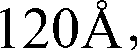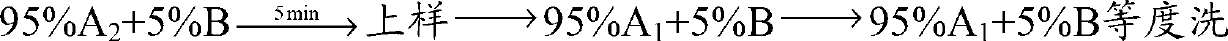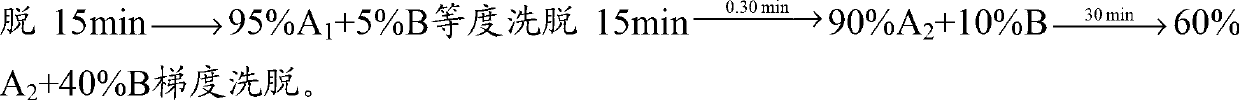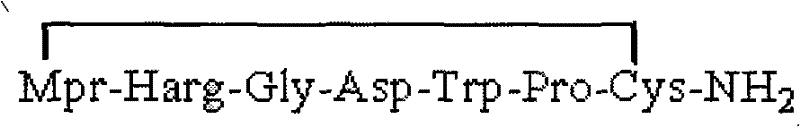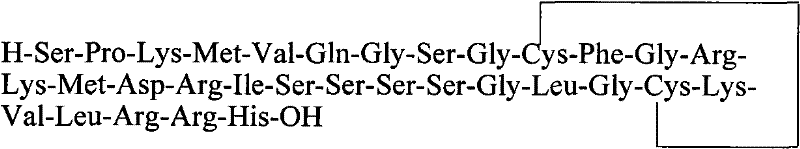Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62results about How to "Considerable economic and practical value" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
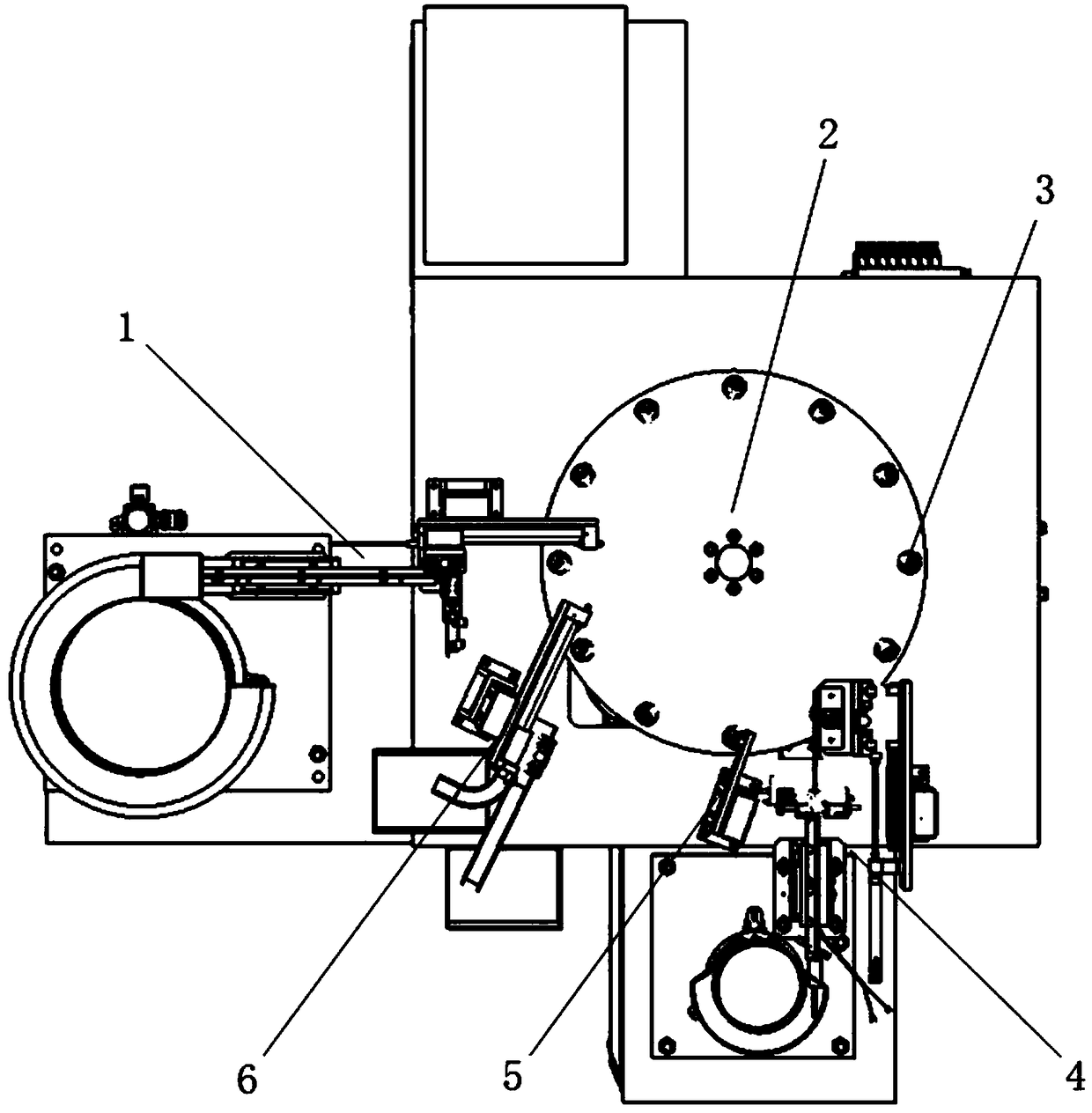
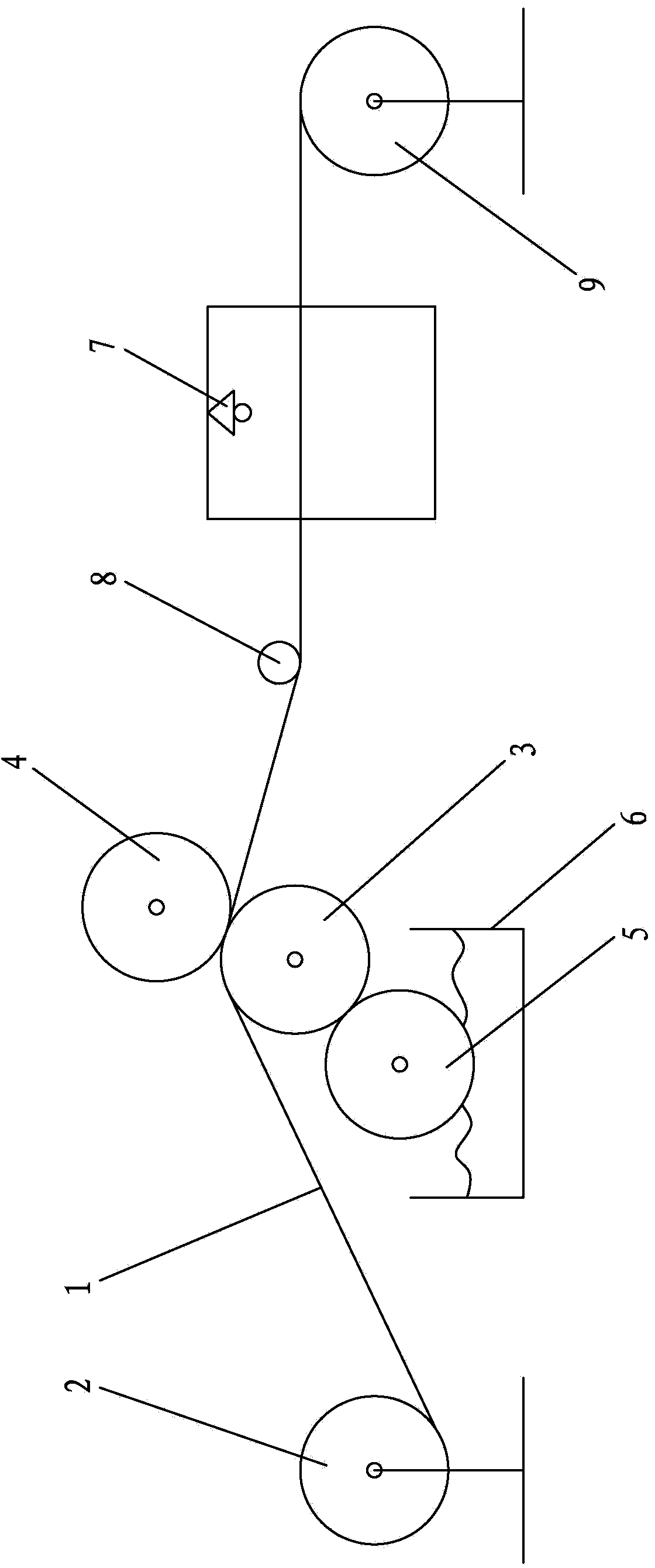
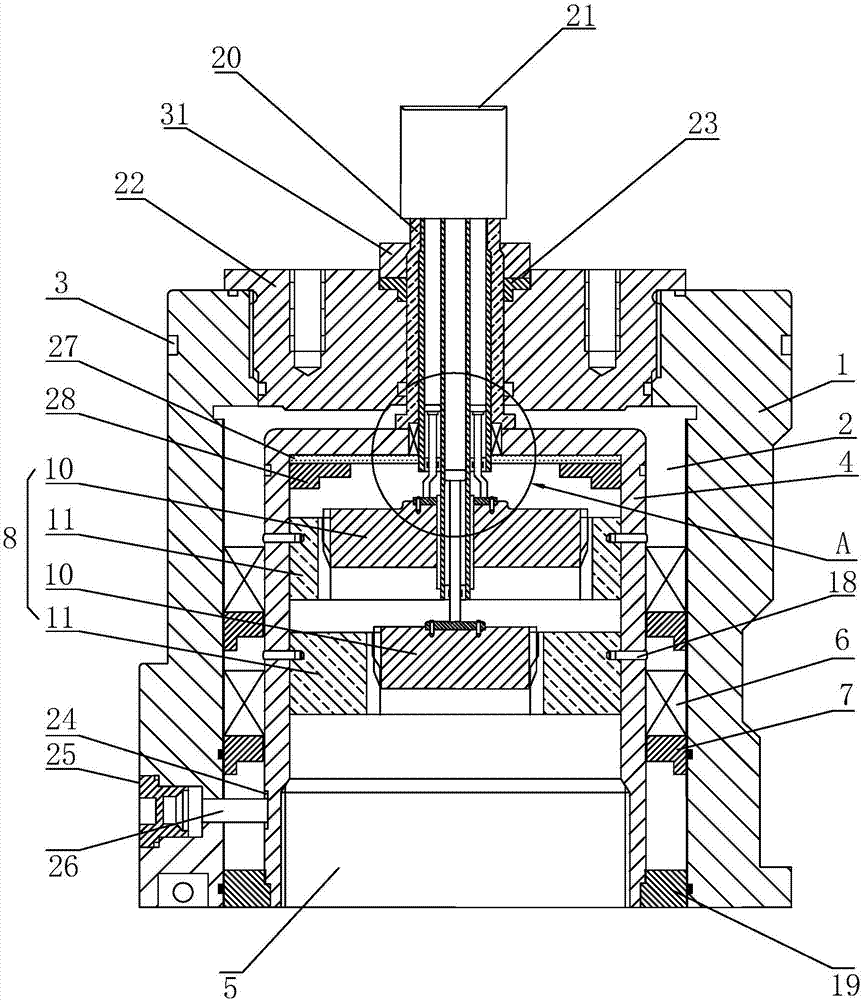
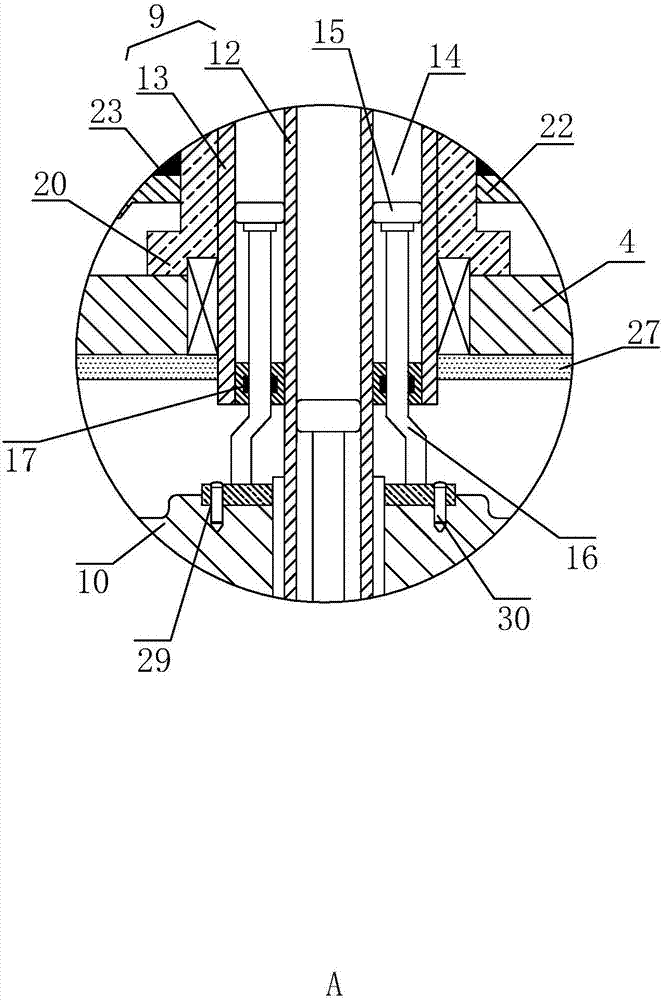
O-shaped sealing ring automatic assembly device and method
InactiveCN108655689AAssembly precisionAccurate assembly tasksMetal working apparatusEngineeringMechanical engineering
The invention discloses an O-shaped sealing ring automatic assembly device and method. The device comprises a sleeve automatic loading module, an O-shaped sealing ring automatic loading module, an automatic detecting module, a finished product sorting module, all of which are arranged on the outer side of a revolving worktable module in the peripheral direction in sequence; the sleeve automatic loading module and the O-shaped sealing ring automatic loading module are in supply connection with the revolving worktable module; the automatic detecting module detects the assembly effect of O-shapedrings on the revolving worktable module in real time; and the finished product sorting module is used for realizing sorting of qualified and unqualified products through chutes according to the detecting result of the automatic detecting module. The device is simple in structure and convenient to use; and through motions of three arc plates on clamping jaws of a three-jaw cylinder and pressing plates with the same apertures as the diameters of sleeves, the clamping and assembly of the O-shaped sealing rings are achieved, the automatic assembly task of the O-shaped sealing rings can be finished with high quality, a lot of manpower and costs are saved, the assembly efficiency is quickly accelerated, and the production demands are met.
