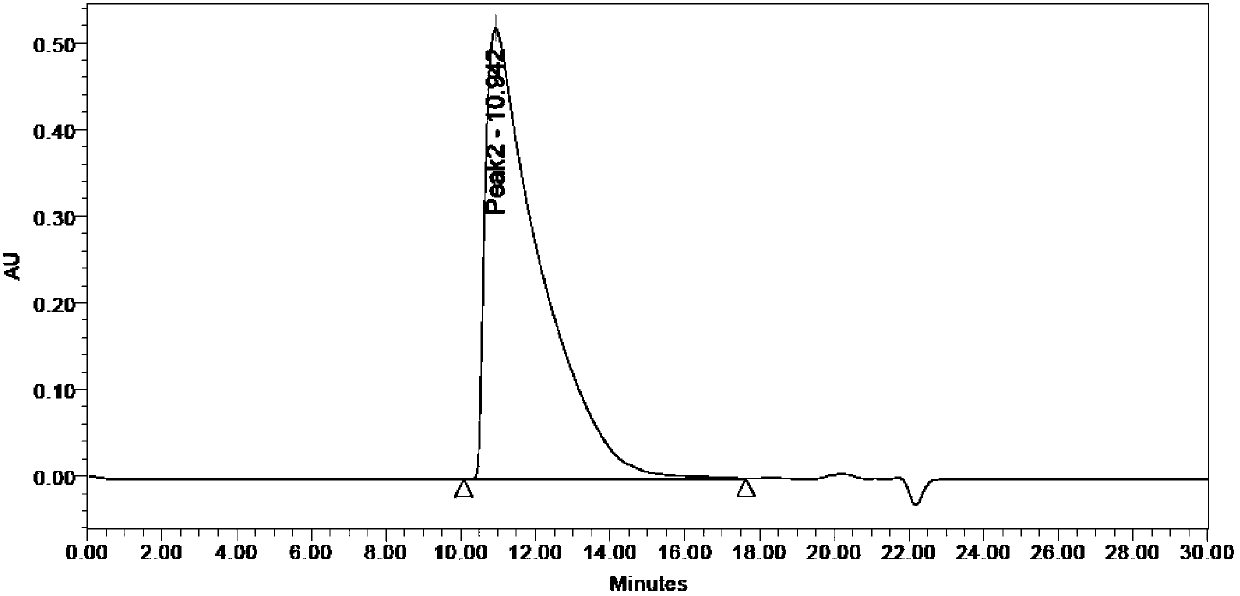
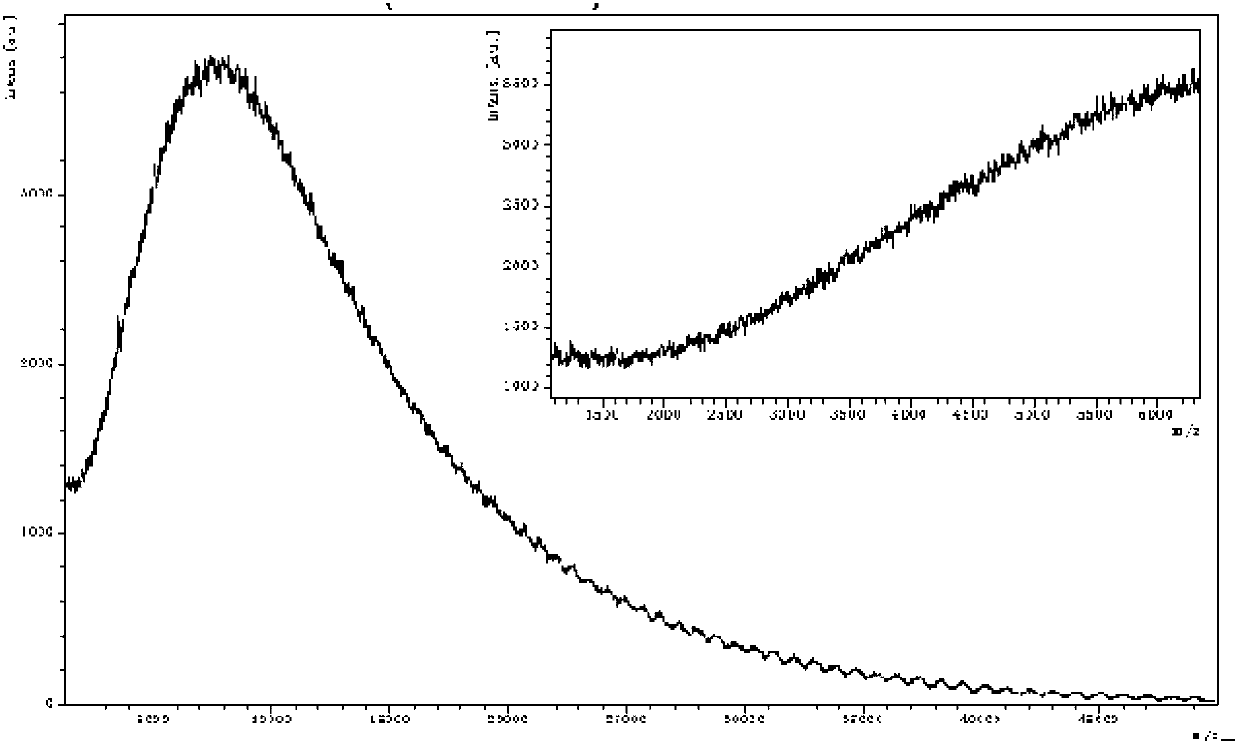
Preparation method of polymer polypeptide
A technology of solution and glatiramer acetate, which is applied in the preparation of polymer polypeptides and the field of preparation of glatiramer acetate, which can solve the problems of unsuitability for the industrial production of glatiramer acetate, complicated process, and low yield of glatiramer acetate. , to achieve considerable economic and practical value, high purity and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: have the preparation of protective group polymer
[0032] Add 103.5g (0.9mol) of alanine carboxylate anhydride, ξ-trifluoroacetyllysine carboxylate anhydride 187.6g (0.70mol), γ-benzyl glutamic acid carboxylate anhydride into a 5L three-necked round bottom flask 78.9g (0.3mol), 41.4g (0.20mol) of tyrosine carboxyl anhydride and 3L of dioxane, stirred to dissolve. Then 0.01% diethylamine was added to initiate the polymerization reaction, stirred at room temperature for 24 hours, then poured into 30 L of water to quench the reaction, the solid was washed with 10 L of water, filtered, and dried to obtain 313.9 g of white solid with a yield of 76.3%.
Embodiment 2
[0033] Embodiment 2: Preparation of trifluoroacetyl glatiramer
[0034] Add 3g of the polymer with protective group prepared in Example 1 into a 50mL round-bottomed flask, add 30mL of 25% hydrogen bromide acetic acid solution, stir at 25°C for 23h, and pour the reaction solution into 300mL of ice water to precipitate a solid after the reaction, filter The collected solid was washed with water (300 mL), filtered and sucked dry. The collected solid was washed three times with 50 mL of diethyl ether, stirred for 25 min each time, filtered and dried to obtain 2.2 g of white solid with a yield of 73.3%.
Embodiment 3
[0035] Embodiment 3: the preparation of trifluoroacetyl glatiramer
[0036] Add 300g of the polymer with protective group prepared in Example 1 into a 5L round bottom flask, add 3000mL of 20-25% hydrogen bromide acetic acid solution, stir at 25°C for 23h, and pour the reaction solution into 30L of ice water to precipitate Solid, collected by filtration, washed with 30 L of water, filtered and sucked dry. The collected solid was washed three times by adding 5 L of ether, stirring for 25 min each time, and the sample was filtered and dried to obtain 216.9 g of white solid with a yield of 72.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com