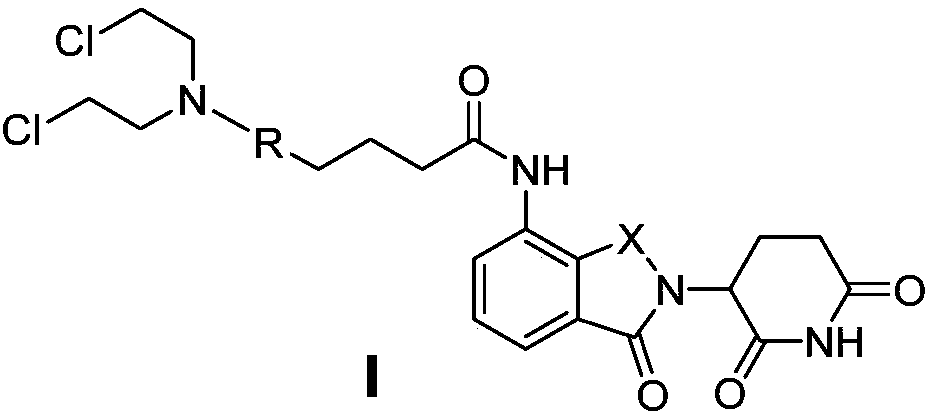
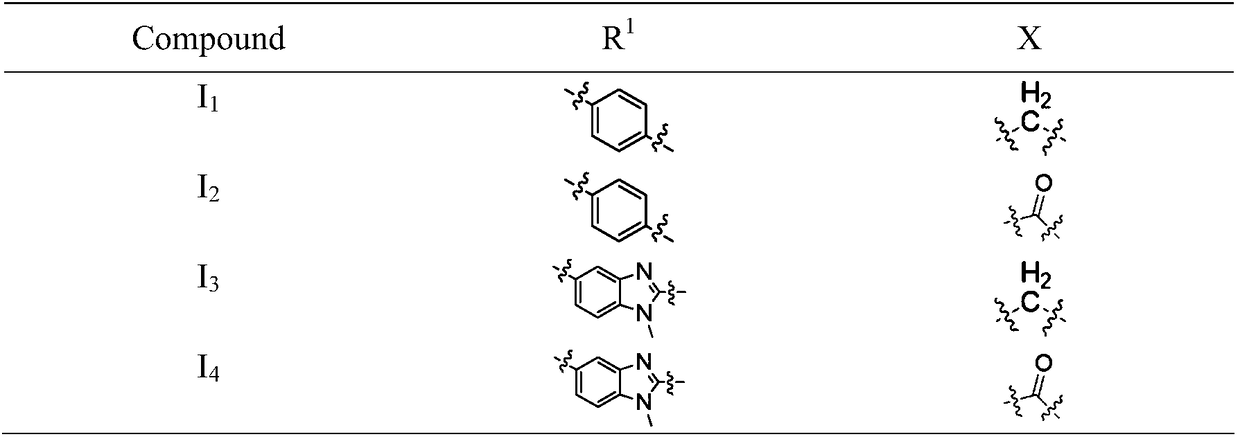
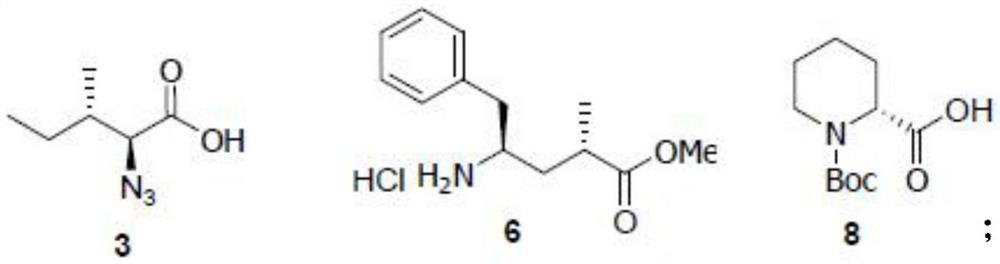
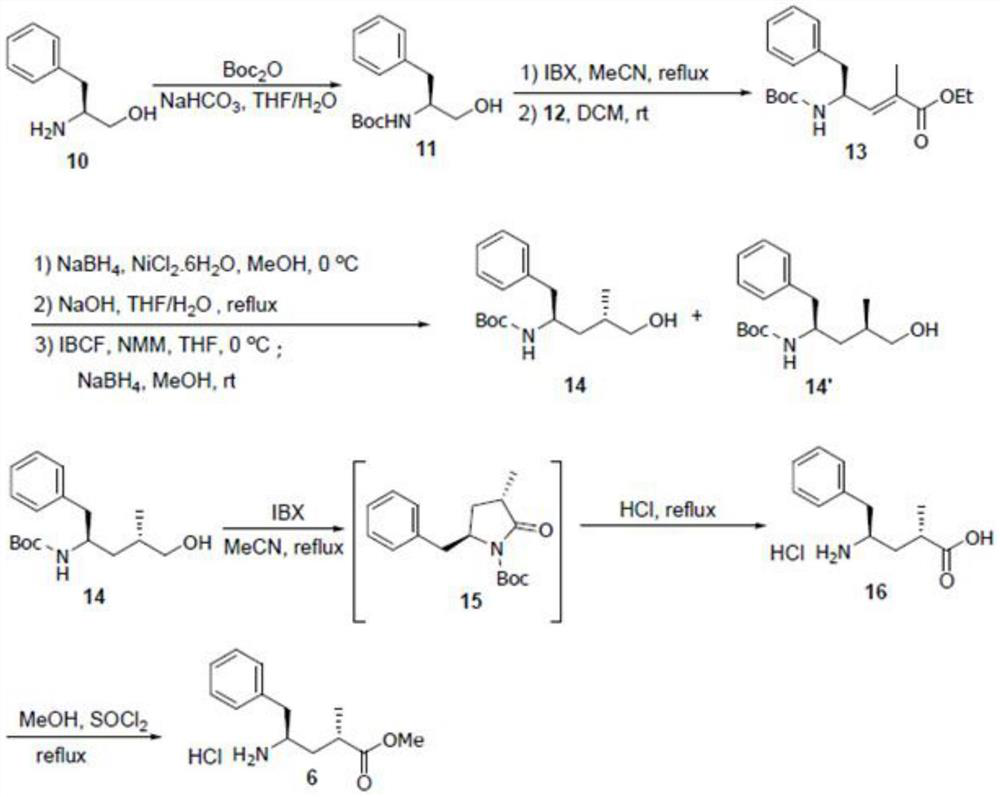
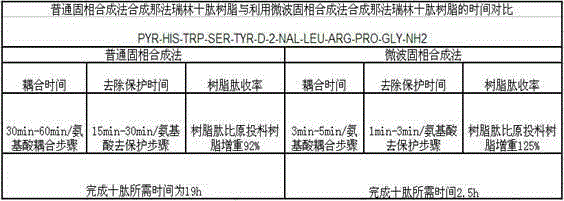
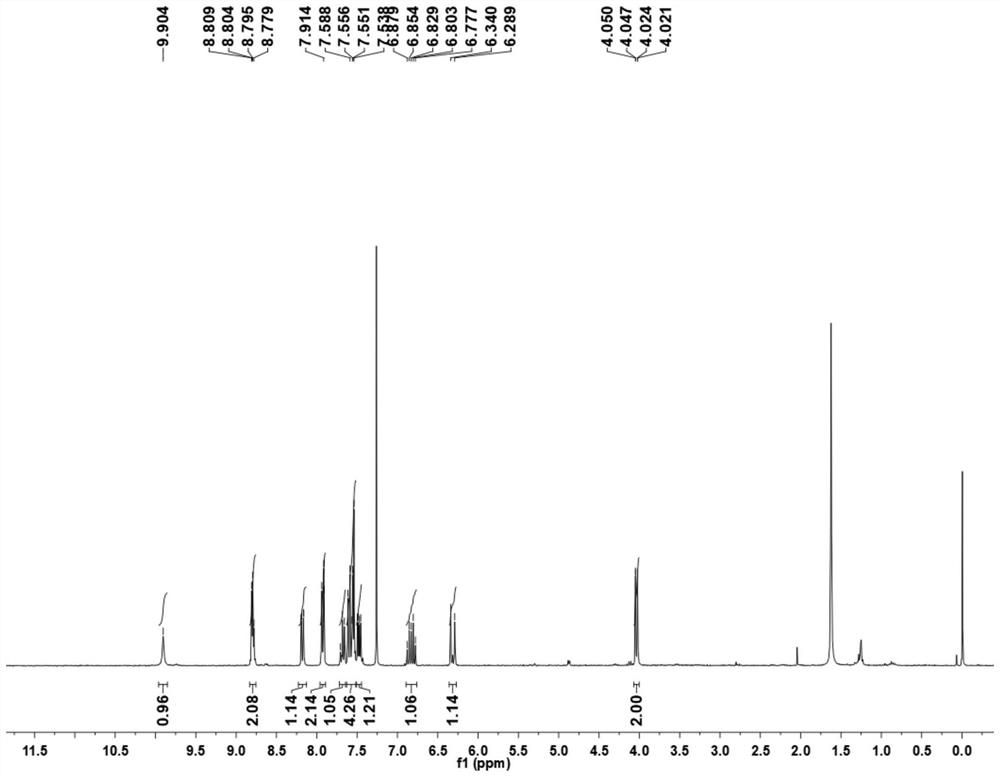
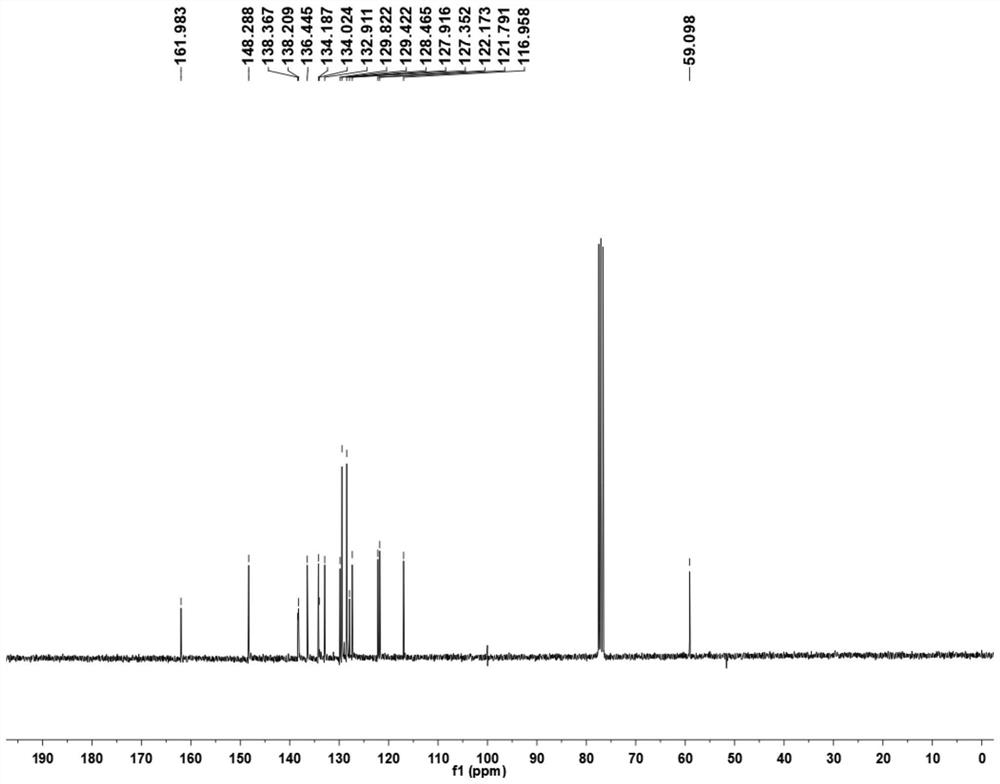
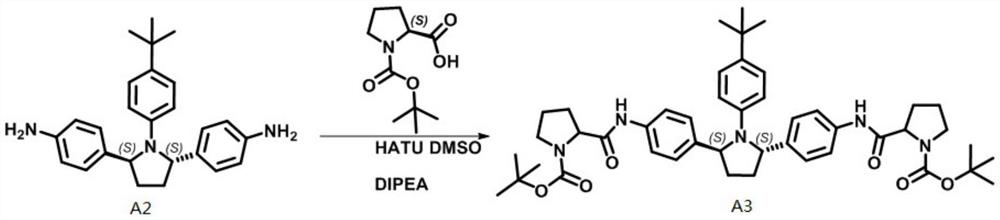
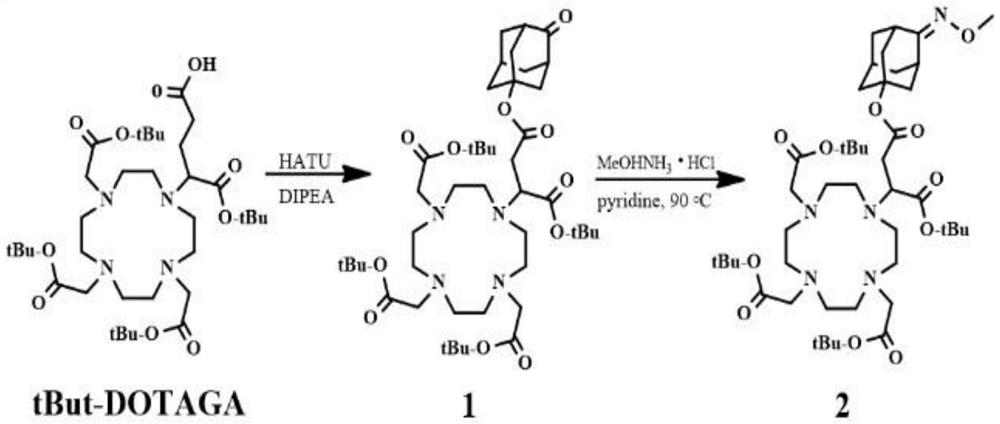
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "HATU" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
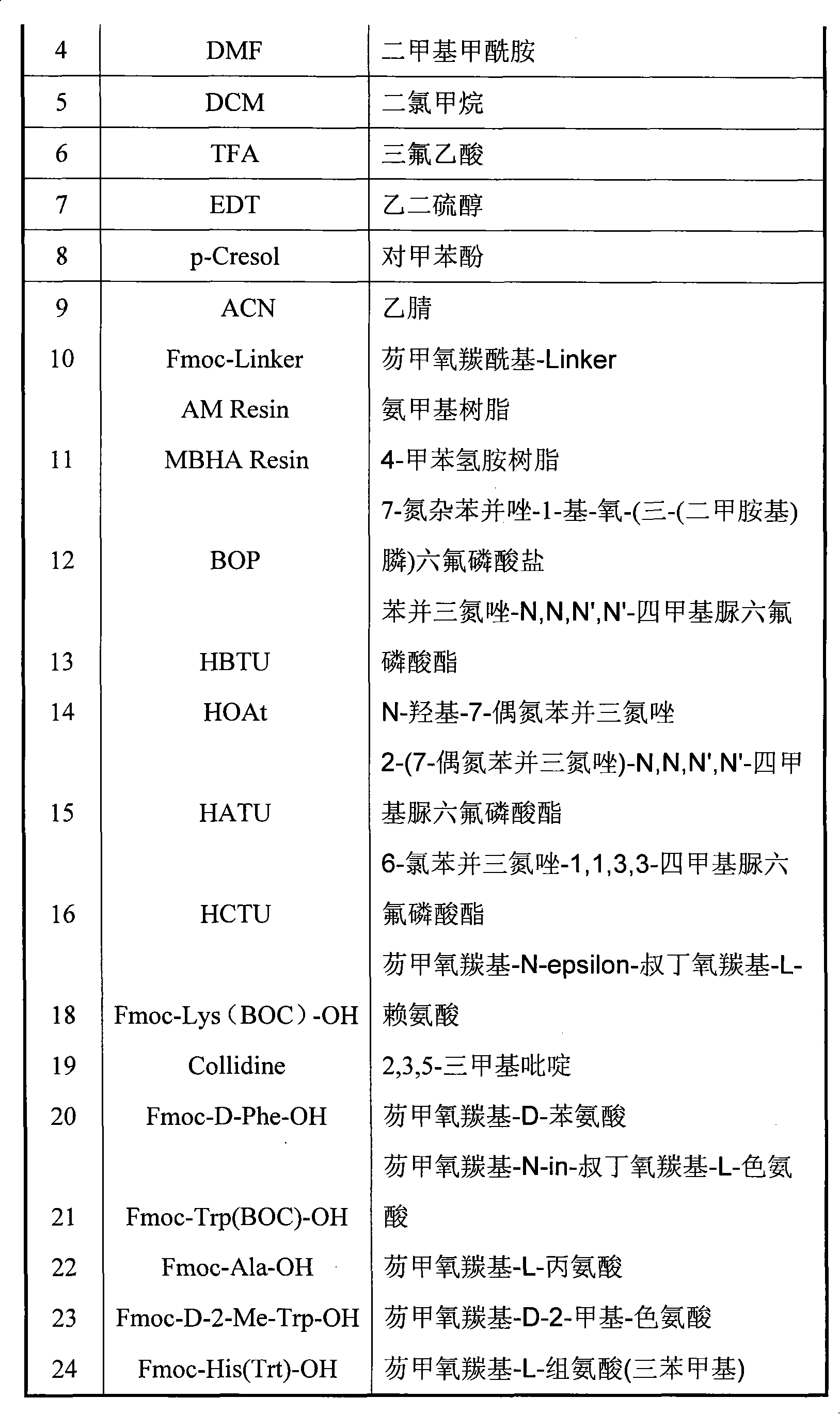
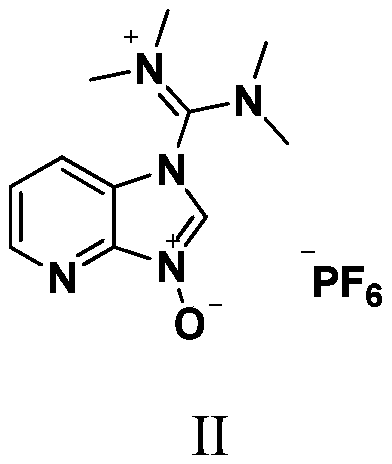
HATU (1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate, Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium) is a reagent used in peptide coupling chemistry to generate an active ester from a carboxylic acid. HATU is used along with Hünig's base (N,N-diisopropylethylamine, DIPEA), or triethylamine to form amide bonds. Typically DMF is used as solvent, although other polar aprotic solvents can also be used.
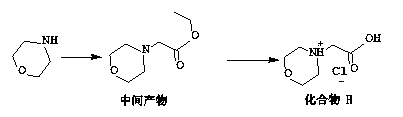
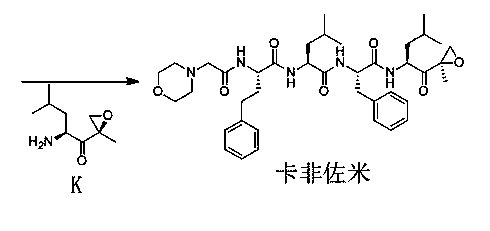
Preparation method for carfilzomib
ActiveCN104086624AShort reaction timeFeeding is simplePeptidesTemperature controlProcess engineering
The invention discloses a preparation method for carfilzomib. The method utilizes HATU as a condensing agent to perform condensation and comprises a plurality of steps. The method has the characteristics of short reaction time, simple feeding, no need for nitrogen protection, appropriate control the feeding temperature, no need for strict temperature control, and easier washing and removal of the by-product of HATU, greatly shortens the preparation time, and improves the work efficiency, thus being suitable for industrial production.
