Levodopa methyl ester and benserazide mixed medicament slow-release microsphere composition and preparation method thereof
A technology of levodopa methyl ester and sustained-release microspheres, which is applied in the directions of drug combinations, pharmaceutical formulations, medical preparations containing active ingredients, etc. Good dispersion effect
Inactive Publication Date: 2010-11-10
XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
View PDF4 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, there is currently no report on the preparation of levodopa methyl ester and benserazide mixed drug sustained-release microspheres, and a single levodopa methyl ester and benserazide mixed drug sustained-release microspheres are easily degraded by enzymes in the body and inactivated
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
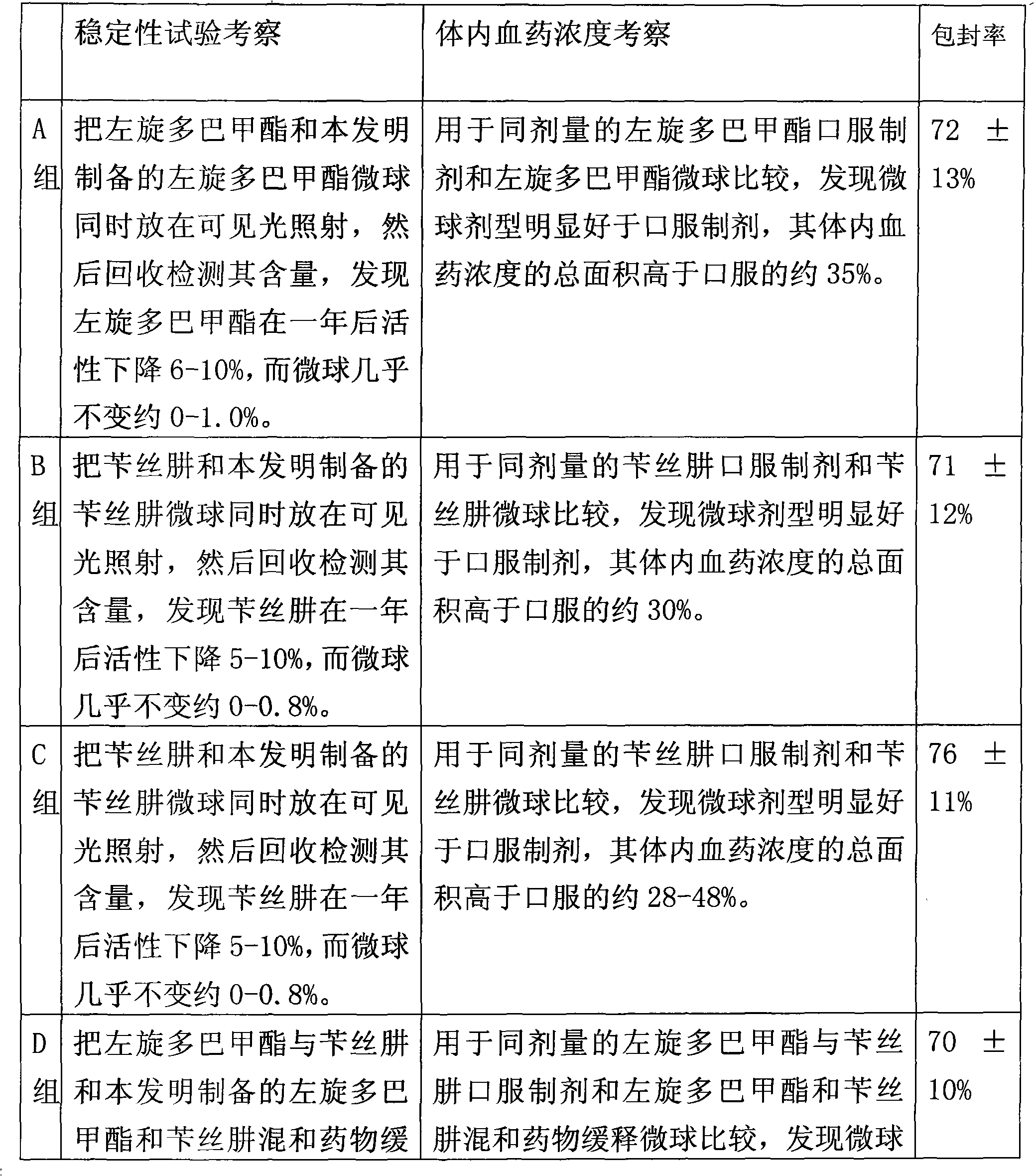
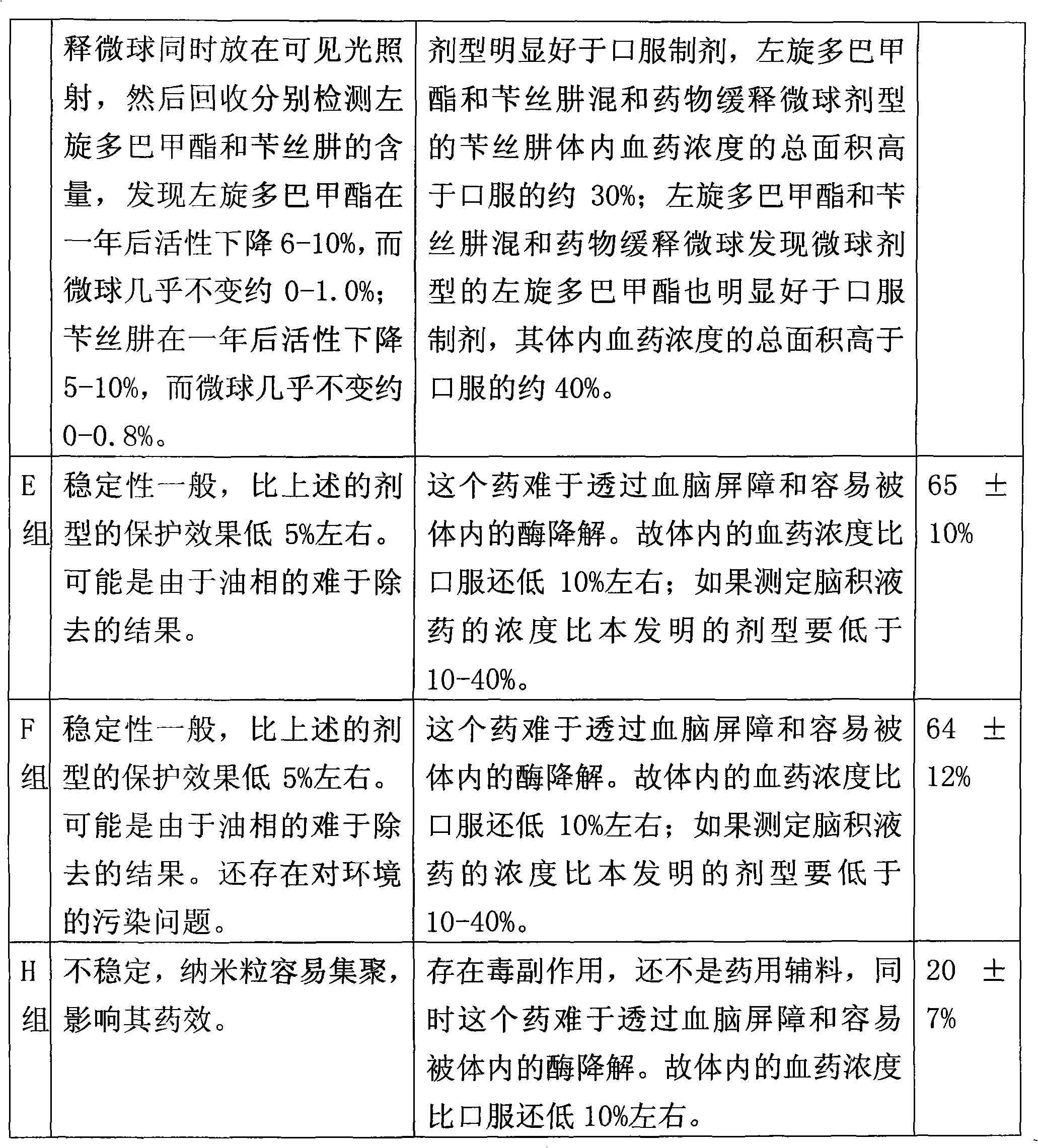
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Login to View More
Abstract
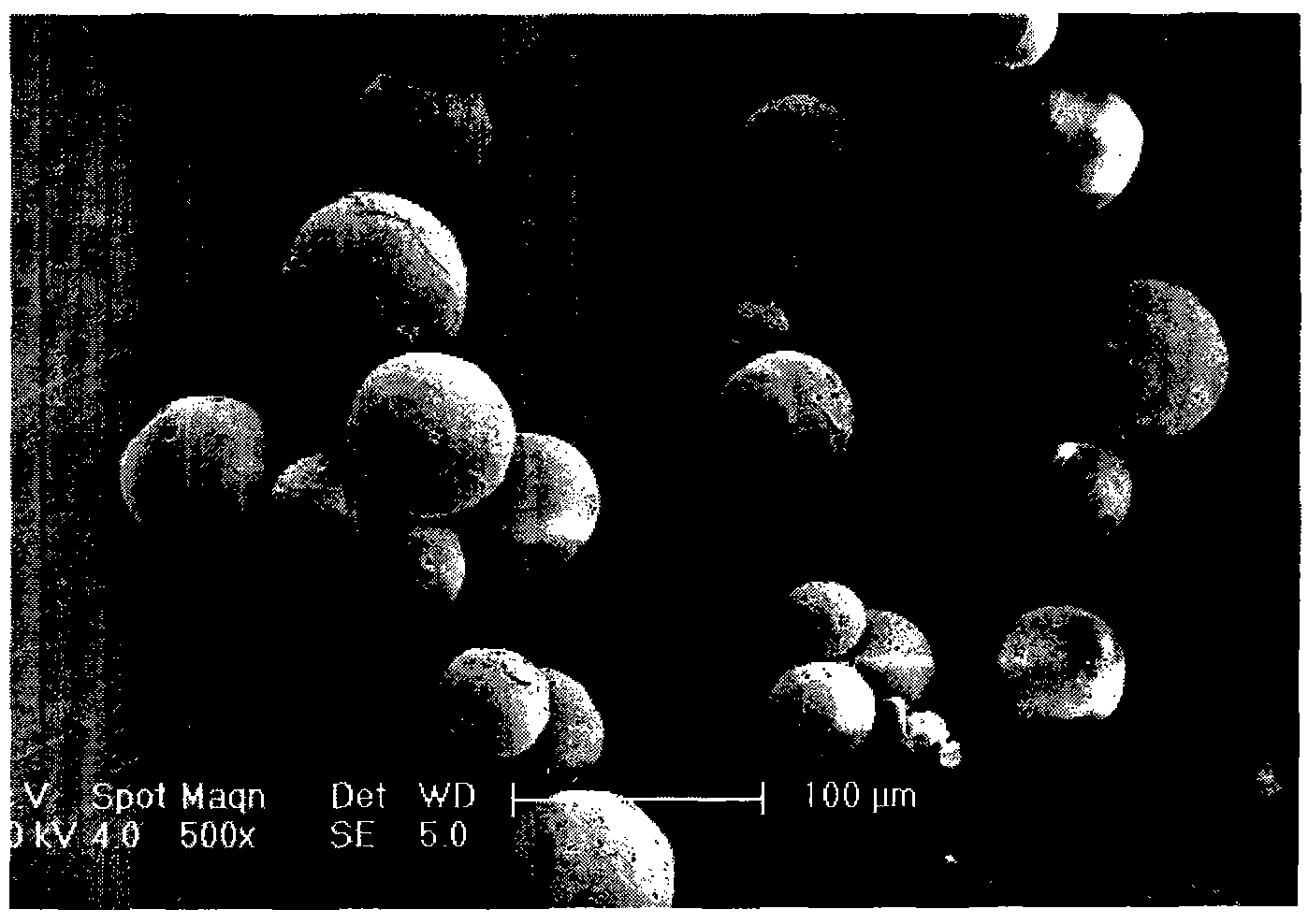
