Pharmaceutical composition for intraocular or oral administration for treatment of retinal diseases
A technology for retinal diseases and intraocular drug delivery, which can be used in drug combinations, sensory diseases, drug delivery, etc., and can solve problems such as low doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
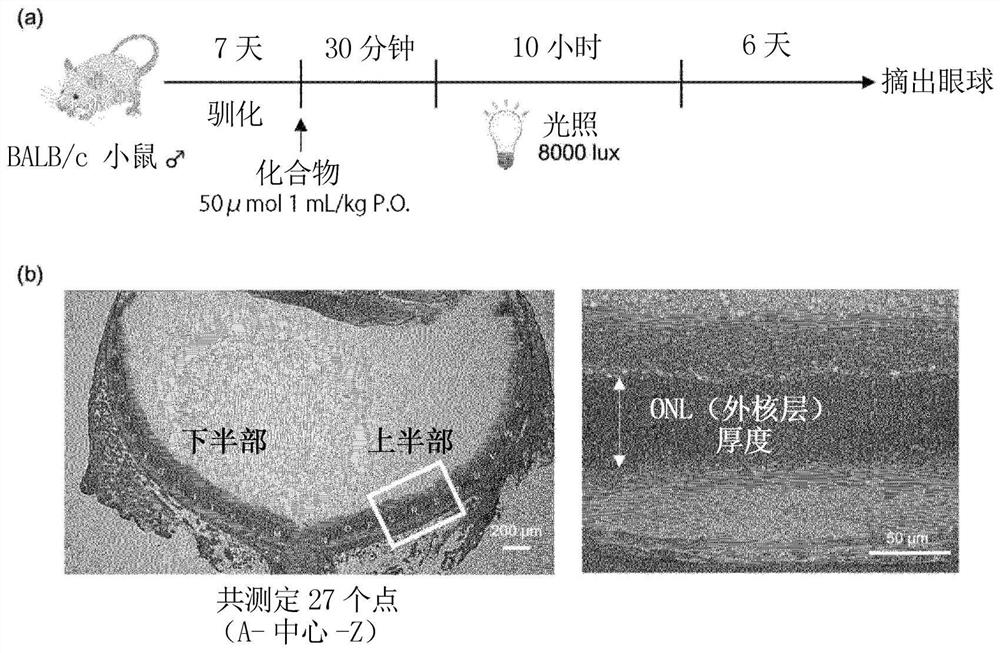
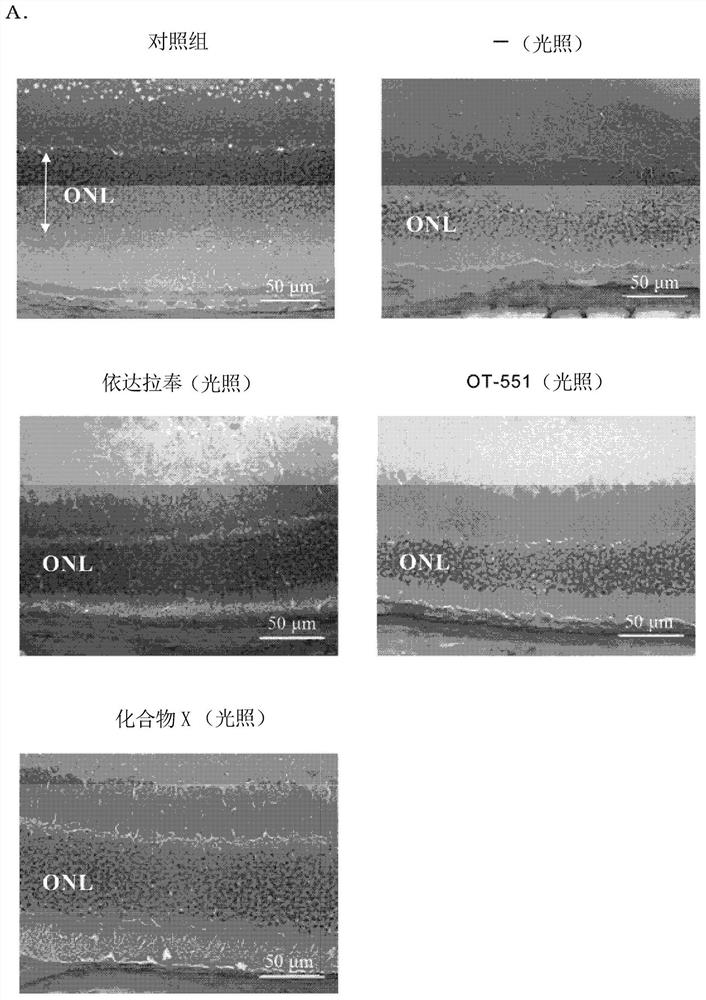
[0196] Although formulation examples and test examples are given below and this invention is demonstrated more concretely, this invention is not limited to these examples.
[0197] Compounds, mice, reagents, etc. used in Examples are commercially available or produced according to known methods.
[0198] Preparation example
[0199] Formulation examples of the pharmaceutical composition of the present invention are shown below, but are not limited thereto.
preparation example 1
[0201] tablet
[0202] [Table 1]
[0203]
[0204] Tablets are manufactured in accordance with a generally known method for tablet formulation. Specifically, mix methyldopa, cornstarch and lactose as the present compound in a mixer, add carboxymethylcellulose calcium and hydroxypropyl cellulose to the mixture for granulation, dry the obtained granules and then granulate granules, magnesium stearate is added to the sized granules for mixing, and the tablets are compressed using a tablet machine. In addition, by changing the amount of the present compound added, tablets with a desired content (for example, 10 mg, 25 mg, or 50 mg) in 100 mg tablets can be produced.
preparation example 2
[0206] eye drops
[0207] [Table 2]
[0208]
[0209]
[0210] Eye drops are produced according to a generally known method for formulating eye drops. Specifically, eye drops were prepared by adding methyldopa as the present compound and the other above-mentioned components to sterilized purified water and mixing them well. By changing the addition amount of this compound such as methyldopa, the desired concentration (for example, 0.3% (W / V), 0.5% (W / V) or 1% (W / V) in 100mL of eye drops can be manufactured. )) eye drops.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com