Owner:NANJING KANGNI NEW ENERGY AUTO PARTS CO LTD +1
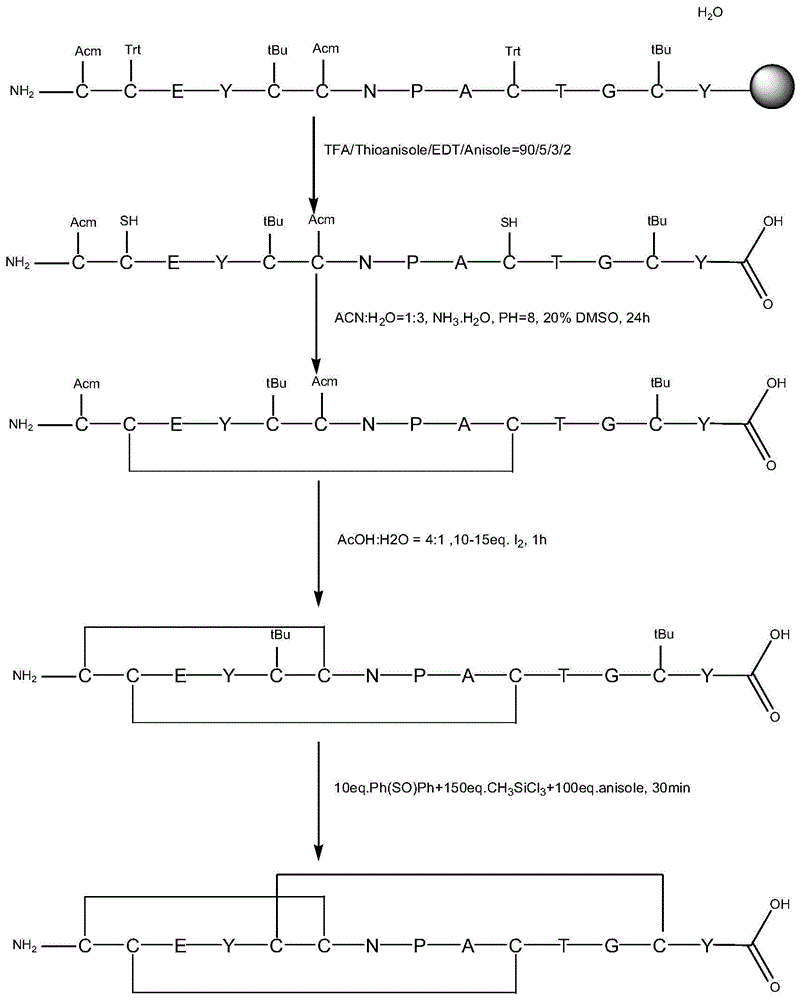
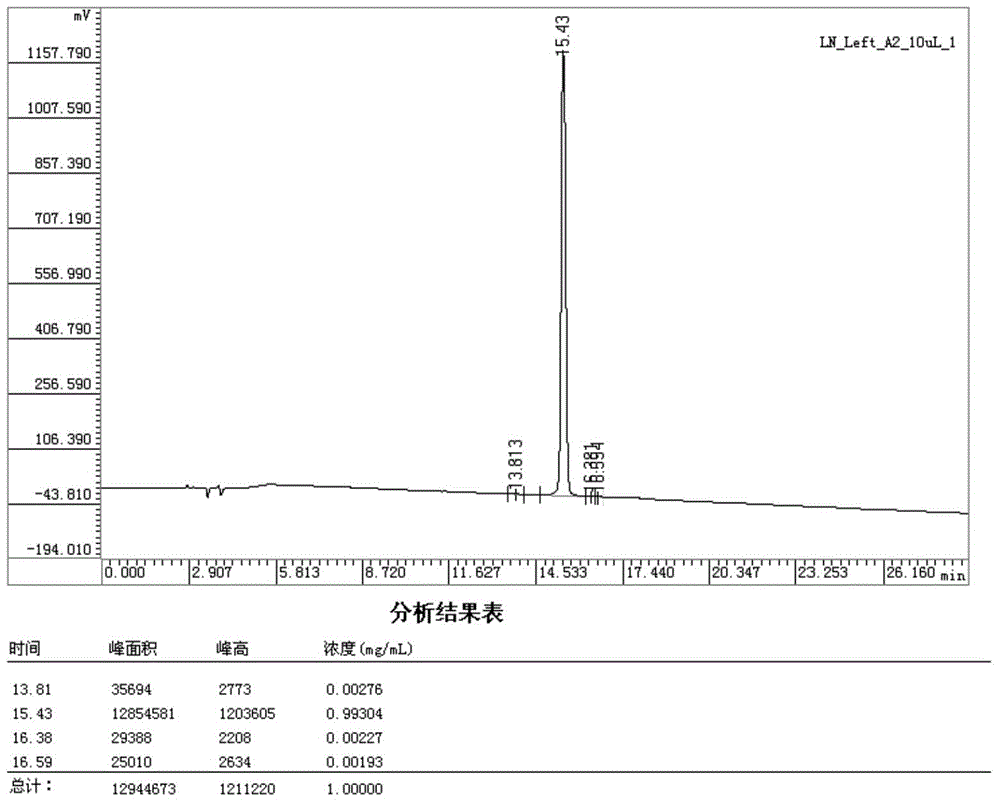
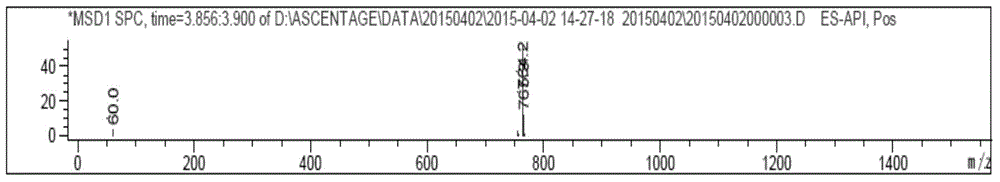
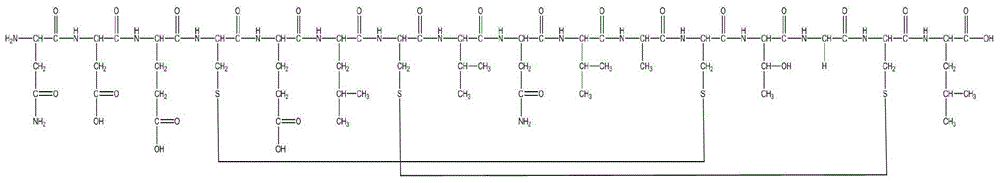
Method for preparing linaclotide
InactiveCN103626849AEasy to operatePost-processing is simplePeptide preparation methodsBulk chemical productionDisulfide bondPeptide
The invention belongs to the technical field of medicine synthesis, and discloses a method for synthesizing linaclotide by three pairs of total selectively formed disulfide bonds. By using the process, formation of three pairs of disulfide bonds in linaclotide can be sequentially completed in the same solution system, so that the operation method of the process is simple, the yield of final fine peptide of linaclotide can be greatly increased, and large-scale production can be realized.
Owner:HYBIO PHARMA
Method for preparing polypeptide used for treating osteoporosis
InactiveCN102731643AShort synthesis cycleEasy to purifyPeptide preparation methodsParathyroid hormonesPeptideSolid phases
The invention belongs to the pharmacochemistry technical field, and discloses a method for preparing polypeptide used for treating osteoporosis, concretely relates to a teriparatide preparation method. The preparation method is characterized by comprising the following steps: preparing each peptide resin fragment for forming teriparatide, gradually coupling each peptide resin fragment to teriparatide on a solid phase, then pyrolysizing to obtain the teriparatide crude product, and purifying to obtain the product teriparatide. Compared with the prior art, the preparation method has the advantages of simple operation, short synthesis cycle, low cost, less environmental pollution and high yield of teriparatide, and the total yield is 30%. The method of the invention is suitable for large-scale industrial production of teriparatide, and the prepared teriparatide has the advantages of high purity and less by-products, and has considerable economical and practical value as well as wide application prospect.
