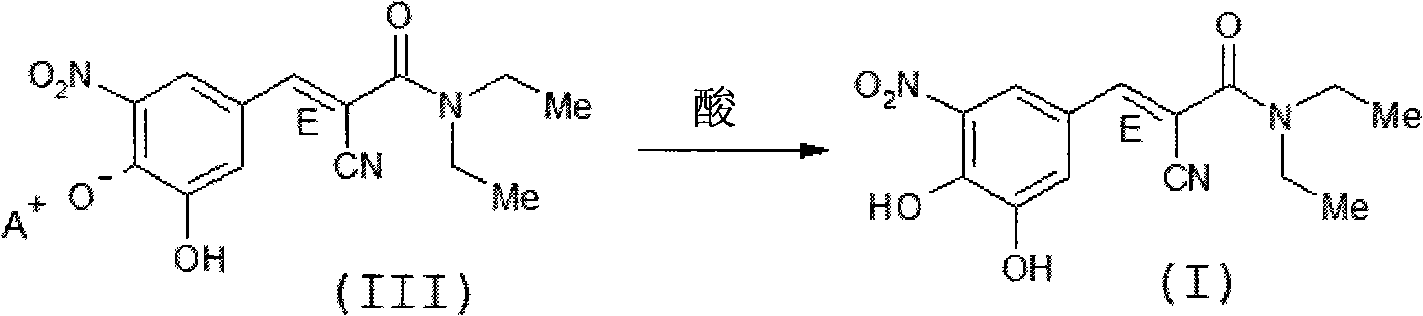
Process for preparing entacapone substantially free of z-isomer, synthesis intermediates thereof and a new crystalline form
A technology of entacapone and isomers, applied in the preparation of carboxylic acid nitrile, organic chemistry methods, chemical instruments and methods, etc., can solve problems such as instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1. Entacapone provided as a synthetic intermediate by using the organic base piperidine From 3,4-dihydroxy-5-nitrobenzoic acid (V) and N,N-dimethylcyanoacetamide (VI) Method for preparing entacapone substantially free of Z-isomer
[0077] a) method for obtaining entacapone piperidine salt (IIIa)
[0078] 3,4-Dihydroxy-5-nitrobenzaldehyde (70g; 382mmol), N,N-diethylhydroxyacetamide (107g; 764mmol), piperidine (56.6ml; 573mmol) and acetic acid (32.8ml; 573 mmol) in isopropanol (700 mL) was heated to reflux for about 3 hours. The resulting solution was cooled to room temperature, and the resulting precipitate was stirred at this temperature overnight. Finally, it was cooled to 0-5°C, filtered and washed with isopropanol (140ml). The resulting product was dried in a vacuum oven at 40°C, yielding 119 g (79.7% yield) of an organic solid (melting point = 152-4°C: HPLC purity = 98.0% (Z-isomer = 0.94%)).
[0079] IR (cm -1 ): 3190, 3038, 2975, 2828, 2723, 254...
Embodiment 2
[0084] Example 2. Entacapone provided as a synthetic intermediate by using the organic base piperidine Piperidinium salt and from crude entacapone (Z / E) to prepare Entacapone substantially free of Z-isomer Methods
[0085] a) method for obtaining entacapone piperidine salt (IIIa)
[0086] Piperidine (6.26g; 73.5mmol) was added to entacapone (E-isomer=75%; Z-isomer=25%) (12.5g; 40.9mmol) in isopropanol (150ml) at room temperature in suspension. The mixture was stirred for about 2 hours and a large precipitate formed. Finally, it was cooled at 0-5° C. for about 2 hours, and the resulting precipitate was filtered and washed with cold isopropanol (20 ml). The resulting product was dried in a vacuum oven at 40°C, yielding 14.2 g (yield = 88.8%) of an organic solid (melting point = 152-4°C (decomposition); (Z-isomer = 1.3%)).
[0087] b) Obtained from entacapone piperidine salt (entacapone crystal form G) substantially free of Z-isomer Entacapone method
[0088] Enta...
Embodiment 3
[0089] Example 3. Provision of Enta as Synthetic Intermediate by Using Inorganic Base Sodium Hydroxide Entacapone sodium salt and prepare Entacapone substantially free of Z-isomer from crude Entacapone (Z / E) Peng's method
[0090] a) method for obtaining entacapone sodium salt (IIIb)
[0091] Add 30% NaOH aqueous solution at room temperature to a suspension of entacapone (E-isomer=69%; Z-isomer=31%) (15.15g; 40.9mmol) in ethanol (100ml) (8.73g ; 65.5 mmol). The mixture was stirred at room temperature, and the resulting precipitate was stirred at this temperature overnight. Finally, it was cooled at 0-5°C for about 2 hours, and the resulting precipitate was filtered and washed with cold ethanol (20 ml). The resulting product was dried in a vacuum oven at 40°C to give 14.13 g (yield = 87.1%) of a red solid (melting point = 260-4°C (decomposition); (Z-isomer = 1.80%)).
[0092] IR (cm -1 ): 3317, 2990, 2201, 1641, 1592, 1538, 1475, 1460, 1443, 1390, 1350, 1265, 1213, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com