Owner:河南海汇药物研究有限公司
Preparation method of organic electrode material based on polyimide structure
ActiveCN108091861AEvenly dispersedParticipate in the improvement of the efficiency of the charging and discharging processCell electrodesSecondary cellsHATUCharge discharge
The invention relates to a preparation method for an organic electrode material based on a polyimide structure. The preparation method comprises the steps: under the nitrogen protection, dispersing anorganic conductive agent into a solvent, adding HATU and diamine monomer to perform stirring reaction and filtering, washing and vacuum drying to obtain an amination conductive agent; ultrasonicallydispersing into a solvent, then sequentially adding diamine monomer, dianhydride monomer and a catalyst to perform stirring reaction and cooling, filtering, washing and vacuum drying to obtain powder;performing thermal treatment under the inert atmosphere to obtain the organic electrode material based on the polyimide structure. When the organic electrode material is utilized as a lithium ion battery anode material, the electrochemical characteristics of high magnification and high cyclic stability are achieved. By means of the preparation method, an efficiency of active matters in the electrode material to participate a charge-discharge process is improved; thus, industrial production cost is reduced, and very large potential and industrial value are achieved.
Owner:DONGHUA UNIV
Cyclo(Phe-Pro-lle-Phe-Pro-Pro-Leu-Val)peptide preparation method
InactiveCN107540728ARaw materials are easy to getEasy to separate and purifyPeptide preparation methodsElutionHATU
The invention discloses a cyclo(Phe-Pro-lle-Phe-Pro-Pro-Leu-Val)peptide preparation method. The preparation method comprises 1) contacting a matrix resin, Fmoc-Phe-OH and DIPEA in a solvent for a reaction to obtain a primary resin, removing Fmoc through methanol end blocking and elution and carrying out piperidine deprotection to obtain a secondary resin, 2) carrying out continuous modification onthe secondary resin orderly with a plurality of modified amino acids multiple times to obtain a tertiary resin, wherein each modification reaction process comprises that the resin and the modified amino acids contact and undergo reactions in the presence of DIPEA, a solvent and HATU, removing Fmoc through elution and carrying out piperidine deprotection, 3) removing the resin group in the tertiary resin through a resin eluent so that linear octapeptide is obtained, and 4) carrying out liquid phase cyclization on the linear octapeptide in the presence of a solvent, PyBOP, HOBt and DIPEA to obtain cyclopeptide. The preparation method realizes synthesis of cyclopeptide.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
Aryl nitrogen mustard-domide conjugate and preparation method and pharmaceutical application thereof
InactiveCN108409718AImprove stabilityPromotes treatment toleranceOrganic active ingredientsOrganic chemistryDrug conjugationHATU
The invention discloses an aryl nitrogen mustard-domide conjugate and a preparation method and pharmaceutical application thereof. Compound (1) is subjected to reaction with compound (2) in the presence of polypeptide condensing agents EDCI and DMAP, or HATU and DIPEA to obtain compound I that is used to prepare antitumor drugs. The two drugs of different target types are combined herein to form aprodrug; multi-action synergic antitumor effect is achieved; antitumor activity and tolerance are improved; drug resistance of tumors is decreased. The domide drugs are linked via amido bonds, so that chances for amino groups on domide benzene rings to be degraded by in-vivo enzymes can be slimmed, and in-vivo bioavailability of the drugs is improved.
Owner:NANTONG UNIVERSITY
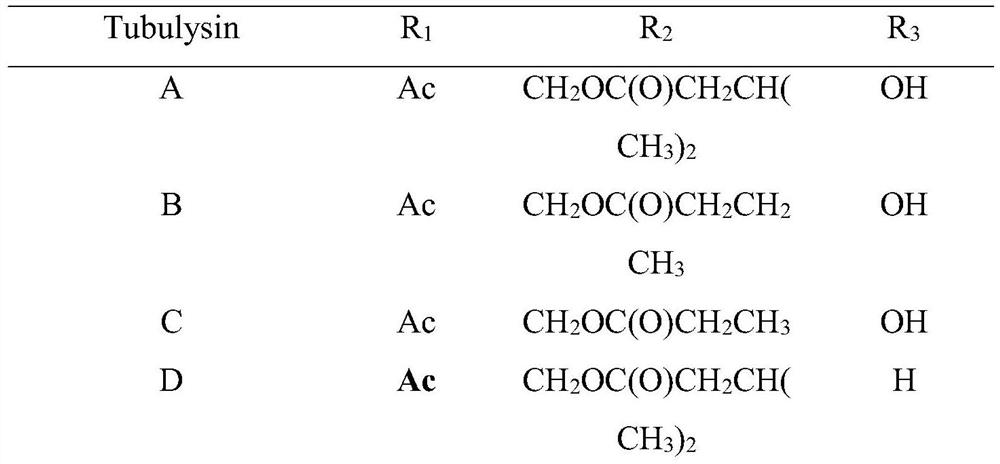
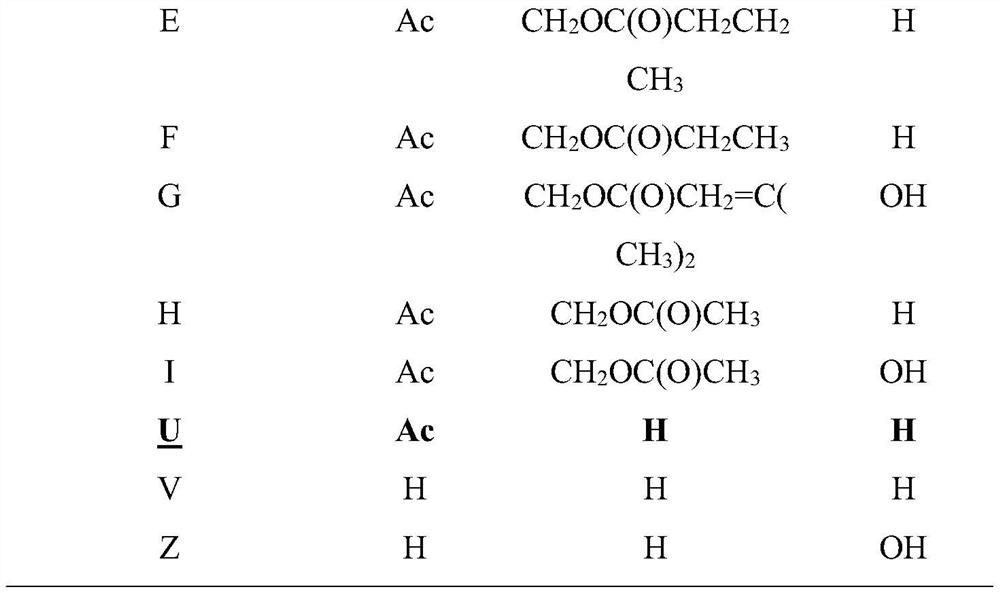
Preparation method of natural active polypeptide Tubulysin U
ActiveCN111647040AOptimizing the Total Synthesis ProcessLow toxicityPeptide preparation methodsAcetic anhydrideEthyl group
The invention provides a preparation method of a novel natural active polypeptide Tubulysin U. The preparation method comprises the following steps: dissolving a compound 2 in trifluoroacetic acid, heating under reflux to prepare an intermediate, reacting with a compound 3 and diisopropylethylamine to obtain a product, reacting the product with 2, 6-dimethylpyridine and tert-butyldimethylsilyltrifluoromethanesulfonate, adding sodium hydroxide after the reaction to prepare an intermediate acid, reacting the intermediate acid with a compound 6, HATU and diisopropylethylamine to obtain a product,adding triphenylphosphine to prepare an intermediate amine, adding a compound 8 and HATU to react, adding ammonium fluoride to prepare a first intermediate, adding sodium hydroxide to the first intermediate to prepare a second intermediate, adding acetic anhydride to the second intermediate to prepare a third intermediate, adding trifluoroacetic acid to the third intermediate to prepare a fourthintermediate, and adding formaldehyde and sodium cyanoborohydride to the fourth intermediate to react, thereby obtaining the target product. The structures of the compound 2, compound 3, compound 6 and compound 8 are disclosed in the specification.
Owner:SHENZHEN ELDERLY MEDICAL RES INST +1
Solid-phase preparation method for exenatide crude product
InactiveCN103122026AEfficient solid phase synthesisEasy Solid Phase SynthesisHormone peptidesPeptide preparation methodsBiochemical engineeringGraft reaction
The invention discloses a solid-phase preparation method for an exenatide crude product. The method comprises the following steps of: taking solid-phase synthetic resin as a starting material; sequentially connecting amino acids or polypeptides with Fmoc protecting groups according to a solid-phase synthesis method, so as to obtain the protected nonatriaconta-peptide resin; sequentially removing the Fmoc protecting groups during the process; taking any one of TBTU / HOBt, HBTU / HOBt, BOP / HOBt, HBTU / HOAt, HBTU / HOAt, DIC / HOBt or BOP / HOAt as a condensing agent, and performing peptide grafting reaction to obtain the protected nonatriaconta-peptide resin; and synchronously removing side-chain protecting groups and cutting peptide to obtain the exenatide crude product. The steps have the following characteristics that: 1, the polypeptides with the Fmoc protecting groups are Fmoc-Gly-Gly-OH; and 2, synthesis for residue 20-19 and residue 17-11 is performed by taking HATU / HOBt as a condensing agent.
Owner:SHANGHAI AMBIOPHARM
Method for synthesizing drug polypeptide nafarelin with microwave solid-phase synthesis method
ActiveCN105153286AShorten the timeIncrease response rateLuteinising hormone-releasing hormonePeptide preparation methodsFreeze-dryingSide chain
The invention relates to a method for synthesizing drug polypeptide nafarelin with a microwave solid-phase synthesis method. According to the solid-phase synthesis method, a raw material is sequentially connected with Fmoc (fluorenylmethoxy carbony) protected amino acids through a microwave reaction instrument, protective decapeptide resin is obtained, meanwhile, Fmos protecting groups are removed sequentially, one of DIC / HOBt, DIC / HOAt, BOP / HOBt, BOP / HOAt, HBTU / HOBt, HBTU / HOAt, HATU / HOBt, HATU / HOAt and HCTU / HOBt is taken as a condensing agent for a peptide synthesis reaction, side chain protecting group removing and peptide cutting are performed synchronously after the protective decapeptide resin is obtained, a crude product of nafarelin is obtained, the crude product is separated and purified by a C18 chromatographic column, the nafarelin is obtained and then subjected to freeze drying, and accordingly, a nafarelin acetate or trifluoroacetate product is obtained.