Owner:HYBIO PHARMA
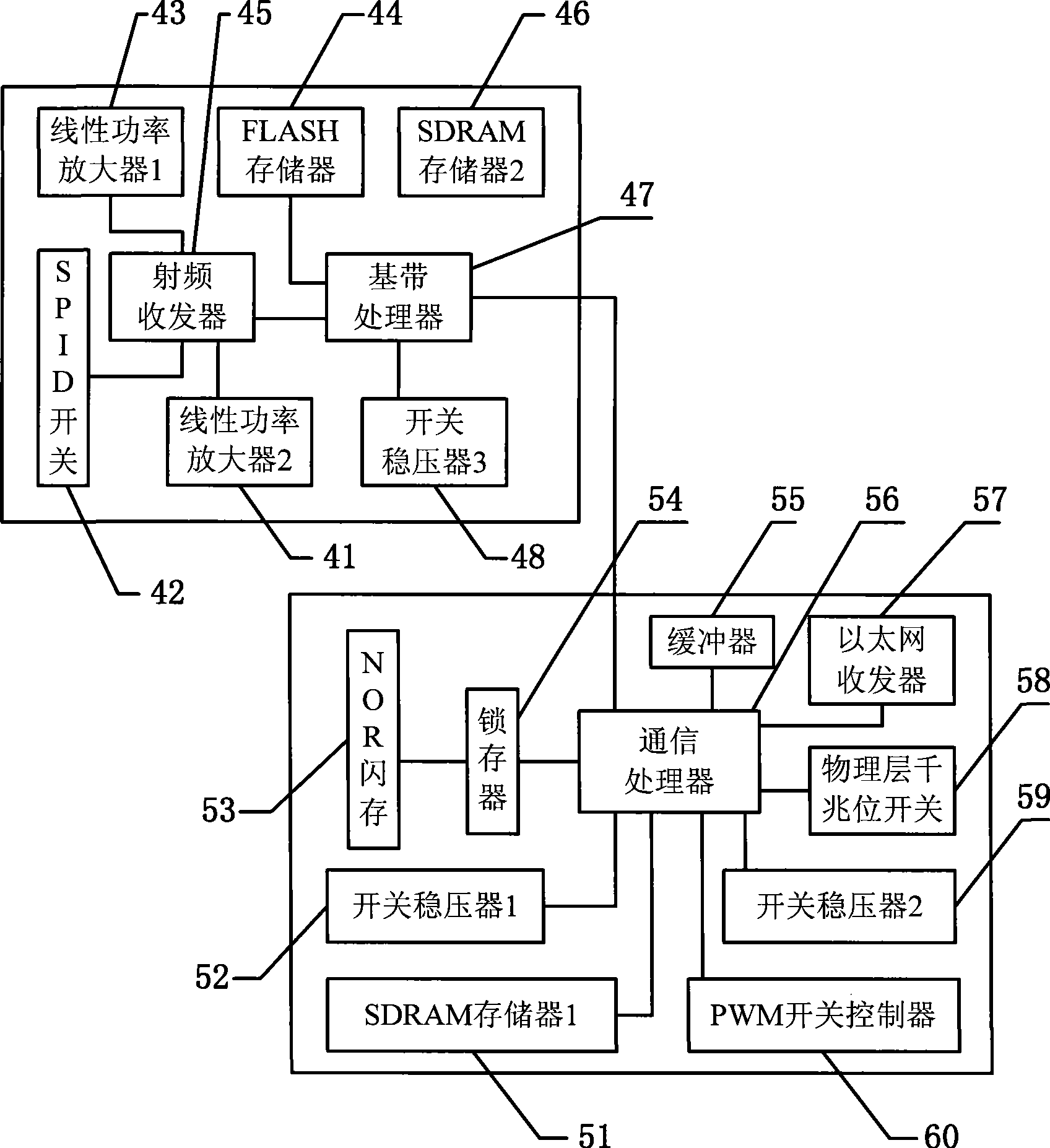
Digital public transport information publishing and cluster controlling method and device
InactiveCN101398975AWide coverageIntegrity guaranteedDetection of traffic movementTelecommunications linkEngineering
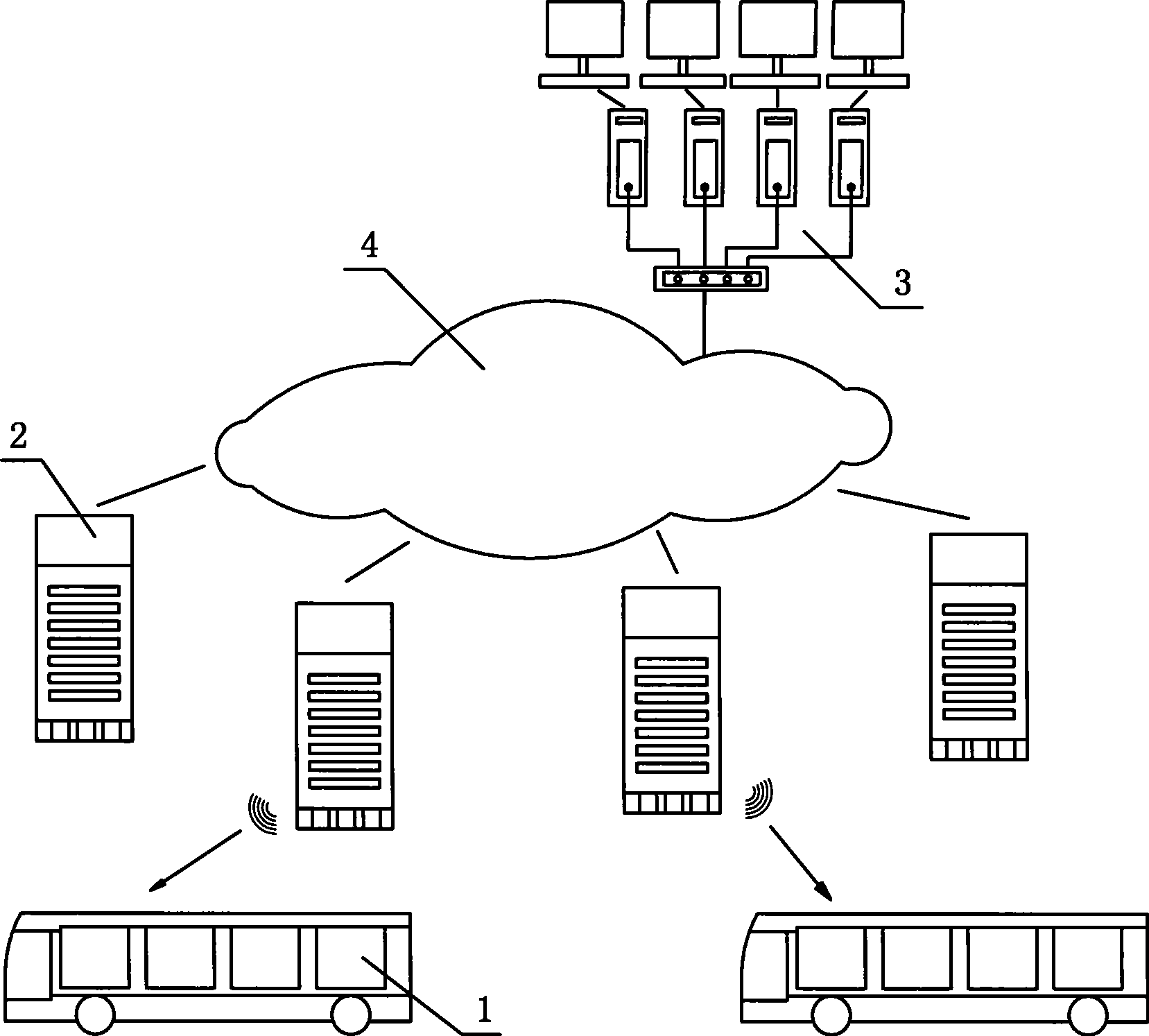
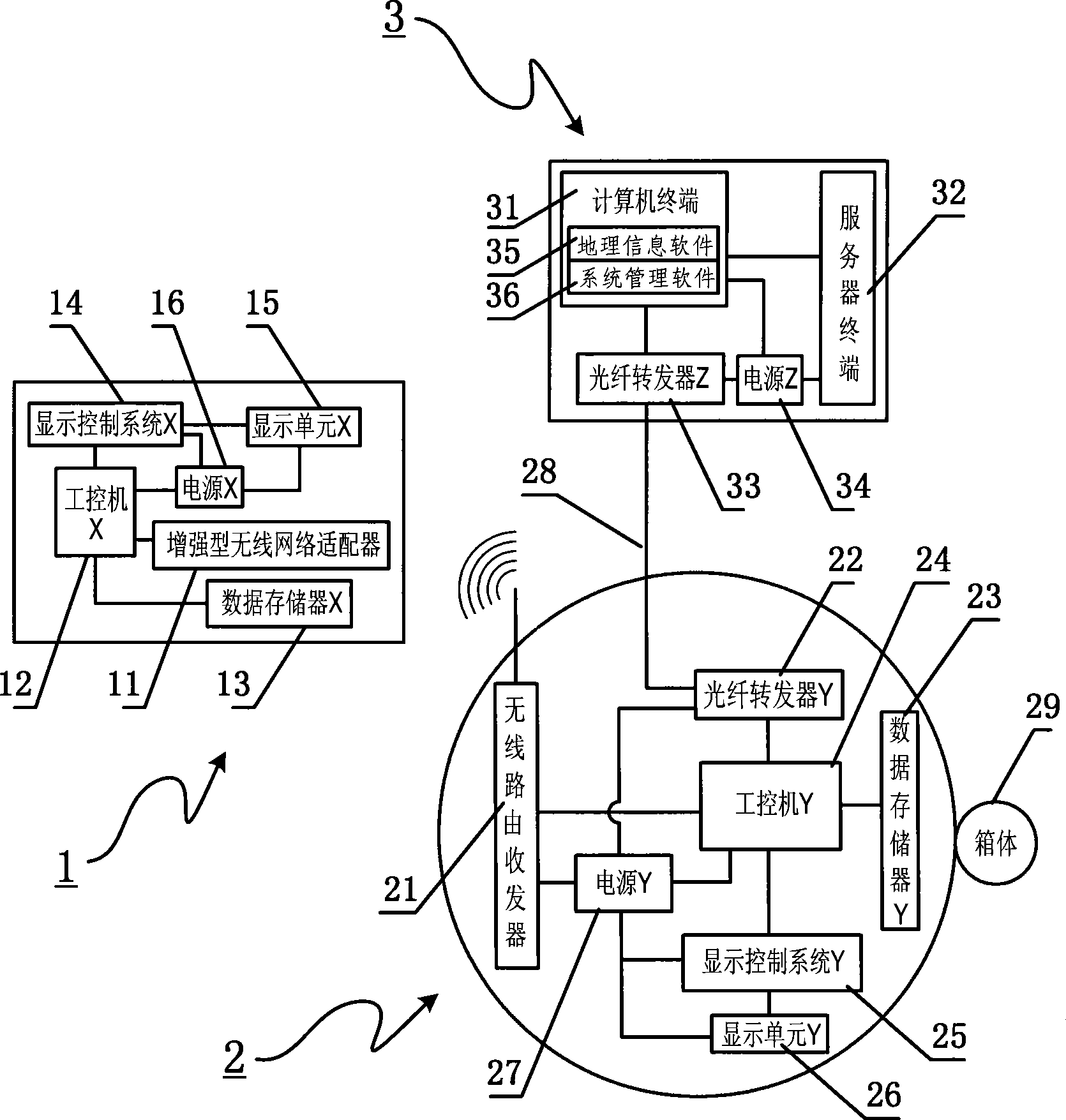
The invention relates to a method used for digital bus information releasing and group control and a device thereof. The technical measure comprises the following steps: a vehicle terminal system, an electronic station board system and a vehicle technical information management centre are arranged, the electronic station board system is the vehicle technical information management centre are connected into a bi-directional communication network by line link, the vehicle terminal system transmits the vehicle information to the electronic station board system in a wireless communication, the electronic station board system reports the information to the vehicle technical information management centre, the bus coming to station at each station can be found out by inquiring a database, the station arriving time of the bus is calculated according to experimental data including average running speed, average running time between the stations and the like, finally, the arriving time is fed back to the electronic station board system, and the arriving time is displayed to the waiting passengers in a digital way. The method can effectively solve the problems of bus reduction in city and dispatching, information releasing, passenger facilitation, intelligent management control and the like. Therefore, the method realizes intelligent bus dispatching.
Owner:JIANGSU HANDSON INTELLIGENT TECH CO LTD
Preparation method of desmopressin acetate
ActiveCN103102395AImprove the oxidation reaction yieldLess side effectsOxytocins/vasopressinsPeptide preparation methodsDrugCombinatorial chemistry
Owner:HYBIO PHARMA
Linaclotide solid-phase synthesis method
InactiveCN104974229AGood effectPrecise positioningPeptide preparation methodsBulk chemical productionFreeze-dryingSolid-phase synthesis
The invention discloses a linaclotide solid-phase synthesis method, and belongs to the biochemical technical field. The method includes the following steps: (1) preparation of linaclotide resin; (2) cutting the linaclotide linear peptide resin obtained in the step (1), to obtain a protection group linear peptide containing Cys(Acm) and Cys(tBu); (3) oxidizing to form a first disulfide bond, to obtain a monodisulfide cyclopeptide; (4) removing an Acm protection group in the monodisulfide cyclopeptide, to obtain a dual disulfide cyclopeptide; (5) removing a tBu protection group of the dual disulfide cyclopeptide, to obtain a trisdisulfide cyclopeptide; and (6) purifying the trisdisulfide cyclopeptide by HPLC, and freeze-drying to obtain linaclotide. The process has the characteristics of simple reaction operation, easy post-processing, low cost, high yield, and considerable economic and practical values, and besides, has wide application prospect in the polypeptide drug design and synthesis field.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Preparation method of Plecanatide
ActiveCN104628827AReduce the impactDifficult to separate and purifyPeptide preparation methodsBulk chemical productionFreeze-dryingSide chain
The invention discloses a preparation method of Plecanatide. The preparation method comprises the following steps: 1) preparing side chain group protected peptide resin from Fmoc-Leu-resin by coupling one by one from the carbon end to nitrogen end by a Fmoc solid-phase synthesis method according to the polypeptide sequence order of Plecanatide; 2) de-protecting the peptide resin respectively according to an order of Cys4-Cys12 and Cys7-Cys15 to obtain all-protected Plecanatide resin; 3) cracking the side chain group protected peptide resin by use of a cracking reagent; and 4) desalting and performing purification and freeze drying to obtain Plecanatide. The Plecanatide preparation method disclosed by the invention has practical industrial application prospects and considerable economic practical values.
Owner:NANJING UNIV OF TECH
Purification method of ganirelix acetate
ActiveCN102993274AGood removal effectHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsGanirelixPurification methods
The invention provides a purification method of ganirelix acetate. The purification method comprises the steps of (1) purification of ganirelix crude peptide, wherein octadecylsilane bonded silica is adopted as a fixed phase, perchlorate / phosphoric acid solution with certain concentration is taken as an A phase and acetonitrile is taken as a B phase, the ganirelix crude peptide is purified by a gradient-elution high performance liquid chromatography (HPLC) method; (2) salt conversion and purification, wherein the alkylsilane bonded silica is taken as the fixed phase, glacial acetic acid solution with a certain concentration is taken as the A phase and the acetonitrile is taken as the B phase, salt conversion and purification are carried out by adopting the gradient elution HPLC method, and the solution collected and subjected to freeze-drying to obtain the ganirelix acetate. The invention aims at providing the purification method of the ganirelix acetate with stable and controllable process, high yield, high purity, and wide practical value and application prospect.
