Method for synthesizing benserazide hydrochloride by utilizing fixed bed hydrogenation equipment
A technology of benserazide hydrochloride and fixed bed, which is applied in the field of chemical industry and can solve the problems of product deterioration and large amount of catalyst.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
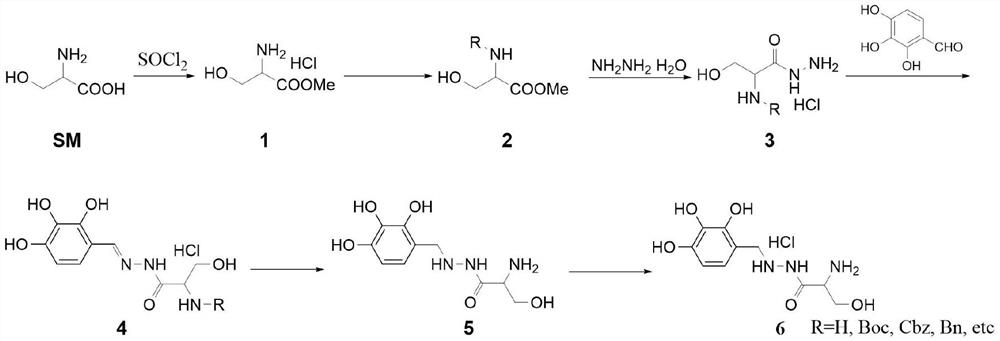
[0029] This embodiment is a method for synthesizing benserazide hydrochloride using fixed-bed hydrogenation equipment, comprising the following steps:
[0030] S11, the synthesis of compound 1:
[0031] Add 100.0g of serine hydrochloride and 400mL of methanol into a 1L three-necked flask, stir mechanically, and add 135.8g of thionyl chloride dropwise into the system at a temperature below 10°C. During the dropping process, control the temperature of the system Not higher than 20°C. After the dropwise addition, stir and react at a temperature of 80°C for 6 hours. Use TLC (MeOH:EA=4:1) color reagent to detect the complete reaction of the raw materials, stop heating, and wait until the temperature of the reaction system drops to 50°C. After ℃, concentrate under reduced pressure to obtain a white solid crude product, which is beaten with 500 mL n-heptane for 20 min, filtered with suction, rinsed once with 50 mL n-heptane, and dried to obtain compound 1 as a white solid , the yiel...
Embodiment 2
[0043] This embodiment is a method for synthesizing benserazide hydrochloride using fixed-bed hydrogenation equipment, comprising the following steps:
[0044] S21, the synthesis of compound 1:
[0045] Add 100.0g of serine hydrochloride and 400mL of methanol into a 1L three-necked flask, stir mechanically, and add 135.8g of thionyl chloride dropwise into the system at a temperature below 10°C. During the dropping process, control the temperature of the system Not higher than 20°C. After the dropwise addition, stir and react at a temperature of 80°C for 6 hours. Use TLC (MeOH:EA=4:1) color reagent to detect the complete reaction of the raw materials, stop heating, and wait until the temperature of the reaction system drops to 50°C. After ℃, concentrate under reduced pressure to obtain a white solid crude product, which is beaten with 500 mL n-heptane for 20 min, filtered with suction, rinsed once with 50 mL n-heptane, and dried to obtain compound 1 as a white solid , the yiel...
Embodiment 3
[0057] This embodiment is a method for synthesizing benserazide hydrochloride using fixed-bed hydrogenation equipment, comprising the following steps:
[0058] S31, the synthesis of compound 1:
[0059] Add 100.0g of serine hydrochloride and 400mL of methanol into a 1L three-necked flask, stir mechanically, and add 135.8g of thionyl chloride dropwise into the system at a temperature below 10°C. During the dropping process, control the temperature of the system Not higher than 20°C. After the dropwise addition, stir and react at a temperature of 80°C for 6 hours. Use TLC (MeOH:EA=4:1) color reagent to detect the complete reaction of the raw materials, stop heating, and wait until the temperature of the reaction system drops to 50°C. After ℃, it was concentrated under reduced pressure to obtain a white solid crude product, which was beaten with 500 mL n-heptane for 20 min, suction filtered, and the filter cake was rinsed once with 50 mL n-heptane, and dried to obtain compound 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com