Owner:苏州强耀生物科技有限公司
Liquid-phase synthesis method of snake venom-like tripeptide
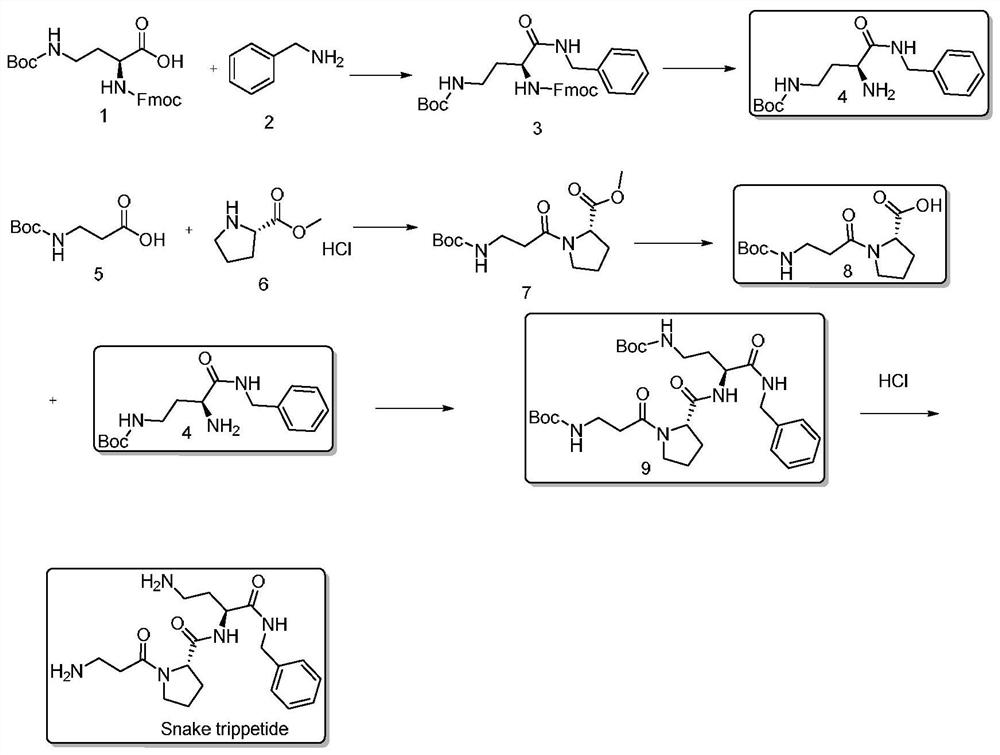
InactiveCN112430253ADoes not involve racemization issuesAvoid racemizationPeptide preparation methodsBulk chemical productionFluid phaseHATU
The invention discloses a liquid-phase synthesis method of snake venom-like tripeptide. The method comprises the following steps: using raw materials Fmoc-Da (Boc)-OH, benzylamine, Boc-beta-Ala-OH andH-Pro-OMe. HCl / H-Pro-OBzl. HCl as raw materials and DIC / HOBt, DIC / HOAt, EDC / HOBT, HATU, HBTU and the like as reagents, synthesizing polypeptide H-beta-Ala-Pro-Dab(Boc)-NHBzl, then cutting off a protective group Boc under an acidic condition and purifying to obtain the product. The product H-beta-Ala-Pro-Dab(Boc)-NHBzl is directly obtained by using a solid-phase synthesis method, the stability problem of CTC resin is solved in the product, the racemization problem of Dab is avoided by adopting a method of connecting Fmoc-Db (Boc)-OH and benzylamine, and the synthesis method is short in processroute, very short in production period, suitable for large-scale production, good in method stability and suitable for large-scale production.
Owner:ZHEJIANG PEPTITES BIOTECH CO LTD
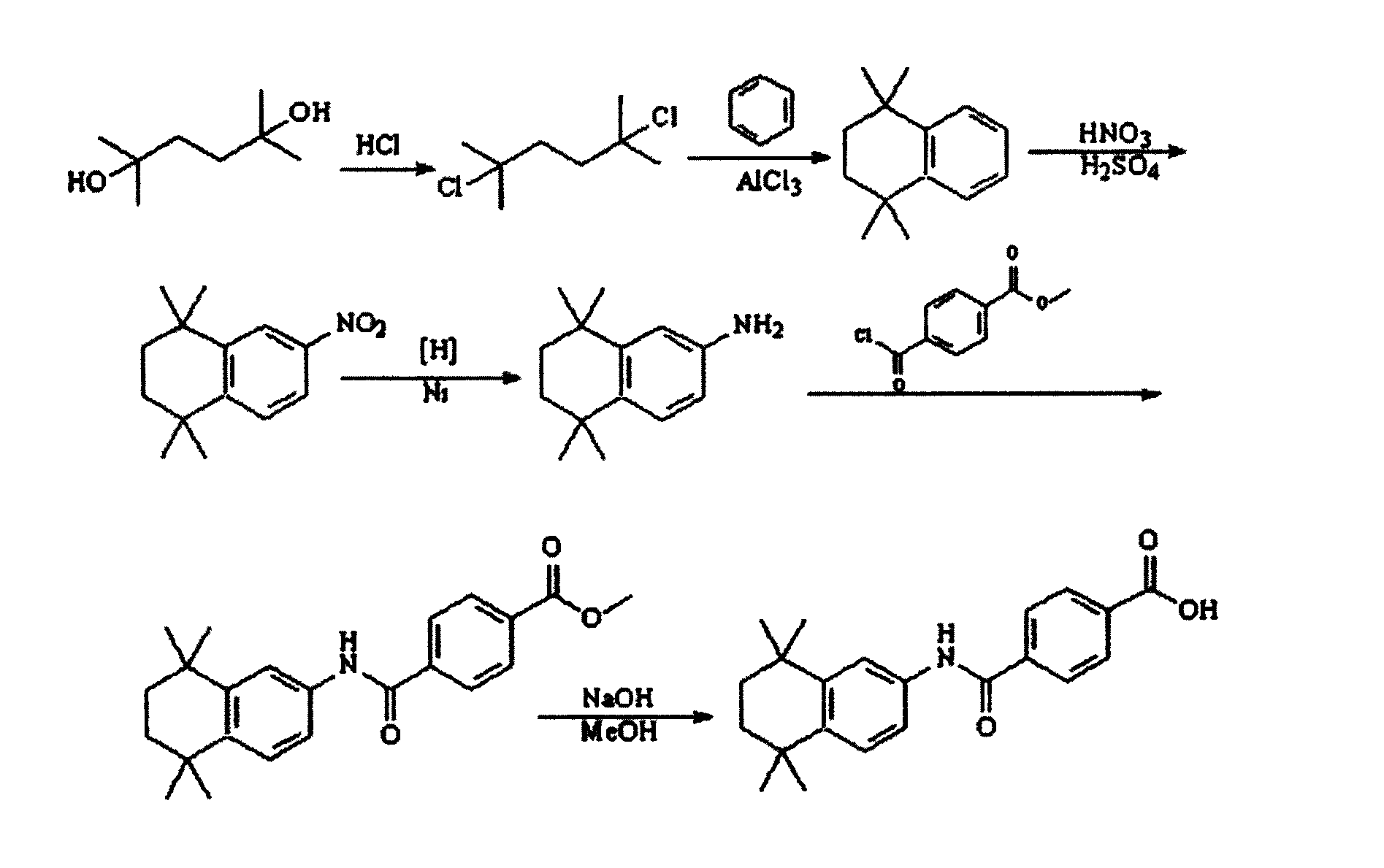
Synthesis method of tamibarotene
InactiveCN102633673AInhibit productionAvoid it happening againOrganic compound preparationCarboxylic acid amides preparationAlkaneSynthesis methods
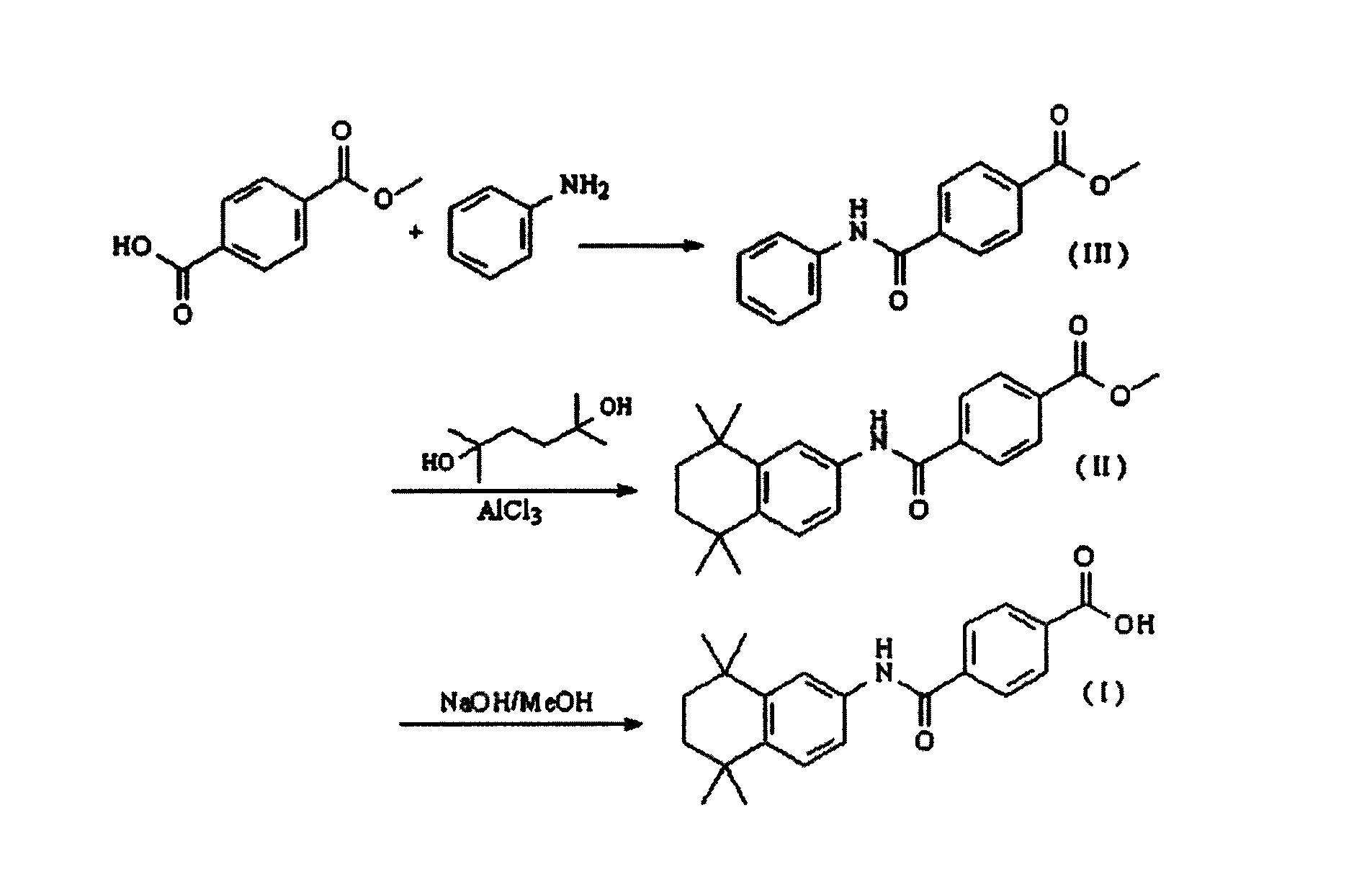
The invention relates to a synthesis method of tamibarotene, which comprises the steps of: 1)taking aniline and monomethyl ester terephthalate as raw materials, and synthesizing p-carbaniloyl methyl benzoate (III); 2) under the protection of nitrogen in an anhydrous condition, carrying out cyclization on the intermediate III and 2, 5-dimethyl-2, 5-hexanediol at a low temperature, to obtain an intermediate II; and 3) hydrolyzing the intermediate II to obtain the tamibarotene (I), wherein dicyclohexyl carbodiimide (DCC) / hydroxyl benzotriazole (HOBt), diisopropylcarbodiimide (DIC) / HOBt, HATU or HBTU taken as a condensing agent as well as triethylamine and diisopropylethylamine (DIEA) taken as acid-binding agents can be added into the step 1); halogen acid taken as a catalyst is added into the step 2); and the reaction solvent is halogenated alkanes. The synthesis method of the tamibarotene avoids the link with high pollution, thus reducing the environmental cost.