Owner:HYBIO PHARMA
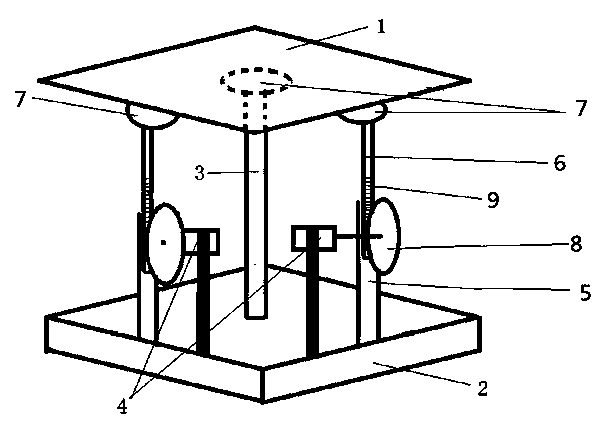
Electronic stability augmentation platform
InactiveCN103453287ASimple structureImprove stabilityStands/trestlesControl using feedbackControl signalEngineering
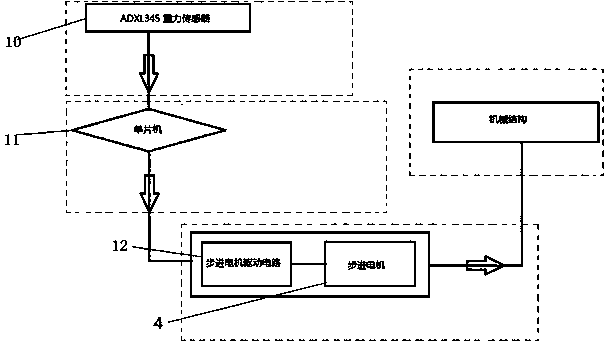
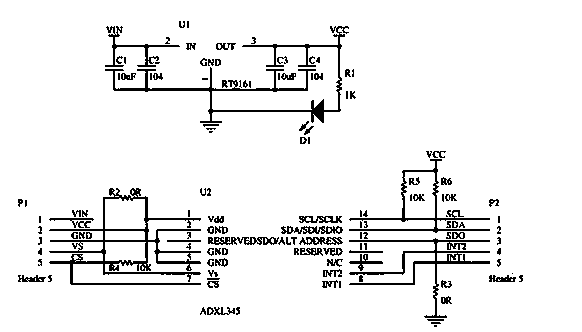
The invention discloses an electronic stability augmentation platform comprising a base, a platform body, and a single chip microcomputer, a gravity sensor and a stepper motor which are arranged on the base. The platform body is arranged on the base in a supporting manner through three supporting rods fixed on the base. When the base is bumped, the gravity sensor detects changes of acceleration signals and sends the detected signals to the single chip microcomputer, the single chip microcomputer outputs corresponding control signals to control the stepper motor to move through calculation, and the stepper motor drives gears to rotate so as to allow adjusting rods to telescope and maintain the upper-layer carrying platform horizontally. The electronic stability augmentation platform is simple in structure and good in stability augmentation, has advantages of small size, convenient for mounting, low cost, promised application prospect and the like, and has great economic practical value.
Owner:NANJING VOCATIONAL UNIV OF IND TECH
Preparation method of polymer polypeptide
The invention belongs to the technical field of pharmaceutical chemistry, relates to a preparation method of polymer polypeptide, and discloses a method for preparing glatiramer acetate. According to the invention, carboxy anhydrides of alanine, zeta-trifluoro-indole-3-acetyl lysine, gamma-benzyl glutamate and tyrosine is used as raw materials; diethylamine is used as a trigger in dioxane to trigger polymerization, so as to obtain a polymer with a protecting group; the obtained polymer with a protecting group experiences debenzylation reaction with 20 to 25% of acetic acid solution of hydrogen bromide at the temperature of 18 to 30 DEG C; the reaction liquid is poured into ice water to separate out solids; the obtained solids are cleaned with diethyl ether; after being filtered, the solids are collected; water solution of piperidine is added to remove trifluoroacetyl, so as to obtain coarse product solution of glatiramer, and after purification, glatiramer acetate is obtained. Compared with the prior art, the preparation method has the advantages of simpleness in operation, little impact on molecular weight of glatiramer acetate, and high yield of glatiramer acetate, so as to be suitable for the large-scale industrial production of glatiramer acetate.
Owner:HYBIO PHARMA
Method for preparing cyclodipeptide cyclo(L-Asp-L-Pro)
InactiveCN101143852AReduce pollutionEasy to operateOrganic chemistryDipeptideTert-Butyloxycarbonyl protecting group
The invention relates to a preparation method of cyclic dipeptide, in particular to a preparation method of aspartic ammonia prolinase cyclic dipeptide. The technical scheme of the invention includes four steps; firstly, starting from proline methyl ester and N-tertbutyloxycarbonyl-Beta-benzyl aspartic acid, the protective aspartic ammonia prolinase cyclic dipeptide methyl ester is obtained; secondly, under the acidic condition, the protective aspartic ammonia prolinase cyclic dipeptide methyl ester is deprotected; thirdly, cyclization occurs under the alkaline condition; fourthly, the aspartic ammonia prolinase cyclic dipeptide is finally generated by catalytic hydrogenolysis. The invention skillfully utilizes a gas phase method to synthesize the aspartic ammonia prolinase cyclic dipeptide, the technique is characterized in simple reaction and operation, easy-processing, little environmental pollution, low cost, etc., and the invention can be widely applied to the field of the design and synthesis of drugs.
Owner:HYBIO PHARMA
Solid phase method for synchronizing Argatroban
InactiveCN101519429AReduce investmentEasy to operatePeptide preparation methodsBlood disorderSide chainOrganic base
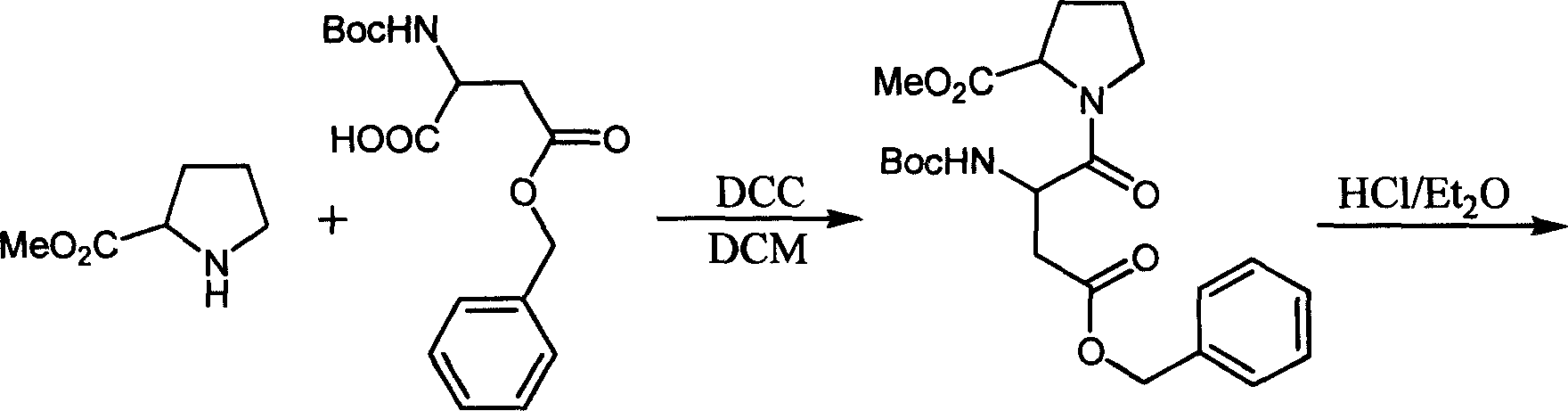
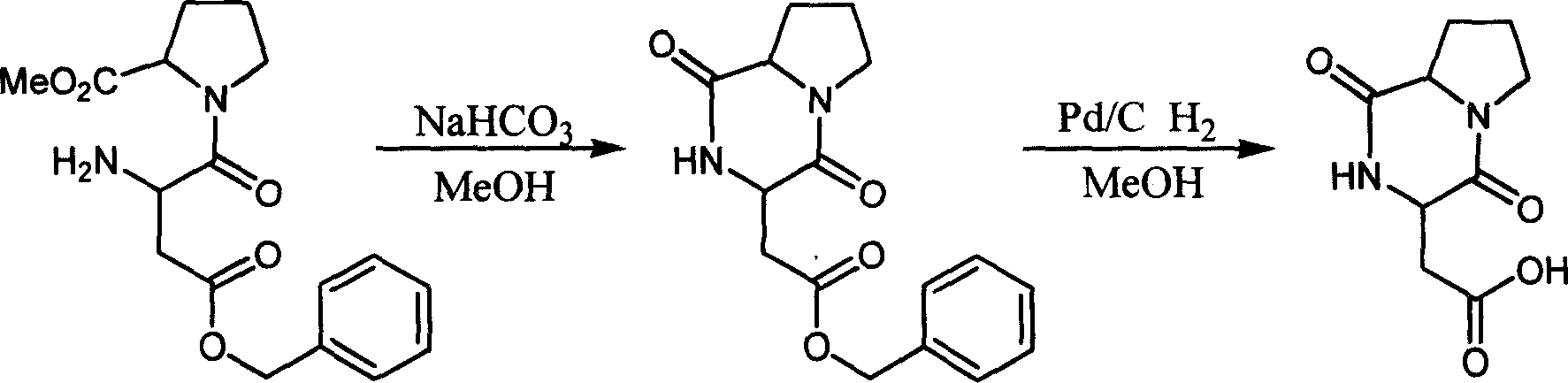
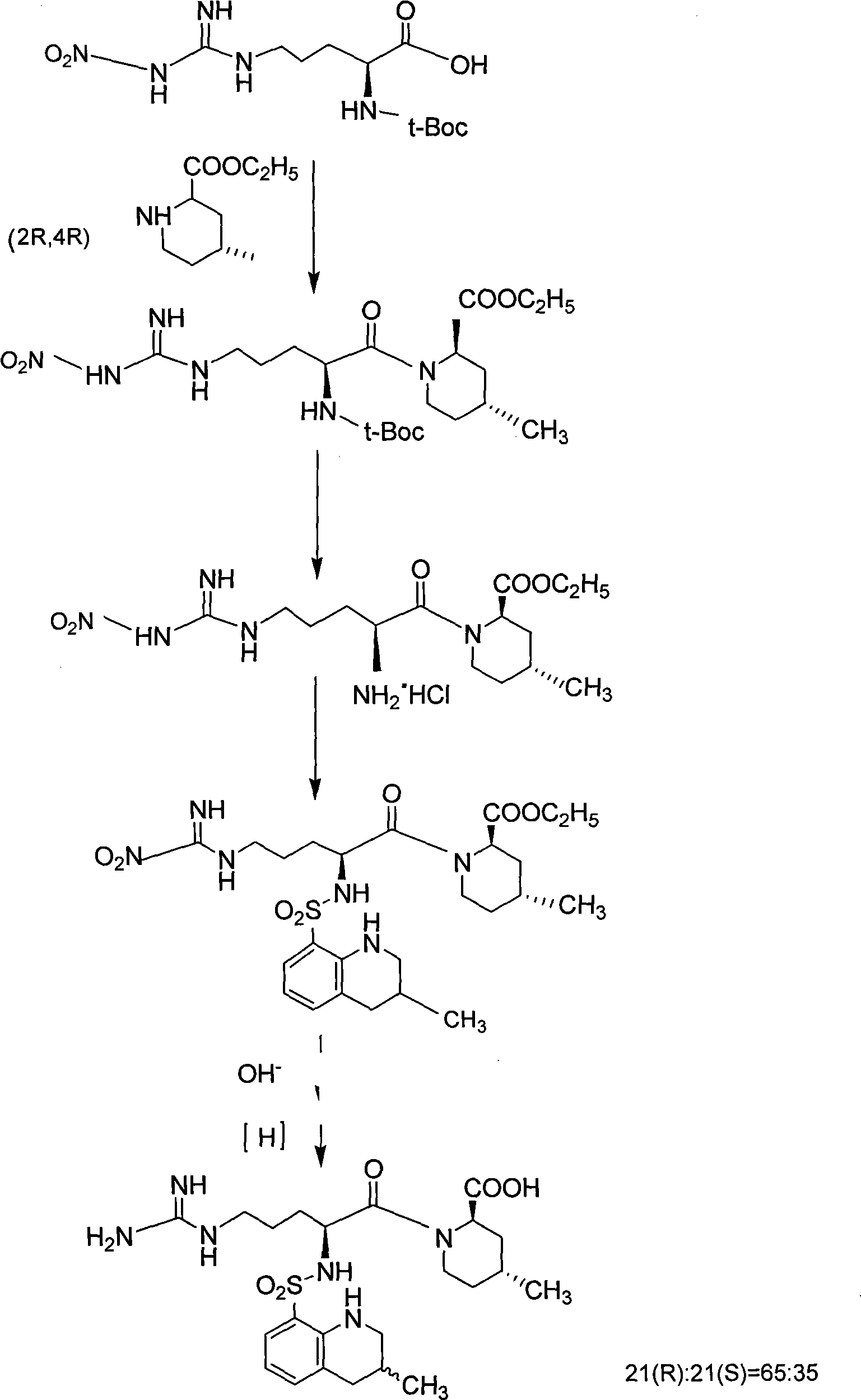
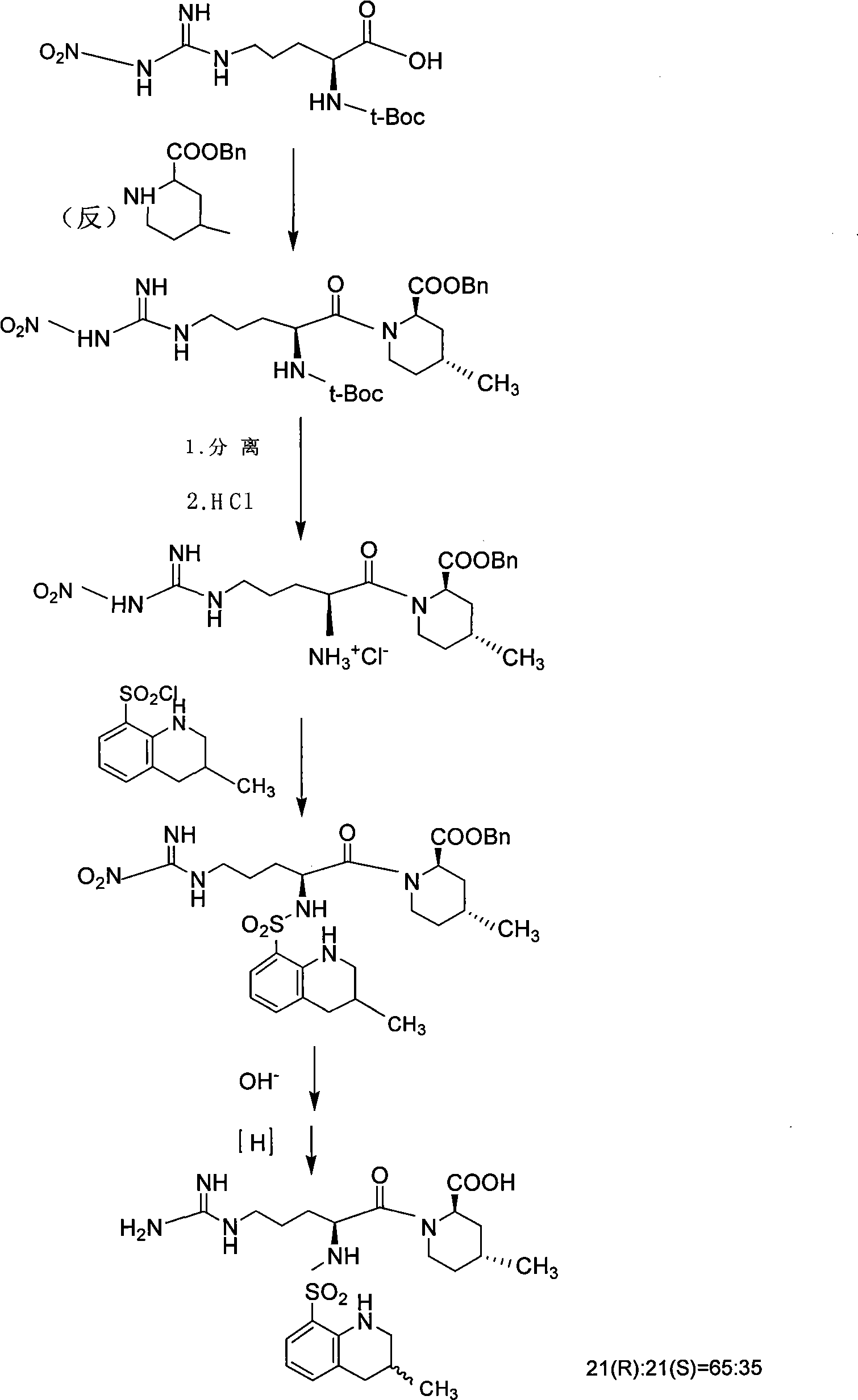
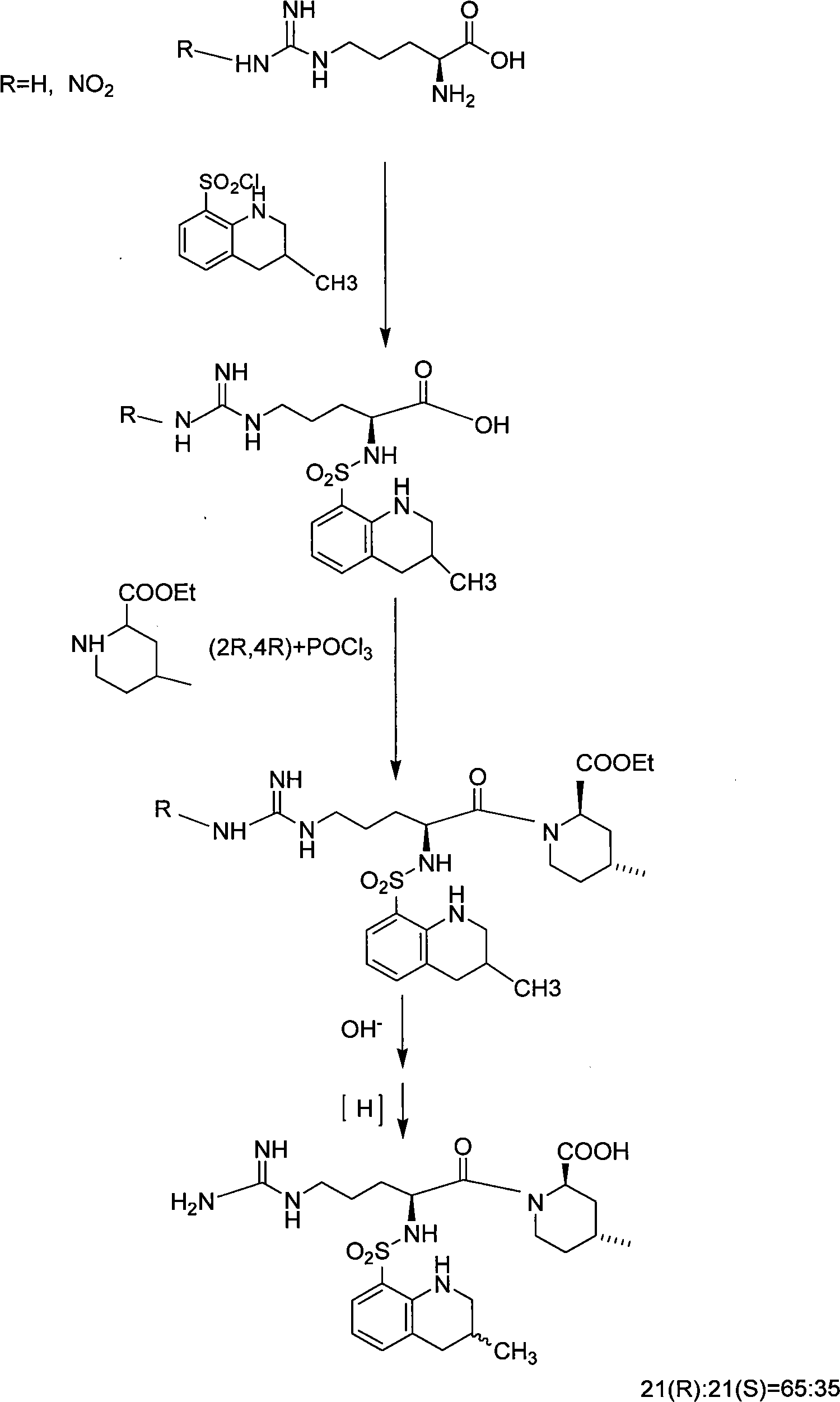
