Method for preparing telomerase polypeptide vaccine
A technology of polypeptide vaccine and telomerase, which is applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., and can solve the problems of unprovided technical process, unlisted product purity, yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
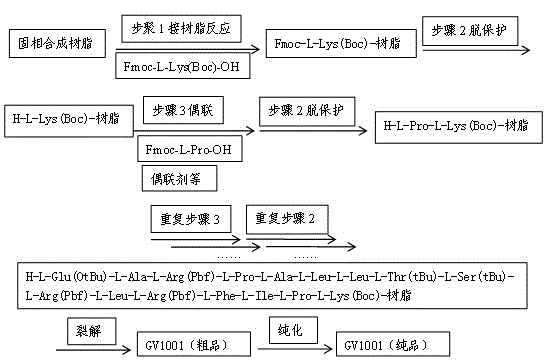
[0055] Such as figure 1 As shown, the preparation method claimed in the present invention comprises the following steps:
[0056] step one,
[0057] 1. Put 10 g of CTC resin with a loading capacity of 0.6 mmol / g in the reactor, add NMP to wash twice, and then use NMP to swell for 1 hour.
[0058] 2. Weigh 2.81g of Fmoc-L-Lys(Boc)-OH, add 50 ml of NMP to dissolve, add 4.18 ml of DIPEA after complete dissolution, mix well and add to the reactor to start the reaction.
[0059] 4. After 6 hours of reaction, the reaction was terminated. Wash 3 times with NMP, 2 times with DCM, and 2 times with methanol, and directly use for the next step of amino acid coupling.
[0060] Step two,
[0061] 1. Add the piperidine NMP solution with a volume ratio of 1:4 to the Fmoc-L-Lys(Boc)-CTC resin for deprotection reaction. After the reaction, the product is washed 3 times with NMP, 2 times with methanol, and 2 times with DCM 2 times.
[0062] 2. Weigh 6.07g Fmoc-Pro-OH, 2.43g HOBt, 6.83g HB...
Embodiment 2
[0072] Such as figure 1 As shown, the preparation method claimed in the present invention comprises the following steps:
[0073] step one,
[0074] 1. Put 10 g of WANG resin with a loading capacity of 1.0 mmol / g in the reactor, add NMP to wash twice, and then use NMP to swell for 1 h.
[0075] 2. Weigh 28.8g Fmoc-L-Lys(Boc)-OH, add 80ml NMP to dissolve, measure 4.67mL DIC to react for 2 hours, add it to the reactor, add 62mg DMAP to start the reaction.
[0076] 4. React for 6 hours, finish the reaction, wash with NMP for three times, measure 3.8ml of acetic anhydride and 3.3ml of pyridine and mix them in 70ml of NMP, add them into the reactor and react for 2 hours, wash with NMP for 3 times, DCM for 2 times, methanol for 2 times, directly used in the next step of amino acid coupling.
[0077] Step two,
[0078] 1. Add the piperidine NMP solution with a volume ratio of 1:4 to the Fmoc-L-Lys(Boc)-WANG resin for deprotection reaction. After the reaction, wash with NMP for 3 ...
Embodiment 3
[0089] Compared with Example 2, the only difference is that the solid-phase synthetic resin in this example is HMPA resin with a loading amount of 0.1 mmol / g.
[0090] The coupling agent used in step 1 was DIC / HOBt; the reaction solvent was NMP / DMF, the amount of DMAP was 12.5 mg, and the reaction time was 1 h. The coupling agents used in step 2 were HOAt and HATU, the organic base was DIPEA, the amount of the corresponding protected amino acid and coupling agent was 1.5 times the amount of resin loading, and the reaction time was 10 h. In step 3, the lysis reagent is TFA / thioanisole / anisole / EDT with a volume ratio of 90 / 5 / 3 / 2.
[0091] The purity of the product obtained in this example is 63%, and the synthesis yield is 48%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com