Owner:SHANGHAI MEDPEP
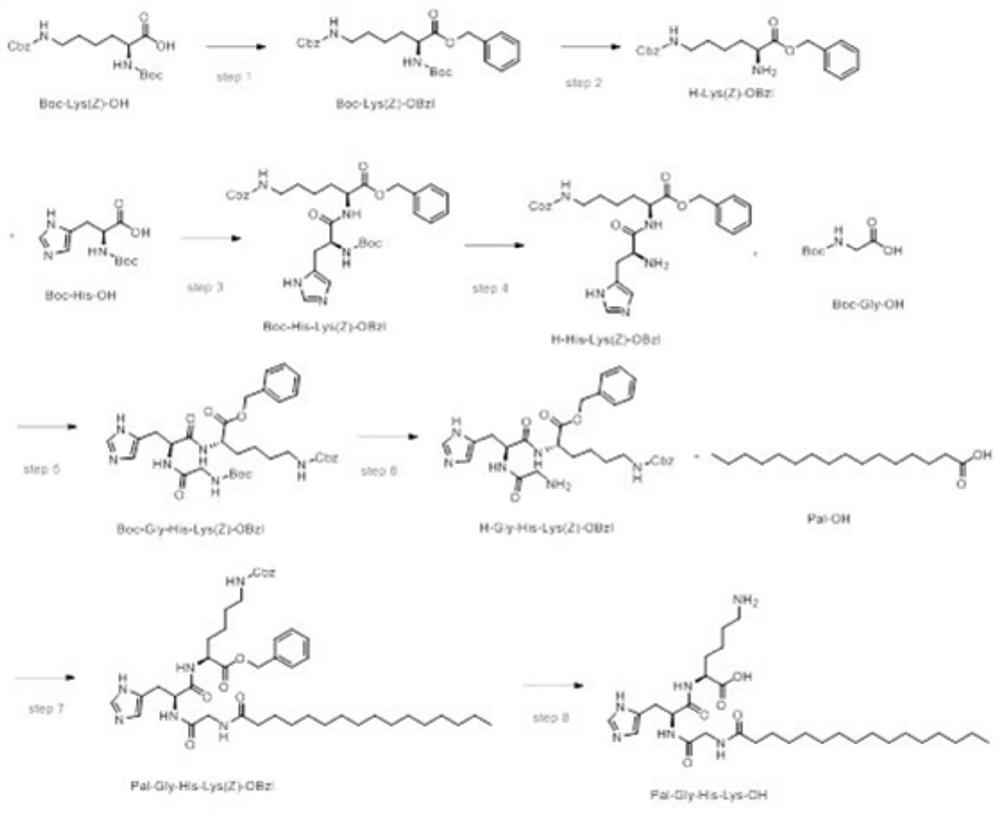
Liquid-phase synthesis method of palmitoyl tripeptide-1
InactiveCN112409444AReduce usageFix stability issuesPeptide preparation methodsBulk chemical productionFluid phaseBiochemical engineering
The invention discloses a liquid-phase synthesis method of palmitoyl tripeptide-1. The method comprises the following steps: synthesizing polypeptide Pal-Gly-His-Lys-OH by taking raw materials Boc-Lys(Z)-OH, MS-OH, Boc-His-OH and Boc-Gly-OH as raw materials and taking DIC / HOBt, DIC / HOAt, EDC / HOBt, HATU, HBTU and the like as reagents; then cutting off a protecting group Boc under an acidic condition; and purifying to obtain a product. The product is obtained by the liquid-phase synthesis method disclosed by the invention; the product is obtained by utilizing simple and conventional reactions in a process; each step of reaction is easy to detect and control; a key intermediate is easy to separate and store; and after hydrogenation, a high-purity product can be obtained without purification.The liquid-phase synthesis method is suitable for industrial large-scale production.
Owner:ZHEJIANG PEPTITES BIOTECH CO LTD
Preparation method of N-phenyl bis (trifluoromethanesulfonyl) imine
ActiveCN113880733ANo wasteHigh purityGroup 5/15 element organic compoundsSulfonic acid amide preparationImideOrganic solvent
The invention relates to a preparation method of N-phenyl bis (trifluoromethanesulfonyl) imine, and belongs to the technical field of chemical engineering. The method comprises the following steps: dissolving trifluoromethanesulfonic acid and organic alkali in an organic solvent, and adding HATU for reaction; the reaction temperature is greater than or equal to 15 DEG C, and the organic solvent is not boiled; after the reaction is finished, a reaction solution containing trifluoromethanesulfonic acid active ester is obtained; adding aniline into the reaction liquid, and reacting at 25 + / -5 DEG C for 6-12 hours; and after the reaction is finished, removing the organic solvent to obtain a crude product containing the N-phenyl bis (trifluoromethanesulfonyl) imine, washing, and recrystallizing and purifying by using an alcohol solvent with 1-3 carbon atoms to obtain the N-phenyl bis (trifluoromethanesulfonyl) imine. The N-phenyl bis (trifluoromethanesulfonyl) imine with high purity and high yield can be prepared by the method, the reaction condition is mild, the utilization rate of raw materials is high, and the method is green and environment-friendly.