The invention discloses a solid phase method for synchronizing Argatroban, including the following steps: (1) (2R, 4R)-N-Fmoc-4-methyl-2-nipecotic acid, macromolecule resin, protective amino acid, coupling reagent and organic base are used as starting raw materials to form (2R, 4R)-N-Fmoc-4-methyl-2-nipecotic acid-resin; (2) Fmoc protection is removed, and a solid phase method is adopted to couple Fmoc-Arg(X)-OH so as to obtain Fmoc-Arg(X)-(2R, 4R)-4-methyl-2-nipecotic acid-resin; (3) the Fmoc protection is removed, and after 3-methyl-1,2,3 4-tetrahydroquinoline-sulfonic acid chloride is coupled, complete full protection Argatroban-resin is obtained; (4) complete peptide protection Argatroban-resin is reacted with a side chain separated blocking group so as to gain the crude product of the Argatroban; and (5) after the operation of recrystallization is carried out, the Argatroban with high purity is obtained. The technical process of the invention has the advantages of simple reaction operation, easy post treatment, less raw material, low cost, more than 80 percent of total yield, considerable economic and practical value as well as broad application prospect in the field of designing and synchronizing polypeptide drugs.
Owner:HYBIO PHARMA
Method for synthesizing cholecystokinin octapeptide by combining solid phase method and liquid phase method
ActiveCN102775471AReduce pollutionReduce investmentPeptide preparation methodsFreeze-dryingSynthesis methods
The invention relates to a preparation method of cholecystokinin octapeptide, in particular to a method for synthesizing the cholecystokinin octapeptide by combining a solid phase method and a liquid phase method. The method mainly solves the technical problem that the existing synthesis method is troublesome in separation of intermediate products, long in preparation period, apt to produce by-products in reaction, high in cost, low in yield and the like. The technical scheme is that the synthesis method comprises the following steps of: (1) synthesizing L-aspartyl-4-tertiary butyl ester-benzene propanamide by using the liquid phase method; (2) synthesizing cholecystokinin octapeptide full-protection fragments by using the solid phase method; (3) carrying out weak acid cutting on the full-protection fragments; (4) carrying out liquid phase condensation on the full-protection fragments and dipeptide fragments to obtain full-protection cholecystokinin octapeptide; (5) cutting, adding the full-protection cholecystokinin octapeptide in cutting fluid for cutting, and then adding ice diethyl ether for sediment to obtain cholecystokinin octapeptide crude products; and (6) purifying the crude products through high-phase liquid chromatogram, preparing, rotatably steaming, carrying out freeze-drying to obtain the cholecystokinin octapeptide competitive products. The method is used for preparing the cholecystokinin octapeptide.
Owner:GL BIOCHEM SHANGHAI +2
Method for preparing vapreotide
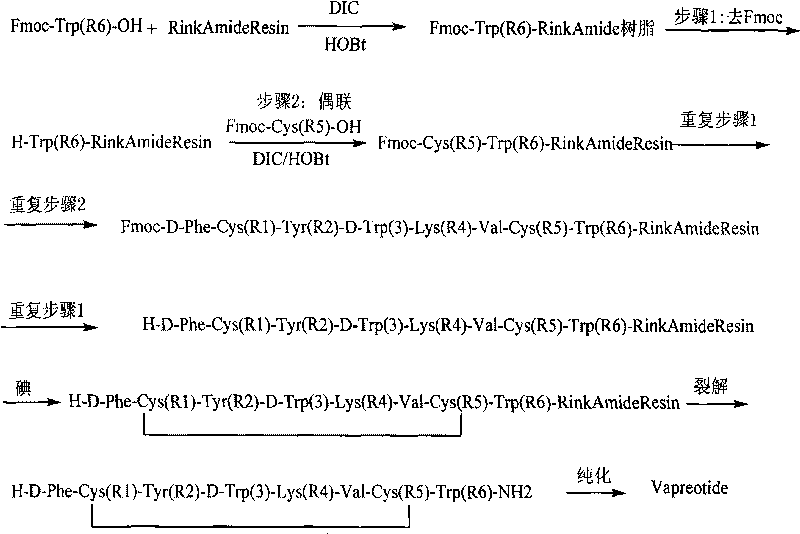
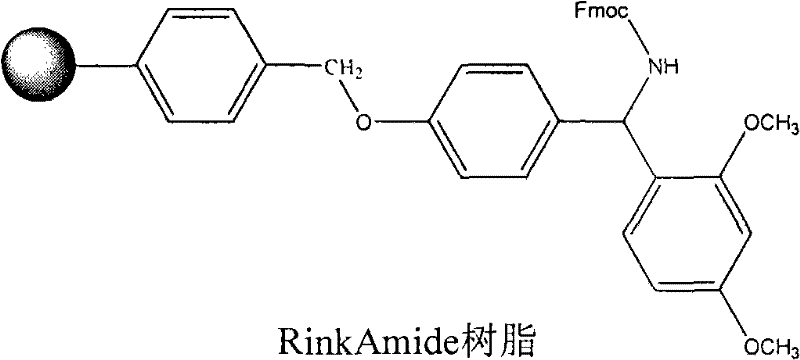
InactiveCN101712716AReduce investmentAvoid operational hasslesPeptide preparation methodsFreeze-dryingRink amide resin
The invention discloses a method for preparing vapreotide, comprising the following steps: 1) starting from Fmoc-Trp(Boc)-OH and Rink Amide resin to obtain Fmoc-Trp(Boc)-Rink Amide resin; 2) coupling the Fmoc-Trp(Boc)-Rink Amide resin by a solid phase synthesis method to obtain linear vapreotide-Rink Amide resin according to the peptide sequence; 3) utilizing a solid phase to oxide the vapreotide-Rink Amide resin; and 4) cracking the vapreotide-Rink Amide resin, purifying and freeze-drying to obtain the vapreotide. The invention utilizes iodine to oxide the linear vapreotide resin in the solid phase, thereby avoiding the trouble brought by traditional liquid phase oxidation and improving oxidation yield by 10%-20%. The process of the invention has the characteristics of simple reaction operation, low raw material investment, low cost, high yield and the like, the total reaction yield can reach 68%, and the purification of crude peptide can reach 85%. Therefore, the method has considerable economic value and practical value.
