A kind of recovery method and application of L-methyldopa intermediate
A technology for methyldopa intermediates and recovery methods, applied in organic chemistry methods, chemical instruments and methods, organic racemization, etc., can solve problems that have not been reported in literature, and achieve strong operability and easy process implementation and the effect of simple production and preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
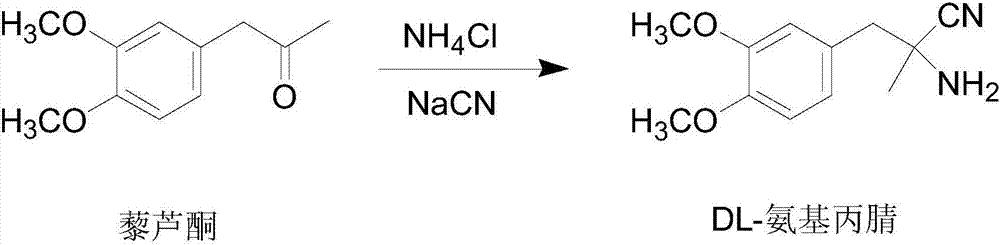
[0039] 1) Synthesis of DL-aminopropionitrile
[0040] Dissolve 154.5g (3.15mol) 96%~98% sodium cyanide and 163g (3.05mol) ammonium chloride in 2200ml (26.8mol) of 12.2mol / L ammonia solution at room temperature, heat to 55℃, and stir quickly Quickly add 582.6g (3mol) of veratrone, stir for 1h, and then cool to below 20℃ within 1.5h, fine particles begin to separate out, keep stirring for 2h, cool to 0℃, filter with suction, the filtrate is DL-amino The mother liquor of propionitrile synthesis, the filter cake is washed with 800ml ice water, and dried to obtain 618.42g (2.81mol) of DL-aminopropionitrile. The molar yield is 93.7%. The melting point is 85℃~87℃. The obtained DL-aminopropionitrile is not Purification and direct resolution.
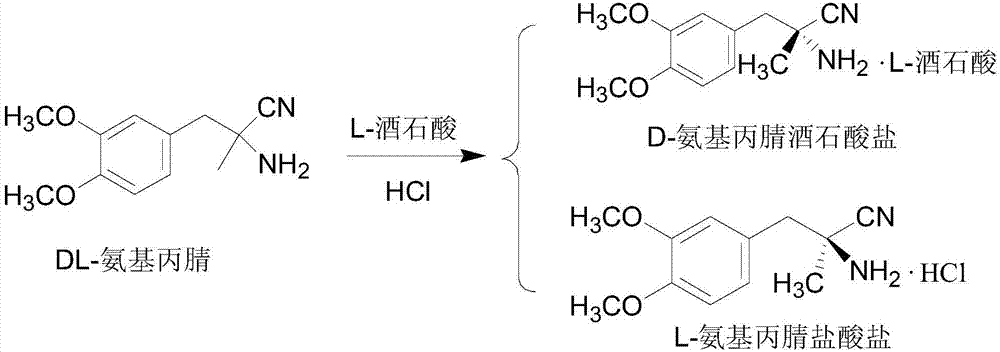
[0041] 2) Resolution of DL-aminopropionitrile
[0042] Dissolve 125g D-tartaric acid (0.83mol) in 500ml water, adjust the pH to 8 with sodium hydroxide to obtain D-tartaric acid disodium salt solution, add 4375ml water, 125g D-tartaric acid, 204.5g c...
Embodiment 1
[0051] Take 262.5ml of mother liquor obtained in step 4) (containing 10.67g of 3-(3,4-dimethoxyphenyl)-2-methylalanine hydrochloride), and add 32.8g of ammonium sulfate (volume to weight ratio of mother liquor) :Salt=8:1), carry out 60℃, vacuum-0.08mpa vacuum distillation, distill 168ml of water (COD:798mg / L), a small amount of crystals will precipitate out, cool to 0℃, filter, add 10ml ice water (0℃) ) Wash to obtain a solid, dry to obtain 9.7g, 3-(3,4-dimethoxyphenyl)-2-methylalanine hydrochloride, the recovery rate is 90.9%, and the HPLC purity is 98.3%; the mother liquor continues to be distilled Distilled water 30ml (COD: 839mg / L), a large number of crystals precipitated, cooled to 0°C, filtered to obtain a solid, dried to obtain 44.1g, containing COD: 1.2g, mother liquor 14ml, COD: 40936mg / L.
Embodiment 2
[0053] Take 262.5ml of mother liquor obtained in step 4) (containing 10.67g of 3-(3,4-dimethoxyphenyl)-2-methylalanine hydrochloride), add 21.8g of ammonium chloride (volume to weight ratio Mother liquor: salt=12:1), carry out 60℃, vacuum-0.08mpa vacuum distillation, distill 200ml of water (COD: 796mg / L), cool to 0℃, filter to obtain solid, dry 10.1g, 3-( The recovery rate of 3,4-dimethoxyphenyl)-2-methylalanine hydrochloride was 95%, and the purity by HPLC was 98.3%; the mother liquor continued to be distilled, and 34ml of water was distilled. A large amount of crystals precipitated, and the temperature was reduced to 0°C , Filtered to obtain solid, dried to obtain 34.2g, containing COD: 0.98g, mother liquor 10.8ml, COD: 23578mg / L
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com