Owner:PERIC SPECIAL GASES CO LTD
Method for preparing compound Lifitegrast
InactiveCN111285855ALow priceShort reaction pathOrganic chemistryBiochemical engineeringCombinatorial chemistry
The invention provides a method for preparing a compound Lifitegrast. The method comprises the following steps of under the action of an alkali, carrying out a reaction on a compound V and HATU to generate a compound VI, optionally separating out the compound VI, reacting the compound VI with a compound III, and after the reaction is finished, carrying out post-treatment to obtain the compound Lifitegrast, wherein the reaction equation is defined in the specification. According to the invention, reaction raw materials adopted by the preparation method do not need to be subjected to protectionand deprotection steps, so that the preparation method is short in reaction route, high in reaction efficiency, low in price of the reaction raw materials and suitable for industrial production; and the single-step yield of the reaction is 80% or above, the total yield of the reaction is remarkably increased, and the product purity is 99% or above.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD
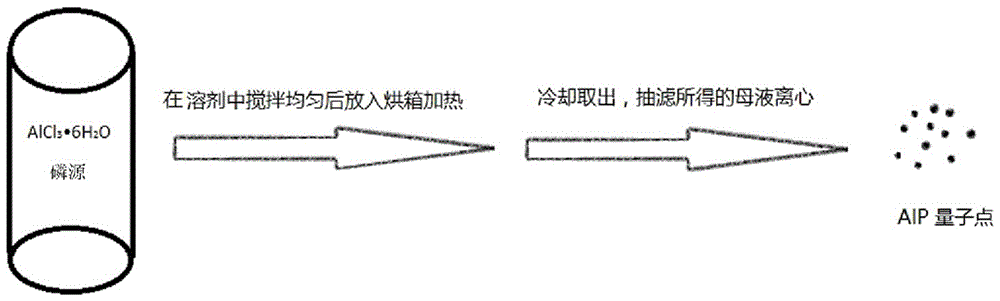
AlP quantum dots and preparing method thereof
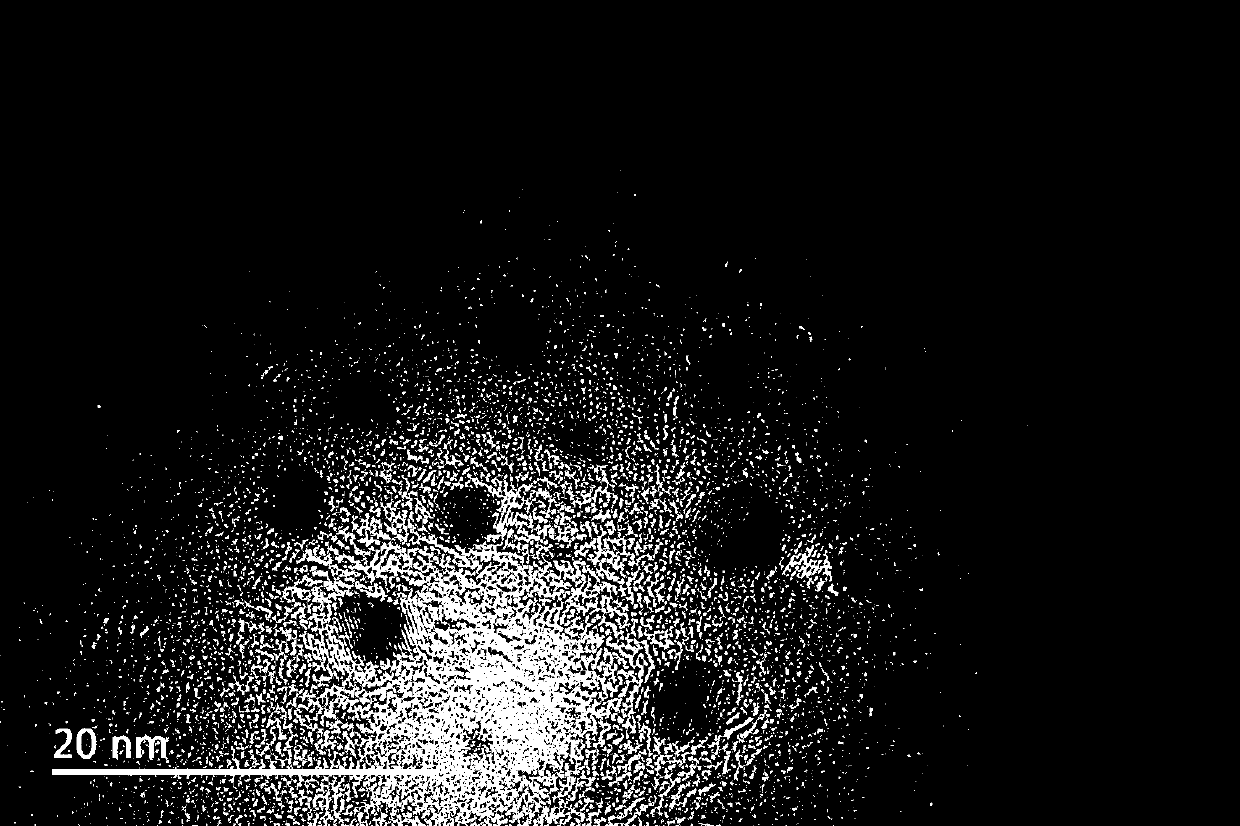
ActiveCN106281321AAvoid complex processEasy to operateNanoopticsLuminescent compositionsSynthesis methodsFluorescence
The invention belongs to the technical field of nano materials, and particularly relates to AlP quantum dots and a preparing method thereof. The preparing method includes the steps that aluminum chloride hexahydrate and a phosphorus source are subjected to solvothermal reaction in organic solvent to obtain the AlP quantum dots which are small in size distribution and high in crystallinity degree and are distributed uniformly, wherein the phosphorus source is selected from one or two of tri-o-methylphenylphosphine, triphenylphosphine, phosphorus pentachloride and HATU and [BMIM]PF6. The synthesis method is easy to operate, mild in reaction and high in repeatability; the obtained quantum dots have strong fluorescence-emission in the blue light region after being irradiated with ultraviolet light, can serve as a blue light emitter applied to a light-emitting diode and can also be applied to biomarking and imaging. In addition, the fluorescence Stock displacement of the quantum is large, interference to transmission signals by exciting light can be effectively avoided, and the quantum dots have extraordinary potential advantages in developing novel optoelectronic devices in the future.
Owner:FUDAN UNIV
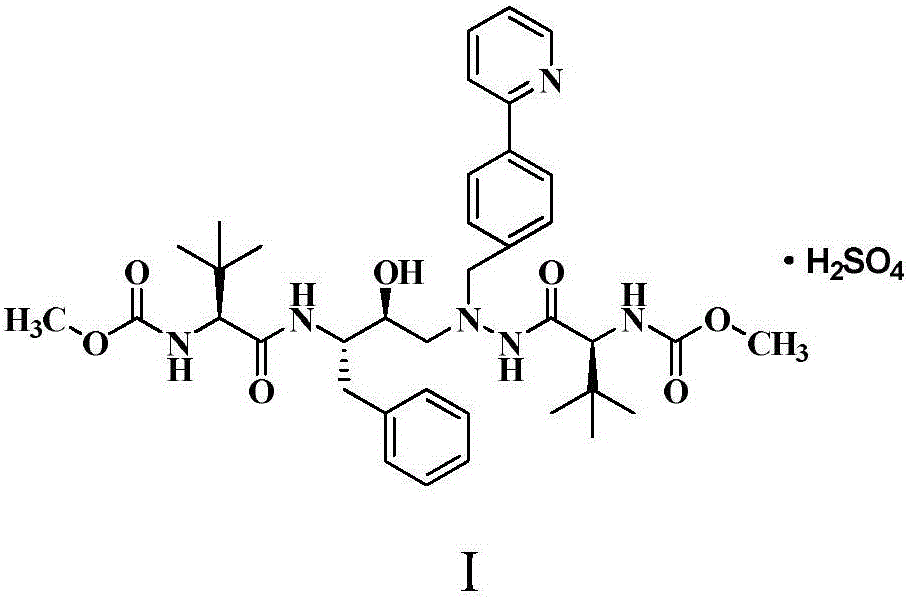
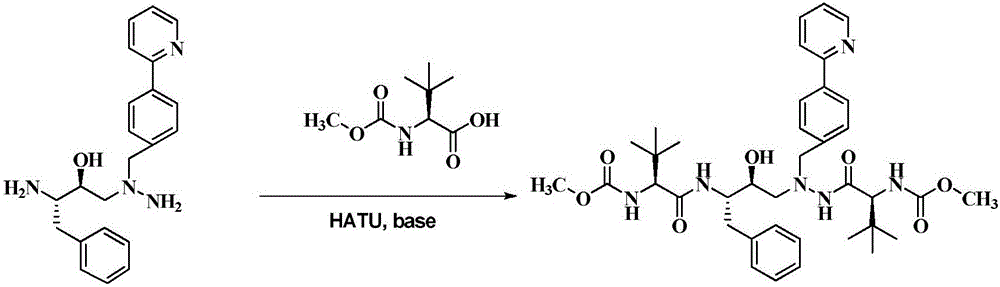
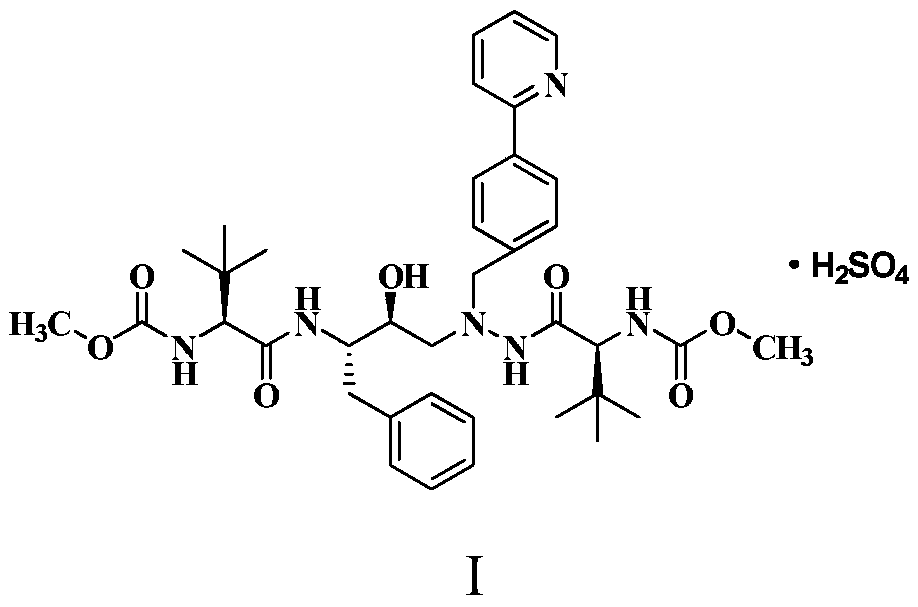
Method for preparing anti-AIDS drug-Atazanavir monomer
The invention discloses a method which is used for preparing an Atazanavir monomer and applied in the technical field of drug synthesis. The method comprises the steps that N-methoxycarbonyl-L-tertiary leucine and 1-[4-(pyridine-2-yl)-phenyl]-4(S)-hydroxyl-5(S)-2,5-diamido-6-phenyl-2-aza-hexane are subjected to an amidation reaction by taking HATU as a condensing agent under the condition of organic alkali and an organic solvent, certain aftertreatment is conducted, and then the Atazanavir monomer is obtained. According to the method, the synthetic yield of Atazanavir is increased, the purity is high, and the cost of raw materials is effectively reduced; meanwhile, the reaction time is short, material putting is easy, nitrogen protection is not needed, the material putting temperature can be properly controlled, by-products of HATU can be washed off more easily, the preparation time is greatly shortened, the working efficiency is improved, and therefore the method is suitable for industrial production.