Owner:HYBIO PHARMA
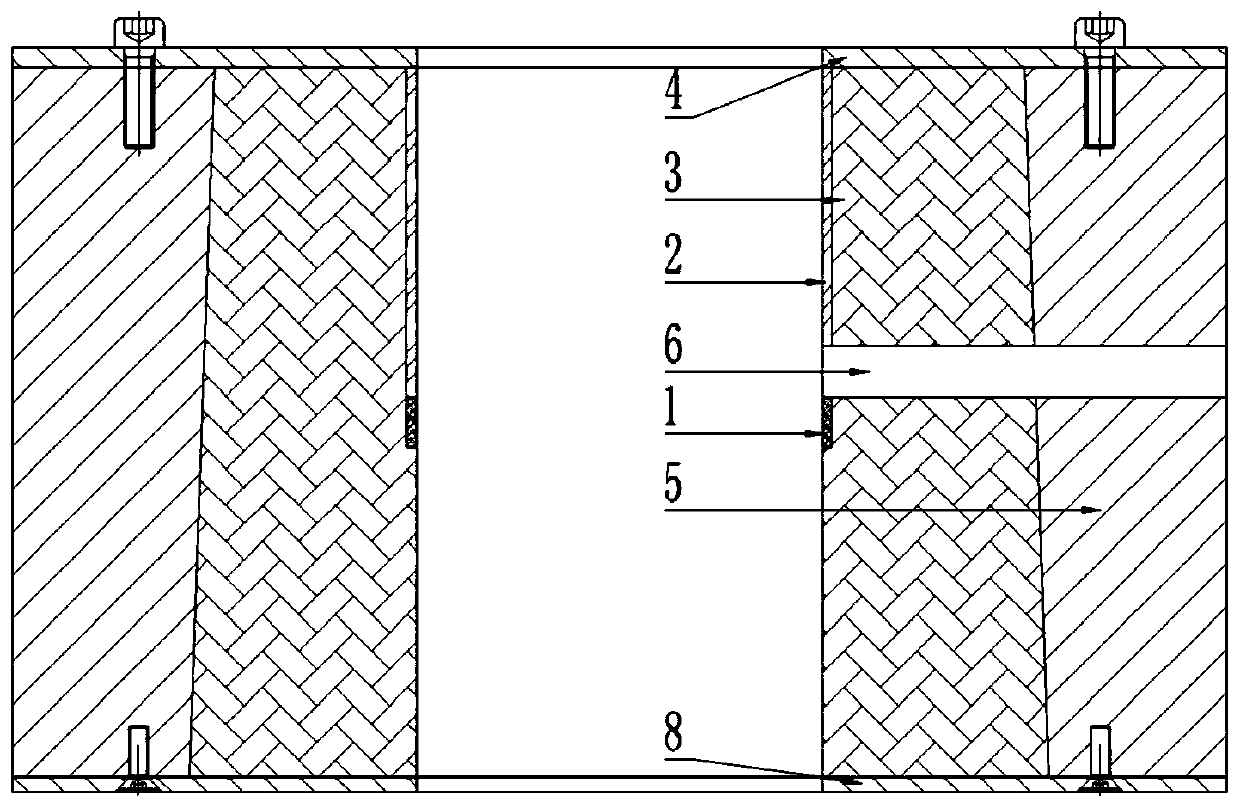
Variable-amplitude constant-thickness sieve
The invention discloses a variable-amplitude constant-thickness sieve. The variable-amplitude constant-thickness sieve comprises a sieve box body, vibration exciters, a sieving surface, damper springs and a bottom support, wherein bearing beams, vibration exciting beams and a strengthening beam are installed at the bottom, the middle and the top between two sieve box side plates separately, the sieving surface is installed on the bearing beams, and the vibration exciters comprises a feeding end vibration exciter which is installed on a feeding end vibration exciting beam and a discharging end vibration exciter which is installed on a discharging end vibration exciting beam; the sieve box body is installed on the bottom support through the damping springs which are symmetrically arranged on the outer sides of the two sieve box side plates; and each vibration exciting beam can rotate by any angle along the center line of the vibration exciting beam, so that adjustment of the vibration exciting direction of the vibration exciters on the vibration exciting beams is achieved, the amplitude and movement locus of the sieving surface from the feeding end to the discharging end are changed, and constant-thickness sieving of materials is achieved. The variable-amplitude constant-thickness sieve is simple in structure, convenient to use, stable and efficient, variable-amplitude sieving from the feeding end to the discharging end is achieved by means of the vibration exciters arranged at the feeding end and the discharging end, the utilization rate of the sieving surface is high, the unit-area treatment capacity is large, and the working condition adaptability is high.
Owner:CHINA UNIV OF MINING & TECH
Method for preparing telomerase polypeptide vaccine
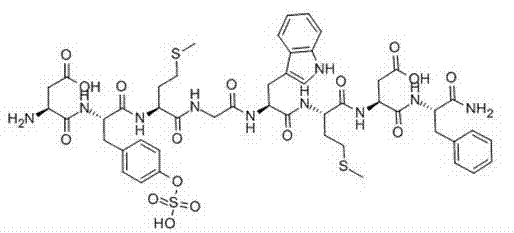
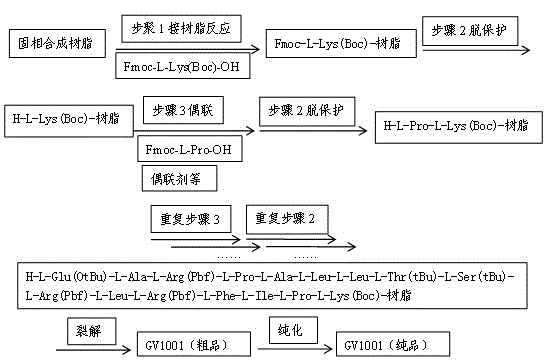
InactiveCN102875657AConsiderable economic and practical valuePeptide preparation methodsAntibody medical ingredientsTelomeraseSide chain
The invention discloses a method for preparing telomerase polypeptide vaccine GV1001. The method includes the steps of firstly, subjecting Fmoc-L-Lys(Boc)-OH and solid synthetic resin as starter materials to reaction so as to obtain Fmoc-L-Lys(Boc)-resin; secondly, subjecting the Fmoc-L-Lys(Boc)-resin to gradual coupling to obtain side-chains-protected GV1001-resin according to a polypeptide sequence of GV1001 by Fmoc solid-phase synthesis; thirdly, decomposing the side-chains-protected GV1001-resin by decomposing reagent, adding precipitating reagent to allow for precipitation so as to obtain crude peptide, and purifying and freeze-drying the crude peptide to obtain the telomerase polypeptide vaccine GV1001. The method for preparing the telomerase polypeptide vaccine GV1001 has practical industrial application prospect and considerable economic and practical values.
Owner:NANJING UNIV OF TECH
A kind of preparation method of plicanatide
ActiveCN104628827BDifficult to separate and purifyEase of industrial productionPeptide preparation methodsBulk chemical productionSide chainCombinatorial chemistry
The invention discloses a preparation method of pulikanatide, comprising the following steps: 1) Fmoc-Leu-resin carrier adopts Fmoc solid-phase synthesis method according to the polypeptide sequence sequence of pulikanatide from carbon end to nitrogen end Coupling one by one to obtain peptide resins protected by side chain groups; 2) Peptide resins are sequentially deprotected according to Cys4‑Cys12 and Cys7‑Cys15 respectively, and then oriented to form a ring to obtain fully protected pulikanatide peptide resins; 3) Cleavage The reagent cleaves the peptide resin protected by the side chain group, and 4) after desalting, it is purified and freeze-dried to obtain plicanatide. The preparation process of pulikanatide adopted in the present invention has practical industrial application prospects and considerable economic and practical value.
Owner:NANJING TECH UNIV
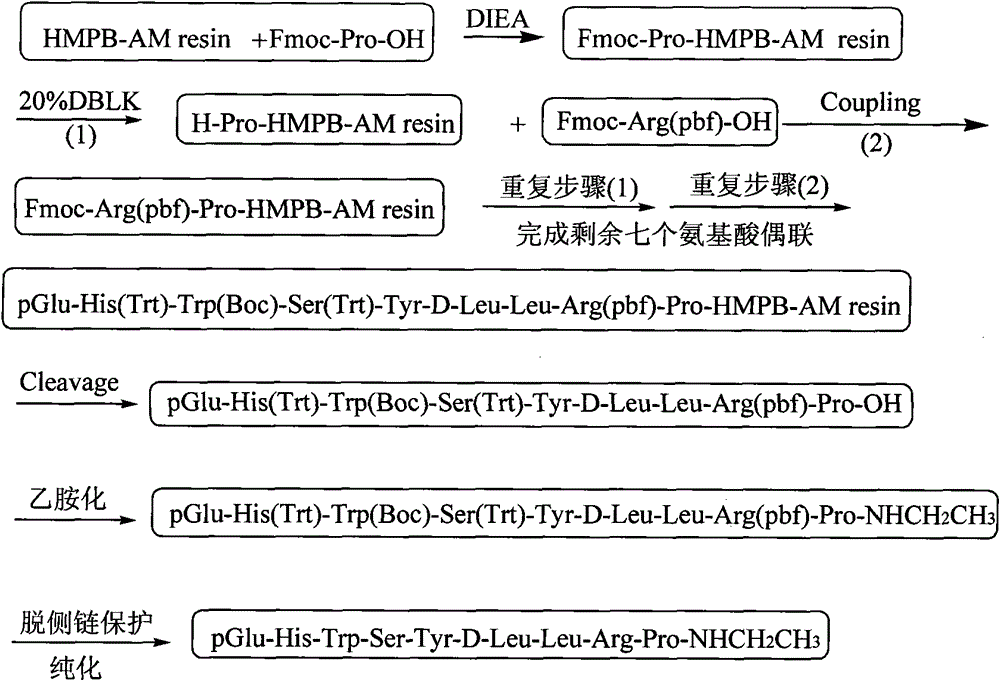
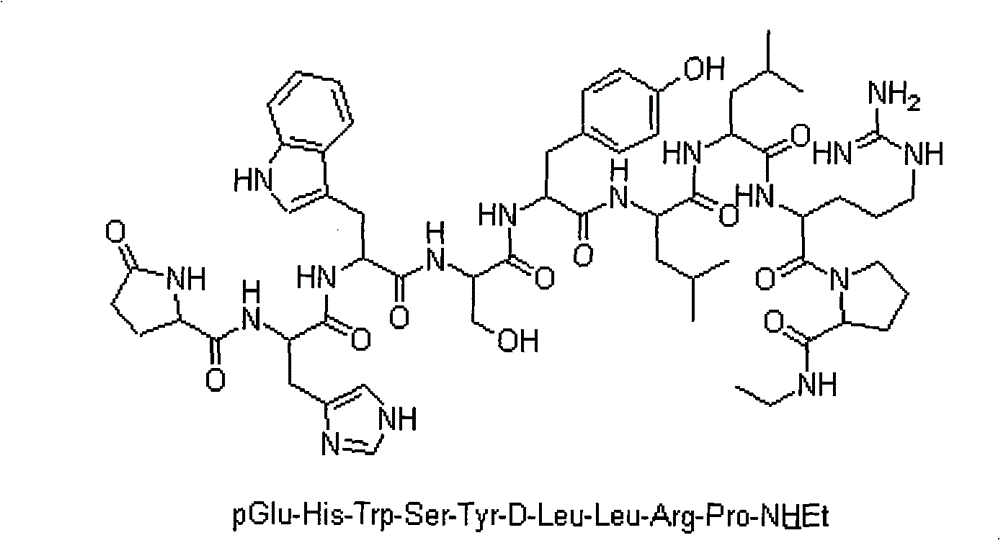
Method for preparing Leuprorelin by combination of solid phase method and liquid phase method
ActiveCN101538315BReduce pollutionReduce investmentPeptide preparation methodsAntineoplastic agentsLeuprorelinSide chain
The invention discloses a new technique for synthesizing Leuprorelin by the combination of a solid phase method and a liquid phase method, which comprises the following steps of: 1) using Fmoc-Pro-OH and HMPB-AM resin as starting material, and obtaining Fmoc-Pro-HMPB-AM resin; 2) coupling in sequence and synthesizing Leuprorelin precursor peptide-HMPB-AM resin with full protective lateral chains;3) cutting the resin and obtaining Leuprorelin precursor peptide with full protective lateral chains; 4) processing the Leuprorelin precursor peptide with full protective lateral chains with methylamine, and obtaining Leuprorelin with full protective lateral chains; 5) removing the protective groups of the lateral chains of Leuprorelin with full protective lateral chains, and obtaining the crude product of Leuprorelin; and 6) conducting separation and purification and freeze drying to the crude product of Leuprorelin, and obtaining refined Leuprorelin peptide. The technology has the capability of large-scale production, easy operation, stable technique, low production cost and total yield of more than 50 percent, and has considerable economical and practical value and wide application prospect.