The invention relates to a levodopa methyl ester and benserazide mixed medicament slow-release microsphere composition. The composition comprises the following components in percentage by weight: 50 to 99 percent of degradable hydrophobic polymer and 1 to 50 percent of levodopa methyl ester and benserazide mixed medicament, wherein the weight ratio of the levodopa methyl ester to the benserazide is 1:1-4:1. The invention also provides a preparation method for the mixed medicament slow-release microsphere composition. The invention overcomes the disadvantage of unavailable effective treatment due to frequent missing of administration caused by the conventional single oral administration scheme and frequent oral administration requirement and provides a W / O / W preparation method. The particle diameter of the composition can be regulated and controlled from 1 to 500mu m according to different requirements without causing environmental pollution; the composition can prevent the influence on the treatment effect of the levodopa methyl ester and benserazide mixed medicament; the solution has the advantages of smooth and round surface, regularity and no adhesion; and the freeze-dried powder of the composition is white, fine, loose, non-collapse and non-adhesive and has high dispersibility.
Description
A kind of levodopa methyl ester and benserazide mixed drug sustained-release microsphere composition and preparation method thereof 【Technical field】 The invention relates to a composition in the technical field of pharmaceutical preparations and a preparation method thereof, in particular to a drug sustained-release microsphere composition of mixed levodopa methyl ester and benserazide and a preparation method thereof. 【Background technique】 In the pharmaceutical industry, from drug discovery to clinical application, the last link is drug preparation. A considerable part of the drugs need long-term frequent administration to be cured; some of them need local administration due to the high toxicity of systemic administration. To achieve these goals, raw materials must be prepared into corresponding dosage forms. For example, drugs that require long-term administration but have a short half-life in the body should be prepared as sustained-release or controlled-release dosa...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/216A61K31/165A61K9/16A61P25/16
Inventor 刘振国袁伟恩任甜甜杨新新陈伟
Owner XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com