Owner:NORTHEAST PHARMA GRP
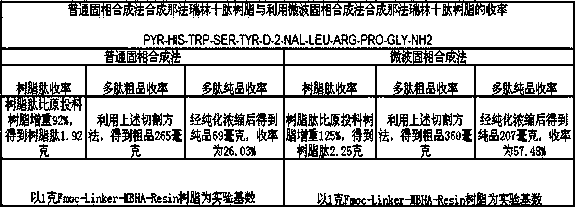
Method for solid phase polypeptide synthesis of hexarelin
ActiveCN101314612BConvenient sourceWith large-scale production capacityPeptide preparation methodsBulk chemical productionSide chainTrifluoroacetic acid
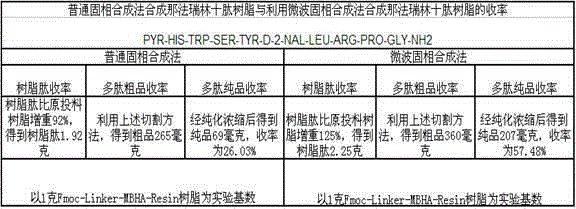
The invention relates to a preparation method of hexarelin, and mainly solves the technical problems with long synthetic period and applications of hypertoxic substances in the prior synthetic method. The method for synthesizing the hexarelin by adopting the solid phase polypeptide synthetic method comprises the steps as follows: Fmoc-Linker-AM-Resin or Fmoc-Linker-MBHA-Resin is adopted as the initial raw material; amino acids with Fmoc protective groups are sequentially connected according to the solid phase polypeptide synthetic method to obtain a protected six peptide resin, while the Fmocprotective groups are sequentially removed and a peptide synthetic reaction is carried out in the presence of one of DIC / HOBt, DIC / HOAt, BOP / HOBt, BOP / HOAt, HBTU / HOBt, HBTU / HOAt, HATU / HOBt, HATU / HOAtor HCTU / HOBt as a condensing agent; side chain protective groups removal and peptide cutting are carried out synchronously to obtain a coarse product; the coarse product is subjected to separation and purification to obtain the hexarelin which is further subjected to lyophilization to obtain hexarelin acetate or trifluoroacetate product. The method is applicable to the large-scale hexarelin production.
Owner:GL BIOCHEM SHANGHAI
A kind of alp quantum dot and preparation method thereof
ActiveCN106281321BAvoid complex processEasy to operateNanoopticsLuminescent compositionsAluminium chlorideFluorescence
The invention belongs to the technical field of nano materials, and particularly relates to AlP quantum dots and a preparing method thereof. The preparing method includes the steps that aluminum chloride hexahydrate and a phosphorus source are subjected to solvothermal reaction in organic solvent to obtain the AlP quantum dots which are small in size distribution and high in crystallinity degree and are distributed uniformly, wherein the phosphorus source is selected from one or two of tri-o-methylphenylphosphine, triphenylphosphine, phosphorus pentachloride and HATU and [BMIM]PF6. The synthesis method is easy to operate, mild in reaction and high in repeatability; the obtained quantum dots have strong fluorescence-emission in the blue light region after being irradiated with ultraviolet light, can serve as a blue light emitter applied to a light-emitting diode and can also be applied to biomarking and imaging. In addition, the fluorescence Stock displacement of the quantum is large, interference to transmission signals by exciting light can be effectively avoided, and the quantum dots have extraordinary potential advantages in developing novel optoelectronic devices in the future.
Owner:FUDAN UNIV
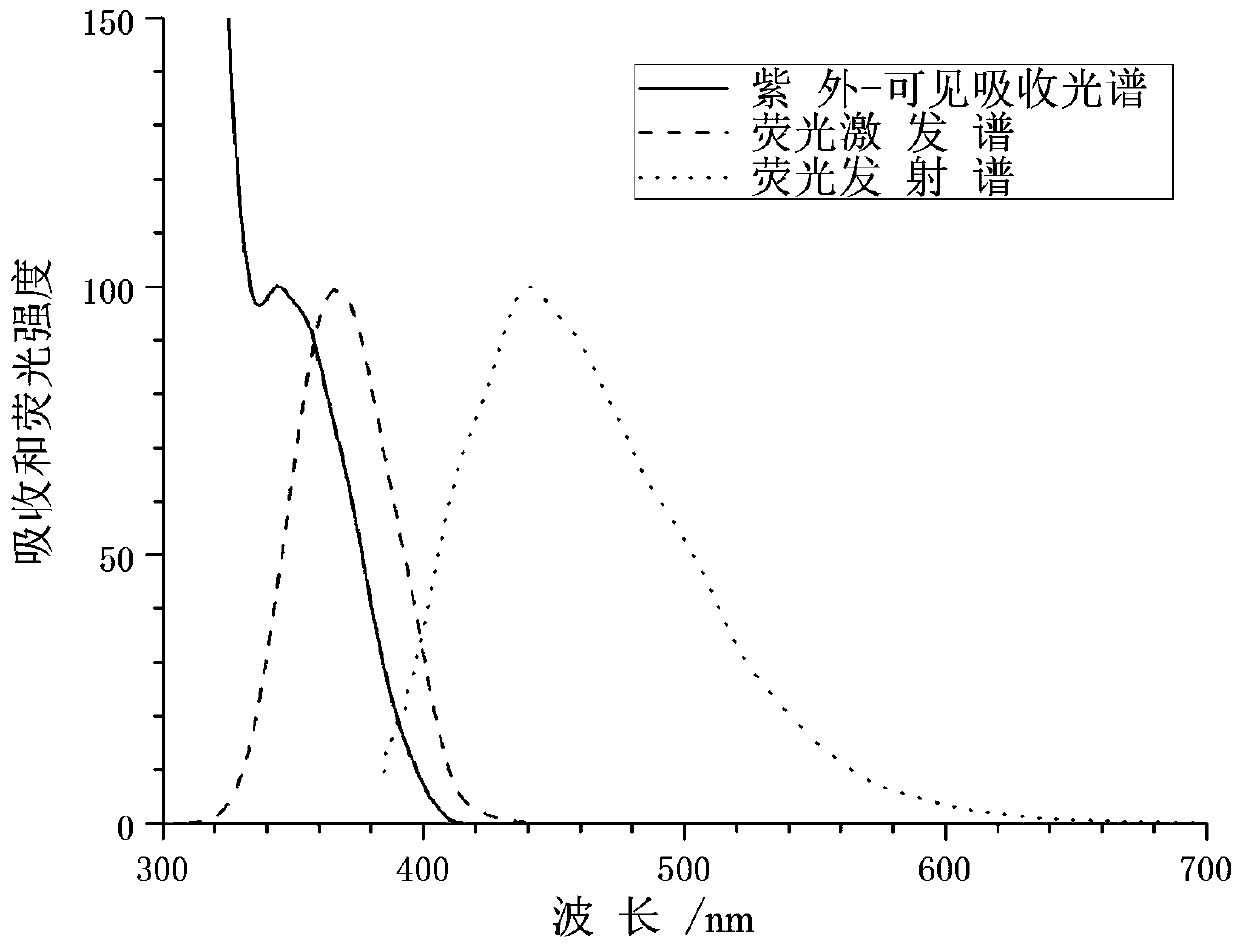
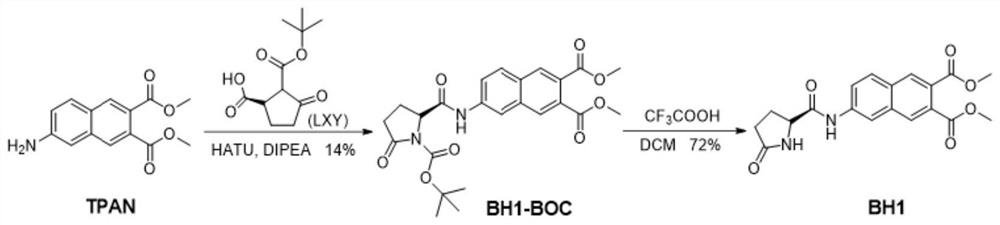
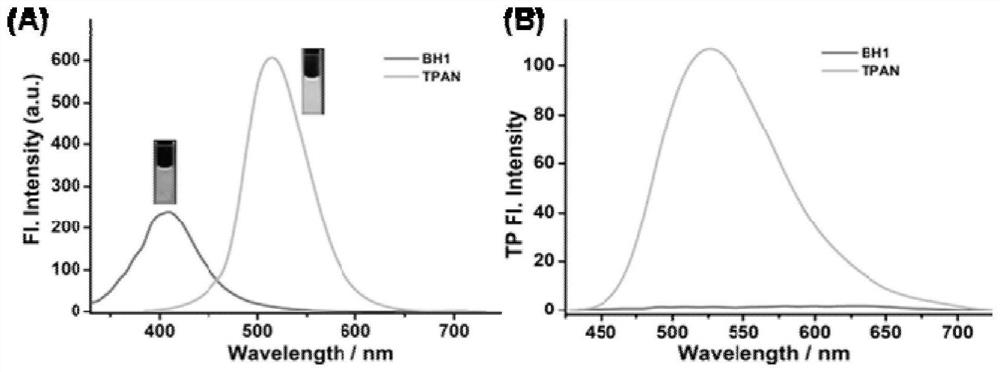
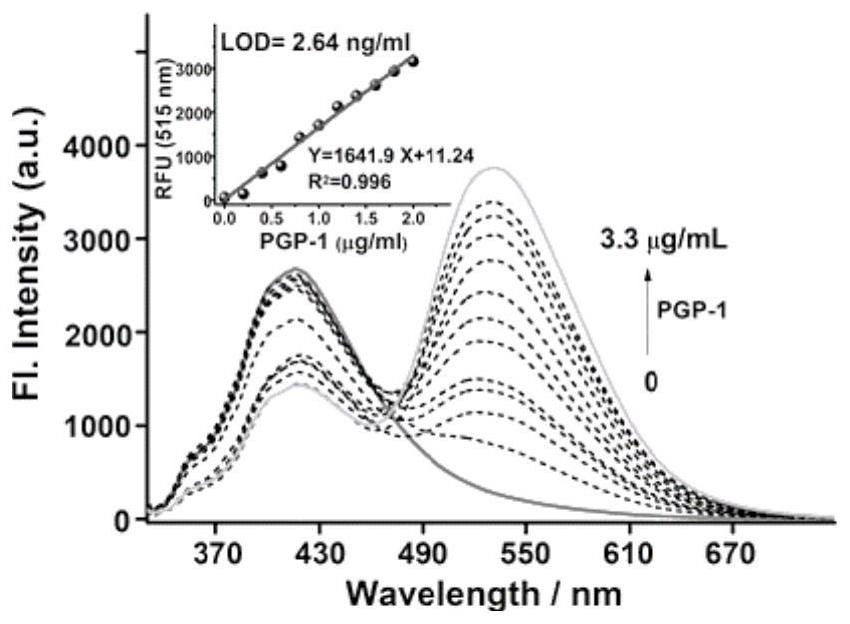
Fluorescent probe for detecting pyroglutamamide aminopeptidase I as well as preparation method and application of fluorescent probe
ActiveCN113845458AExcellent photophysical propertiesGood biocompatibilityOrganic chemistryFluorescence/phosphorescenceFluoProbesAminopeptidase I
The invention discloses a fluorescent probe for detecting pyroglutamamide aminopeptidase I as well as a preparation method and application thereof. The method comprises the following steps: mixing LXY, HATU and DIPEA, completely dissolving the mixture in a dry dichloromethane solution, and carrying out a stirring reaction at 0 DEG C; then adding a TPAN solution, further conducting stirring and reacting at room temperature, and carrying out purifying after the reaction is finished so as to obtain a dark red solid BH1-BOC; dissolving BH1-BOC in anhydrous dichloromethane, adding a prepared trifluoroacetic acid solution under an ice salt bath condition, and carrying out room-temperature stirring reaction after dropwise adding is completed; spin-drying a solvent, adding dichloromethane, and repeating the above operations multiple times until the trifluoroacetic acid is completely spin-dried; and purifying a crude product to obtain a red solid BH1.