Owner:HYBIO PHARMA
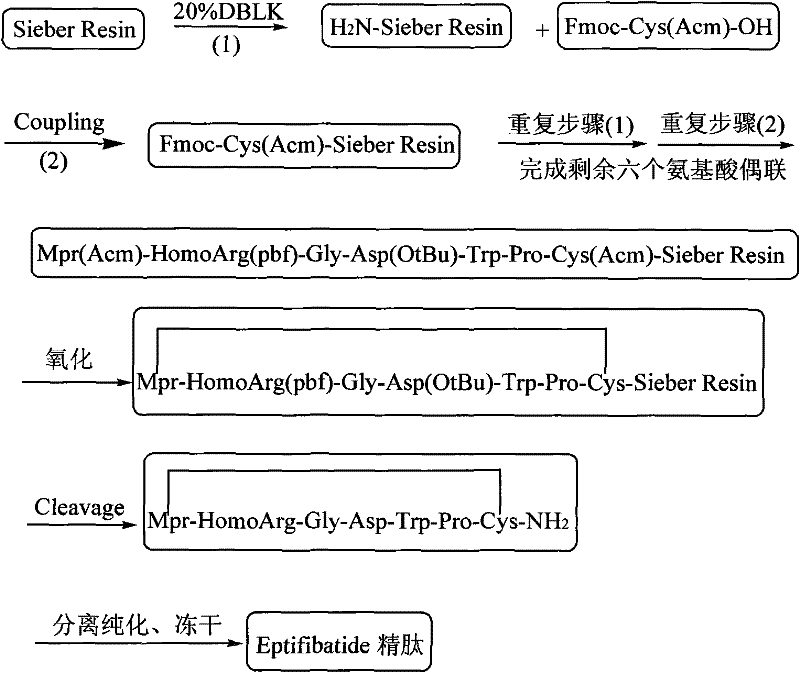
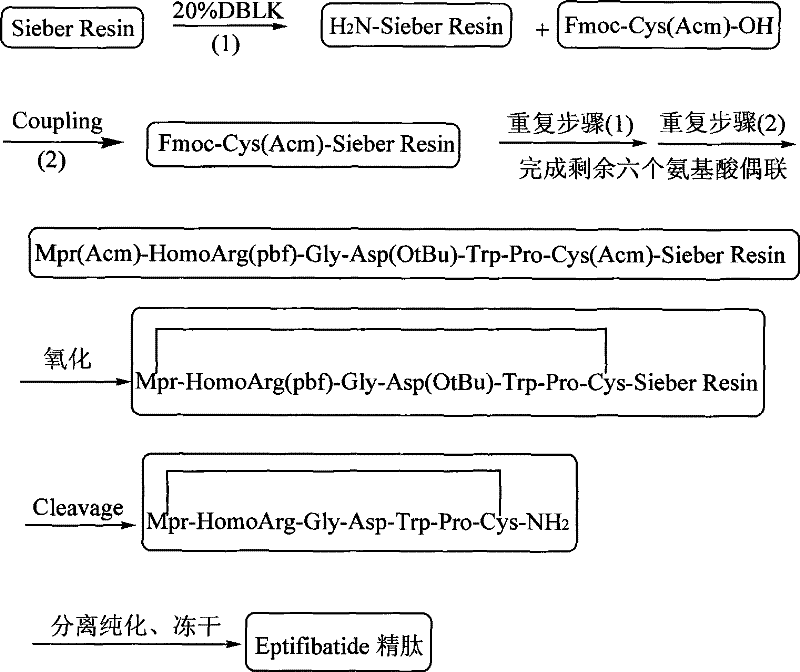
Method for preparing Eptifibatide with solid phase method
InactiveCN101538316BMild reaction conditionsReduce pollutionPeptide preparation methodsBlood disorderSide chainCombinatorial chemistry
The invention discloses a method for preparing Eptifibatide with a solid phase method, which comprises the following steps of: 1) selecting Sieber resin to remove Fmoc, and obtaining H2N-Sieber resin; 2) adopting Fmoc / tBu solid phase method to couple and synthesize linear peptide Eptifibatide-Sieber resin with full protective lateral chains in sequence; 3) conducting solid phase oxidation to the resin, and obtaining oxidant peptide Eptifibatide-Sieber resin with full protective lateral chains; 4) cutting the resin and removing the lateral chain protection, and obtaining crude product of Eptifibatide; and 5) conducting separation and purification, and breeze drying by a freeze dryer, and obtaining refined Eptifibatide peptide. The technology is characterized by simple operation, easy post treatment, less investment of raw material, low cost, high yield and the like, and has considerable economical and practical value and wide application prospect in the field of polypeptide drug design and synthesis simultaneously.
Owner:HYBIO PHARMA
Entire roll embossing production process of IML (in mould labeling) film
The invention provides an entire roll embossing production process of an IML (in mould labeling) film. The entire roll embossing production process comprises the following steps: unrolling a film; passing the film to be embossed between an embossing roll and an auxiliary clamping roll; drying the film. The entire roll embossing production process breaks through a conventional process of embossing corresponding film sheets of the IML film one by one by using a template block; in the entire roll embossing production process, a rolled film is continuously unrolled through an unrolling roll, and the film to be embossed is continuously glued and embossed by the embossing roll coated with glue and provided with a pattern and is continuously dried, so that a continuous embossing production line is formed; by the entire roll embossing production process, the defects in the conventional embossing process that the template block is easy to break, and easy to deflect in the printing process, the embossing stability and the embossing quality are difficult to be guaranteed, and the working procedure is complex, complicated in operation, high in manpower consumption and resource consumption, high in cost and low in production efficiency and the like are overcome; compared with the prior art, the entire roll embossing production process has the advantages of embossing continuousness and stability, high embossing quality, simple process flow, simple process, conservation of manpower and resources, low cost and high production efficiency and significant economical and practical values.
Owner:FUJIAN SHISHI TONGDA ELECTRICAL APPLIANCE
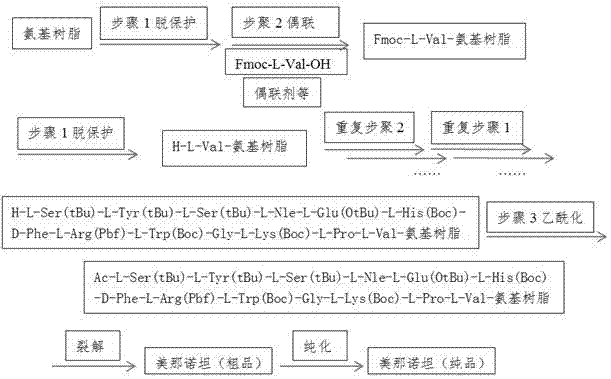
Preparation method of melanotan-II
InactiveCN102816211AImprove protectionEasy to operatePeptide preparation methodsBulk chemical productionSide chainPeptide sequence
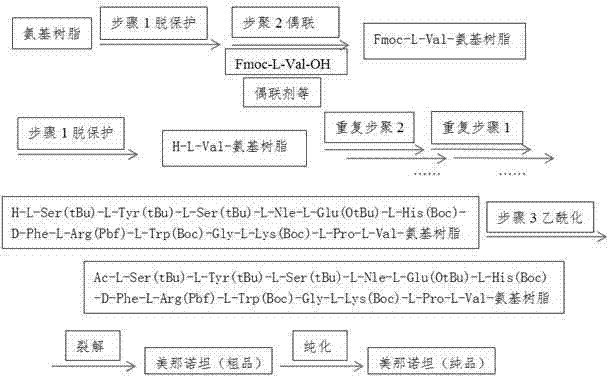
The invention discloses a preparation method of melanotan-II, and the method comprises the following steps of: 1) obtaining Fmoc-L-Val-amino resin according to Fmoc-L-Val-OH and amino resin; 2) coupling the Fmoc-L-Val-amino resin to side chain protection group-containing melanotan-II-amino resin of which the N end is a free amino group one by one by a solid phase synthesis method according to the peptide sequence of the melanotan-II; 3) having a acetylation reaction to generate the side chain protection group-containing melanotan-II-amino resin; and 4) carrying out pyrolysis on the side chain protection group-containing melanotan-II-amino resin by a pyrolysis reagent, and precipitating the ice ether to obtain coarse peptide; and purifying and dryly freezing the coarse peptide to obtain the melanotan-II. The preparation technology method of the melanotan-II provided by the invention is wide in application prospect and considerable in economic and practical value.
Owner:NANJING UNIV OF TECH
Preparation method of melanotan-II
InactiveCN102816211BImprove protectionEasy to operatePeptide preparation methodsBulk chemical productionMelanotan IISide chain
The invention discloses a method for preparing menanotan, which includes the following steps: 1) starting from Fmoc-L-Val-OH and amino resin, obtaining Fmoc-L-Val-amino resin; 2) converting Fmoc-L-Val- Val-amino resin adopts solid-phase synthesis method and is coupled one by one according to the sequence of Menanotan polypeptide to obtain Menanotan-amino resin with a free amino group at the N-terminus and containing a side chain protecting group; 3) Acetylation reaction generates a Menanotan-amino resin containing Menanotan-amino resin with side chain protective groups; 4) Use cleavage reagent to cleave Menanotan-amino resin with side chain protective groups, and precipitate with glacial ether to obtain crude peptide; the crude peptide is obtained after purification and freeze-drying Menanotan. The process for preparing menanotan adopted in the present invention has broad application prospects and considerable economic and practical value.
Owner:NANJING TECH UNIV
Linaclotide purifying method
InactiveCN107266535ALong-term purificationStable purificationPeptide preparation methodsFreeze-dryingGradient elution
The invention mainly relates to a linaclotide purifying method and belongs to the technical field of bioseparation. According to the linaclotide purifying method, an ion exchange chromatography method and a high-performance liquid chromatography method are combined, an anion exchange column is adopted as a stationary phase, a Tris HCl buffering solution is adopted as a mobile phase A, a NaCl-contained Tris-HCl buffering solution is adopted as a mobile phase B, a gradient eluting method is adopted to treat a crude linaclotide solution, eluate is subjected to desalination and acetate changing by means of the high-performance liquid chromatography method, a C18 column is adopted as a separating medium, after a sample is loaded, gradient elution is performed by adopting an aqueous acetic acid solution as the mobile phase A and acetonitrile as the mobile phase B, and eluate is subjected to freeze-drying to obtain pure linaclotide. The linaclotide purifying method is simple to operate, low in separation cost, high in yield and suitable for large-scale linaclotide production; purity of the obtained pure linaclotide can reach to above 99%, so that the obtained pure linaclotide is low in impurity content and has favorable economic and practical values and an extensive application prospect.
