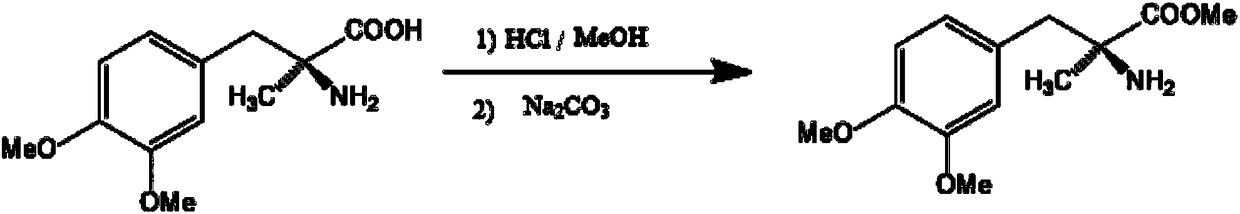
Preparation process of L-methyldopa methyl ester
A technology of methyl dopa methyl ester and preparation process, which is applied in the preparation of organic compounds, cyanide reaction preparation, organic chemistry and other directions, can solve the problems of difficult environmental protection, reduced product quality and yield, and high reaction temperature, and achieves The effect of easy industrial production, less side reactions and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In the reaction flask, add 240g of L-methyldopa and 1440g of methanol, stir, control the temperature at 4°C, pass 1440g of HCl gas, and keep the temperature at 20°C for 72h after passing through. After the reaction, the reaction liquid was centrifuged, and the speed of the centrifuge was controlled to 1200r / min, and the centrifuged mother liquor was used for recycling, and at the same time, the centrifuged material was transferred to another reaction bottle, 560g of purified water was added, and stirred and dissolved at 50°C for 2h. After dissolving and clarifying, adjust the pH value with 20 wt.% ammonia water, keep the temperature at 40° C., and adjust the pH value to 6. After neutralization, lower the temperature to 5 °C, stir for 1 h, control the centrifuge speed at 1500 r / min, centrifuge, wash, and dry at 50 °C for 12 h to obtain L-methyldopa methyl ester with a yield of 70.3%. The analysis and detection carried out the content detection, and the content was 99.5%....
Embodiment 2
[0034] In the reaction tank, add the methanol mother liquor filtered in Example 1, 24g of methanol and 240g of L-methyldopa, stir, control the temperature at 15°C and feed 24g of HCl gas, and keep the temperature at 30°C for 72h after passing through. After the reaction, centrifuge the reaction solution, control the speed of the centrifuge to 1200r / min, centrifuge the mother liquor for recycling, and transfer the centrifuged material to another reaction tank, add 560g of purified water, stir and dissolve at 70°C for 0.5h . After dissolving and clarifying, adjust the pH value with 30% sodium hydroxide, keep the temperature at 45° C. when adjusting the pH value, and adjust the pH=8. After neutralization, lower the temperature to 15°C, stir for 1 hour, control the speed of the centrifuge to 1500r / min, centrifuge, wash, and dry at 70°C for 6 hours to obtain L-methyldopa methyl ester with a yield of 67.3%. The analysis and detection carried out the content detection, and the conte...
Embodiment 3
[0036] In the reaction tank, add the methanol mother liquor filtered in Example 1, 24g of methanol and 240g of L-methyldopa, stir, control the temperature at 40°C and feed 24g of HCl gas, and keep the temperature at 30°C for 48h after passing through. . After the reaction, centrifuge the reaction liquid, control the speed of the centrifuge to 1200r / min, centrifuge the mother liquor for recycling, and transfer the centrifuged material to another reaction tank, add 560g of purified water, stir and dissolve at 50°C for 1h. After dissolving and clarifying, adjust the pH value with 10% sodium carbonate, keep the temperature at 50° C. when adjusting the pH value, and adjust the pH=7. After neutralization, lower the temperature to 8 °C, stir for 1 h, control the centrifuge speed at 1500 r / min, centrifuge, wash, and dry at 55 °C for 10 h to obtain L-methyldopa methyl ester with a yield of 71.7%. The analysis and detection carried out the content detection, and the content was 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com