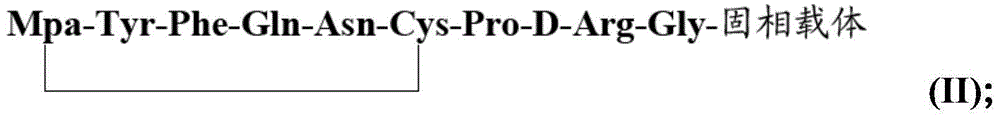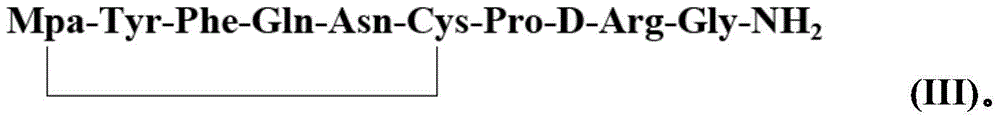Synthesis method of desmopressin
A technology of desmopressin and a synthesis method, which is applied to the synthesis field of desmopressin, can solve the problems of unfavorable industrial sustainable development, consumption of large organic solvents, and high production cost, and achieves considerable economical and practical value. Easy to handle, low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The present invention provides a kind of synthetic method of desmopressin, and described synthetic method comprises:
[0035] 1) Preparation of desmopressin linear peptide peptide resin: firstly mix the amino acid monomer PG-Gly-OH and coupling agent with water evenly, let it stand, and then react the obtained mixture on the solid phase carrier from which the protecting group is removed, Obtain PG-Gly-solid phase carrier, then remove PG to obtain H-Gly-solid phase carrier; repeat the above steps, amino acid monomers and Mpa are sequentially coupled on H-Gly-solid phase carrier, and the coupling sequence is sequentially Coupling PG-D-Arg-OH, PG-Pro-OH, PG-Cys(Trt)-OH, PG-Asn-OH, PG-Gln-OH, PG-Phe-OH, PG-Tyr-OH, Mpa , the PG is a water-soluble amino protecting group; thus obtain the desmopressin linear peptide peptide resin Mpa-Tyr-Phe-Gln-Asn-Cys-Pro-D-Arg-Gly-solid phase of the formula (I) Carrier (I);
[0036] 2) Preparation of desmopressin cyclic peptide resin: Oxid...
Embodiment 1
[0080] Preparation of H-Rink-PEG Amide Resin
[0081] Place 100g (50mmol, 1eq) of Fmoc-Rink-PEG Amide resin with a substitution degree of 0.5mmol / g in a solid-phase reaction column (1000ml), add a mixture of 800ml Triton X-100 and water (molar ratio Triton X-100 : water = 12:2000), nitrogen bubbling and swelling for 60 minutes; then, add DBLK to the solid phase reaction column to remove Fmoc, then add 800ml Triton X-100 and water mixture to the above solid phase reaction column ( molar ratio as above), repeated 4 times to obtain H-Rink-PEG Amide resin.
[0082] Preparation of linear peptide resins of desmopressin
[0083] Add 35.9g (150mmol, 3eq) Esc-Gly-OH, 32.3g (180mmol, 3.6eq) HONB, 32.4 g (180mmol, 3.6eq) EDC·HCl, mix well, then add 23.3g (180mmol, 3.6eq) DIPEA under ice-water bath at 0°C, mix well and let stand for 5 minutes, put the resulting mixture into the above solid phase reaction column , react for 2 hours to obtain Esc-Gly-Rink-PEG Amide resin; then, add 5...
Embodiment 2
[0093] Preparation of H-Rink-PEG Amide Resin
[0094] The same method as in Example 1, except that 83.3 g (50 mmol, 1 eq) of Fmoc-Rink-PEG Amide resin with a substitution degree of 0.6 mmol / g was used.
[0095] Preparation of linear peptide resins of desmopressin
[0096] The same method as in Example 1, except that the amount of amino acid monomer protected by Esc and Mpa(Trt)-OH is 23.9g (100mmol, 2eq) respectively; the amount of HONB, EDC·HCl, and DIPEA are respectively 21.5g (120mmol, 2.4eq), 21.6g (120mmol), 15.5g (120mmol, 2.4eq).
[0097] Preparation of Desmopressin Cyclic Peptide Resin
[0098] Add a mixture of iodine 76.2g (300mmol, 6eq) and 400mlDMF (5188mmol, 103.8eq) to the solid-phase reaction column obtained from the previous step, react for 2h, then add 800mlDMF to wash, repeat 3 times, then add 800ml methanol to shrink, Repeat 3 times, and obtain desmopressin cyclic peptide resin 175g after draining.
[0099] Preparation of desmopressin
[0100] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com