Owner:NANJING UNIV OF TECH +1
High-precision valve actuator
ActiveCN107131347AEasy to driveReduce volumeOperating means/releasing devices for valvesLinear motionValve actuator
The invention discloses a high-precision valve actuator. The high-precision valve actuator comprises a shell, a sleeve and a driving assembly for driving the sleeve to rotate. The driving assembly comprises multiple coaxially arranged hydraulic barrels and main bevel gears driven by the hydraulic barrels to move up and down. The main bevel gears are used in cooperation with auxiliary bevel gears arranged in the sleeve, linear motion of piston plates in the hydraulic barrels is converted into rotational motion of the sleeve, and therefore a valve rod is driven to rotate. Due to the fact that the rotation precision of all the main bevel gears is different, the multiple main bevel gears are used in cooperation to rotate, the valve rod can be easily and rapidly rotated to a set angle, and the response speed is high. Fewer drive parts needed by a driving manner are needed, control is also convenient, and the whole valve actuator is small in size and low in weight. All the parts are detachably arranged, and great convenience is also brought to later maintenance of the whole valve actuator.
Owner:SHANGHAI AOZ PETROLEUM EQUIP CO LTD
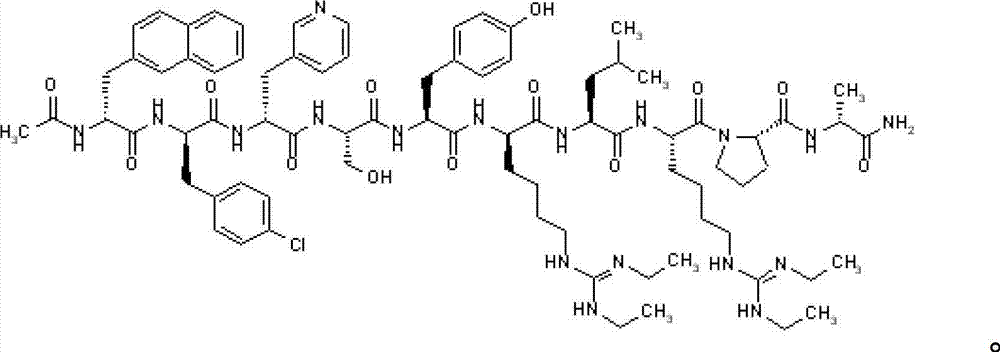
Method for preparing ziconotide
InactiveCN101709082BPurity is easy to controlPrecise positioningPeptide preparation methodsAnimals/human peptidesFreeze-dryingSide chain
The invention discloses a method for preparing ziconotide. The technical scheme of the invention comprises the following steps: (1) obtaining an Fmoc-Cys(Acm)-amino resin from Fmoc-Cys(Acm)-OH and an amino resin; (2) obtaining a linear-ziconotide-amino resin of which a Cys side chain comprises Acm by performing the solid phase synthesis on the Fmoc-Cys(Acm)-amino resin and an amino acid adopting Fmoc group protection; (3) obtaining a linear crude peptide of which the Cys side chain comprises the Acm by performing cracking on the linear-ziconotide-amino resin of which the Cys side chain comprises the Acm, and obtaining linear ziconotide by removing the Acm, purifying and freeze-drying; (4) and obtaining the ziconotide by performing cyclization, purifying and freeze-drying on the linear ziconotide. The method for preparing ziconotide has the characteristics of simple reaction operation, easy subsequent treatment, low raw material investment, low cost, high yield and the like, and has considerable economic and practical value, and also has wide application prospect in the field of design synthesis of polypeptide drugs.
Owner:HYBIO PHARMA
Pressure cavity structure for comprehensive dielectric property measurement under high pressure and measurement method thereof
ActiveCN111257704AEasy to makeNot easy to damageDielectric property measurementsTesting dielectric strengthPolymer scienceBasalt fiber
The invention relates to a pressure cavity structure for comprehensive dielectric property measurement under high pressure and a measurement method thereof. The pressure cavity structure comprises a ceramic ring, a die steel sleeve, a basalt fiber sleeve and an anti-expansion protection steel sleeve. The anti-expansion protective steel sleeve is arranged on the outer side of the basalt fiber sleeve in a sleeving manner; a through hole is formed in the basalt fiber sleeve; the side wall of the through hole is connected with the ceramic ring and the die steel sleeve, the lower end of the ceramicring is supported by the side wall of the through hole, the upper end of the ceramic ring is connected with the die steel sleeve, the pressure cavity structure is provided with a low-pressure hole and a high-pressure hole, and the low-pressure hole and the high-pressure hole penetrate through the through hole in the basalt fiber sleeve from the outer side of the pressure cavity structure. Compared with the prior art, the pressure cavity structure can bear higher pressure and can be used for measuring various dielectric properties and has the advantages of convenience in measurement, firmness,reliability, low manufacturing cost and the like.
Owner:TONGJI UNIV +2
Purification method of ganirelix acetate
ActiveCN102993274BHigh yieldHigh purityLuteinising hormone-releasing hormonePeptide preparation methodsGanirelixPurification methods
The invention provides a purification method of ganirelix acetate. The purification method comprises the steps of (1) purification of ganirelix crude peptide, wherein octadecylsilane bonded silica is adopted as a fixed phase, perchlorate / phosphoric acid solution with certain concentration is taken as an A phase and acetonitrile is taken as a B phase, the ganirelix crude peptide is purified by a gradient-elution high performance liquid chromatography (HPLC) method; (2) salt conversion and purification, wherein the alkylsilane bonded silica is taken as the fixed phase, glacial acetic acid solution with a certain concentration is taken as the A phase and the acetonitrile is taken as the B phase, salt conversion and purification are carried out by adopting the gradient elution HPLC method, and the solution collected and subjected to freeze-drying to obtain the ganirelix acetate. The invention aims at providing the purification method of the ganirelix acetate with stable and controllable process, high yield, high purity, and wide practical value and application prospect.
Owner:HYBIO PHARMA
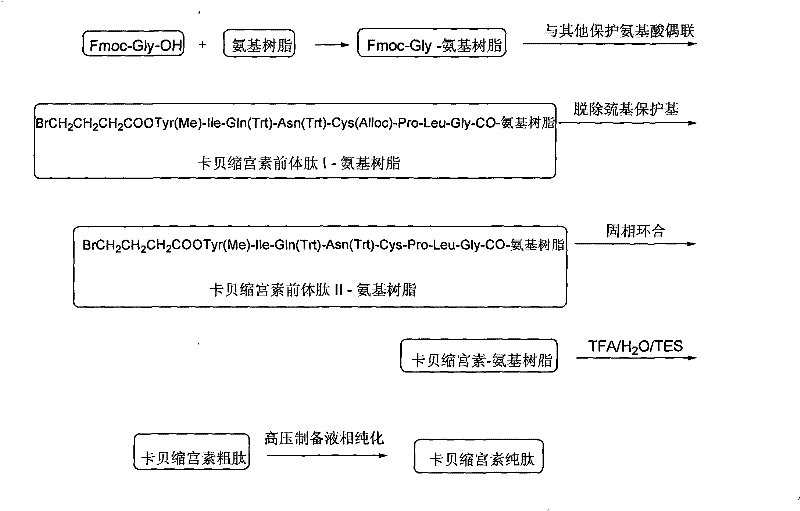
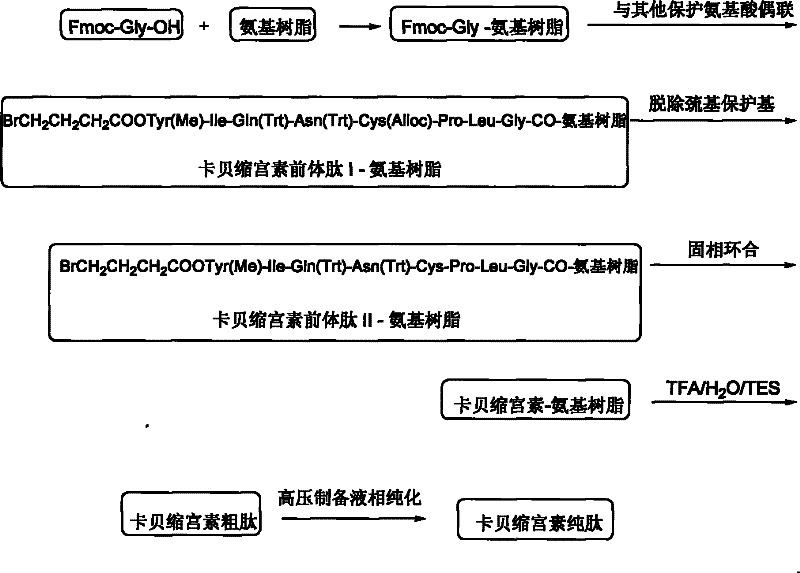
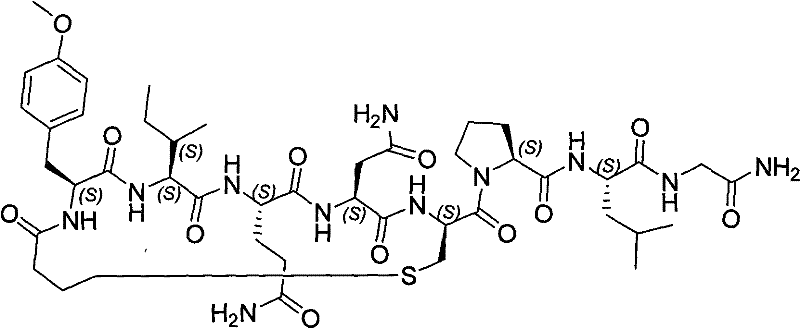
Solid phase preparation method of carbetocin
ActiveCN101555272BReduce investmentEasy to operatePeptide preparation methodsSexual disorderLithium chlorideFreeze-drying
The invention discloses a solid phase synthesis method of carbetocin. The technical proposal comprises the following steps of: obtaining Fmoc-Gly-amino resin by reaction of Fmoc-Gly-OH and amino resinwith the substitutability being 0.2 mmol / g-0.9 mmol / g; sequentially connecting amino acids with Fmoc protecting groups by the solid phase synthesis method to obtain carbetocin precursor peptide I-amino resin; stripping off cysteine side chain protecting groups to obtain carbetocin precursor peptide II-amino resin; adding organic alkali and lithium chloride in solvent for cyclization to obtain carbetocin-amino resin; cracking to obtain carbetocin crude peptide; and purifying and freeze-drying to obtain the carbetocin. The method adopts the amino resin to synthesize carbetocin by the solid phase cyclization technology. The process is characterized by simple operation, easy post-treatment, high yield, low cost, and the like, and has considerable economical and practical value and broad application prospect.
Owner:HYBIO PHARMA
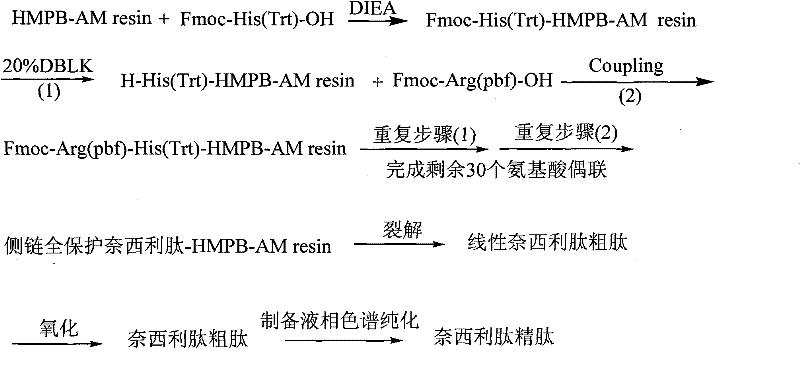
A kind of method for preparing nesiritide
InactiveCN101519444BReduce investmentEasy to operatePeptide preparation methodsAnimals/human peptidesFreeze-dryingSide chain
The invention relates to a method for preparing Nesiritide. In the method, HMPB-AM resin is taken as an initial raw material, amino acids with Fmoc blocking groups are sequentially connected in a solid phase synthesis way under the action of condensing agent and transpeptidase reagent to obtain side chain full-protection linear Nesiritide HMPB-AM resin; linear Nesiritide raw peptides are obtained by schizolysis, Nesiritide raw peptides are obtained by liquid phase oxidation, and Nesiritide fine peptides are obtained by purification, salt transfer and freeze drying. The invention has the advantages of simple operation, easy post treatment, few raw materials, low cost, high yield coefficient, and the like, thereby having considerable economy and utility value and also having extensive application prospect in the polypeptide drug design synthesis field.
Owner:HYBIO PHARMA
Method for preparing lepirudin
InactiveCN102731647AReduce difficultyShort synthesis timePeptide preparation methodsLeech-based protease inhibitorsCombinatorial chemistryDisulfide bond
The present invention relates to the field of polypeptide synthesis, and particularly to a method for preparing lepirudin. According to the method, combination of stepwise synthesis and fragment synthesis is adopted so as to greatly reduce synthesis difficulty, save synthesis time, reduce final impurities, improve purity and yield, provide considerable economic and practical values, and provide wide application prospects in the field of design and synthesis of multiple disulfide bond ring closing polypeptide drugs.
Owner:HYBIO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com