Owner:西安天工生物医药研究所有限公司
A kind of preparation method of natural active polypeptide tubulysin U
ActiveCN111647040BOptimizing the Total Synthesis ProcessLow toxicityPeptide preparation methodsAcetic anhydrideEthyl group
The invention provides a method for preparing a novel natural active polypeptide Tubulysin U. In the preparation method, compound 2 is dissolved in trifluoroacetic acid, heated to reflux to prepare an intermediate, and reacted with compound 3 and diisopropylethylamine to obtain The product is reacted with 2,6-lutidine and tert-butyldimethylsilyl trifluoromethanesulfonate. After the reaction, sodium hydroxide is added to treat the intermediate acid. The intermediate acid is mixed with compound 6, HATU and diiso Propylethylamine reaction, the obtained product is added with triphenylphosphine to make intermediate amine, and compound 8 and HATU are added to react, ammonium fluoride is added to make the first intermediate, and the first intermediate is added with sodium hydroxide to make the second intermediate , the second intermediate adds acetic anhydride to prepare the third intermediate, the third intermediate adds trifluoroacetic acid to obtain the fourth intermediate, and the fourth intermediate adds formaldehyde and sodium cyanoborohydride to react to produce the target product; compound 2, The structures of compound 3, compound 6 and compound 8 are:
Owner:SHENZHEN ELDERLY MEDICAL RES INST +1
Preparation method of medicine RIP1183 for resisting multi-drug resistance staphylococcia
ActiveCN107188937AProcess stabilityQuality is easy to controlAntibacterial agentsPeptide preparation methodsSide chainHATU
Disclosed is a preparation method of medicine RIP1183 for resisting multi-drug resistance staphylococcia. Any one of Rink, AmideMBHA Resin, MBHA Resin and Rink Amide-AM Resin is adopted as a starting raw material, on the basis of a solid-phase synthesis method, according to a RIP1183 amino acid sequence, corresponding amino acids with Fmoc protection are connected in sequence, Fmoc protecting groups are removed in sequence in the process, one of DIEA / HOBt or DIEA or TBTU / HOAt / HOBt or HBTU / HOBt or HBTU / HOAt or HATU / HOBt or HATU / HOAt or HCTU / HOBt is adopted as a condensing agent for performing a transpeptidase reaction, aftr corresponding RIP1183 resin is obtained, meanwhile, side chain removal protecting groups and final peptide cut-off are performed, a crude RIP1103 product is obtained, and separation and purification are performed through a C18 or C8 chromatographic column to obtain the needed purified RIP1183 product.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for preparing (E)-4-(benzenesulfonyl) butyl-3-olefine acid
PendingCN114835613AGo greenReduce the impactOrganic compound preparationOrganic chemistry methodsBenzoic acidQuinoline
The invention provides a synthesis method of (E)-4-(benzenesulfonyl) butyl-3-olefine acid, which comprises the following steps: 1) taking 8-aminoquinoline and 3-butenoic acid as raw materials, taking dichloromethane as a solvent, and obtaining a compound 3: N-(quinoline-8-yl) butyl-3-enamine under the action of HATU and 2, 4, 6-trimethylpyridine; 2) taking acetonitrile as a solvent, and reacting the compound 3 with sodium benzenesulfinate under the action of palladium acetate, benzoic acid and silver hexafluoroantimonate to obtain a compound 5: (E)-4-(benzenesulfonyl)-N-(quinoline-8-yl) butyl-2-enamine; and 3) hydrolyzing the compound 5 under the action of an HCl aqueous solution. The method comprises the following steps: carrying out dehydration condensation on 3-butenoic acid and 8-aminoquinoline to obtain amide; under the catalysis of palladium, olefinic bonds in the amide react with sodium benzenesulfinate to obtain allyl sulfone; amido bonds in allyl sulfone are hydrolyzed under the acidic condition, meanwhile, double bonds are shifted, and a target product is obtained and has excellent regioselectivity.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
A kind of preparation method of crude drug olbitasvir
ActiveCN107474105BEasy to separate and purifyHigh yieldDipeptide ingredientsDigestive systemEthyl groupHATU
The invention discloses a preparation method of a bulk drug olbitasvir, which comprises bis Nitro reduction reaction S1, diamino proline peptide coupling reaction S2, pyrrolidine amino deprotection reaction S3, and pyrrolidine amino valine peptide coupling reaction S4. 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride is used as the valine peptide coupling reagent in the preparation method of the raw material drug obitavir, which is disclosed in the prior art Compared with peptide coupling reagents such as EDAC / HOBT, PyBOP, HATU, and T3P, the final product synthesis step has a higher yield, and the separation and purification of the product is easier.
Owner:安徽拜善晟制药有限公司
Synthesis method and application of Fe (II)-based specific MRI contrast agent
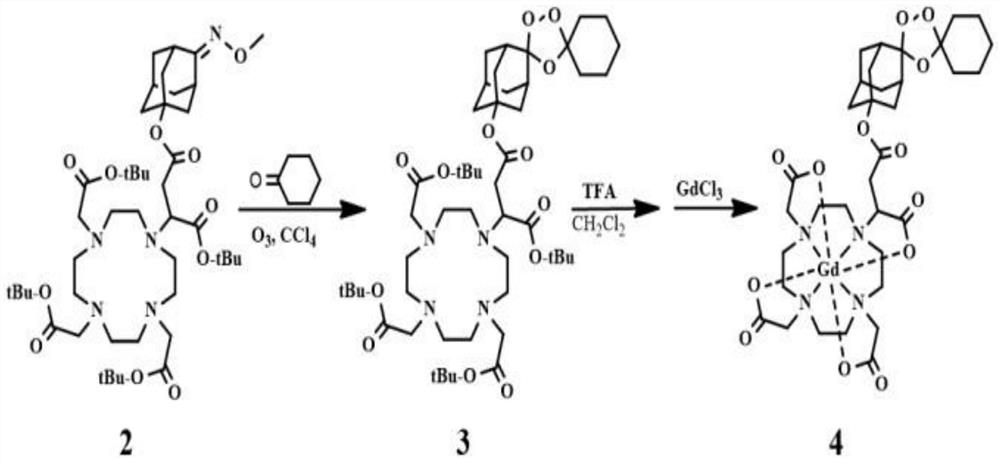
PendingCN114181164AEnabling Quantitative MRI ImagingIncrease concentrationOrganic chemistryAnalysis using nuclear magnetic resonancePhosphoric Acid EstersMeth-
The invention discloses a synthesis method and application of a Fe (II)-based specific MRI contrast agent, and the synthesis method comprises the following steps: carrying out a reaction on tBut-DOTAGA, N, N-diisopropylethylamine DIPEA, 2-(7-azabenzotriazole)-N, N, N ', N'-tetramethylurea hexafluorophosphate HATU and 5-hydroxy-2-adamantanone to synthesize a first compound; performing reaction synthesis on the first compound, a pyridine solution and methoxy ammonia hydrochloride to obtain a second compound; introducing ozone gas into the second compound under an anaerobic condition, and carrying out column separation to obtain a third compound Fer-DOTA; and stirring the third compound and trifluoroacetic acid (TFA) for reaction, adjusting the pH value, adding gadolinium chloride GdCl3 for stirring reaction, and performing high performance liquid chromatography separation to obtain the Fe (II)-based specific MRI contrast agent. Gd-DOTA is covalently bound to a Fe (II) selective group through a chemical bond, so that the Gd-DOTA has Fe (II) selective enrichment capacity, Fe (II) responsive enrichment of Gd-DOTA is realized, and the contrast agent concentration and T1WI signal intensity of a Fe (II)-rich tissue are improved in a targeted manner, so that spatial and temporal distribution of Fe (II) in an ICH edema region is established, and quantitative MRI imaging of Fe (II) is realized.
Owner:ZHONGNAN HOSPITAL OF WUHAN UNIV
A method for preparing anti-AIDS drug atazanavir monomer
A method for preparing atazanavir monomers applied in the technical field of pharmaceutical synthesis, using HATU as a condensation agent under organic base and organic solvent conditions, N-methoxycarbonyl-L-tert-leucine and 1- [4‑(pyridine‑2‑yl)‑phenyl]‑4(S)‑hydroxy‑5(S)‑2,5‑diamino‑6‑phenyl‑2‑azahexane undergoes an amide reaction, After certain post-processing, atazanavir monomer is obtained. The method of the invention improves the synthesis yield of atazanavir, has high purity, and effectively reduces the cost of raw materials. At the same time, the reaction time is short, the feeding is simple, no nitrogen protection is required, and the feeding temperature can be properly controlled. The by-products of HATU are easier to be washed and removed, which greatly shortens the preparation time and improves work efficiency. It is suitable for industrial production.
Owner:NORTHEAST PHARMA GRP
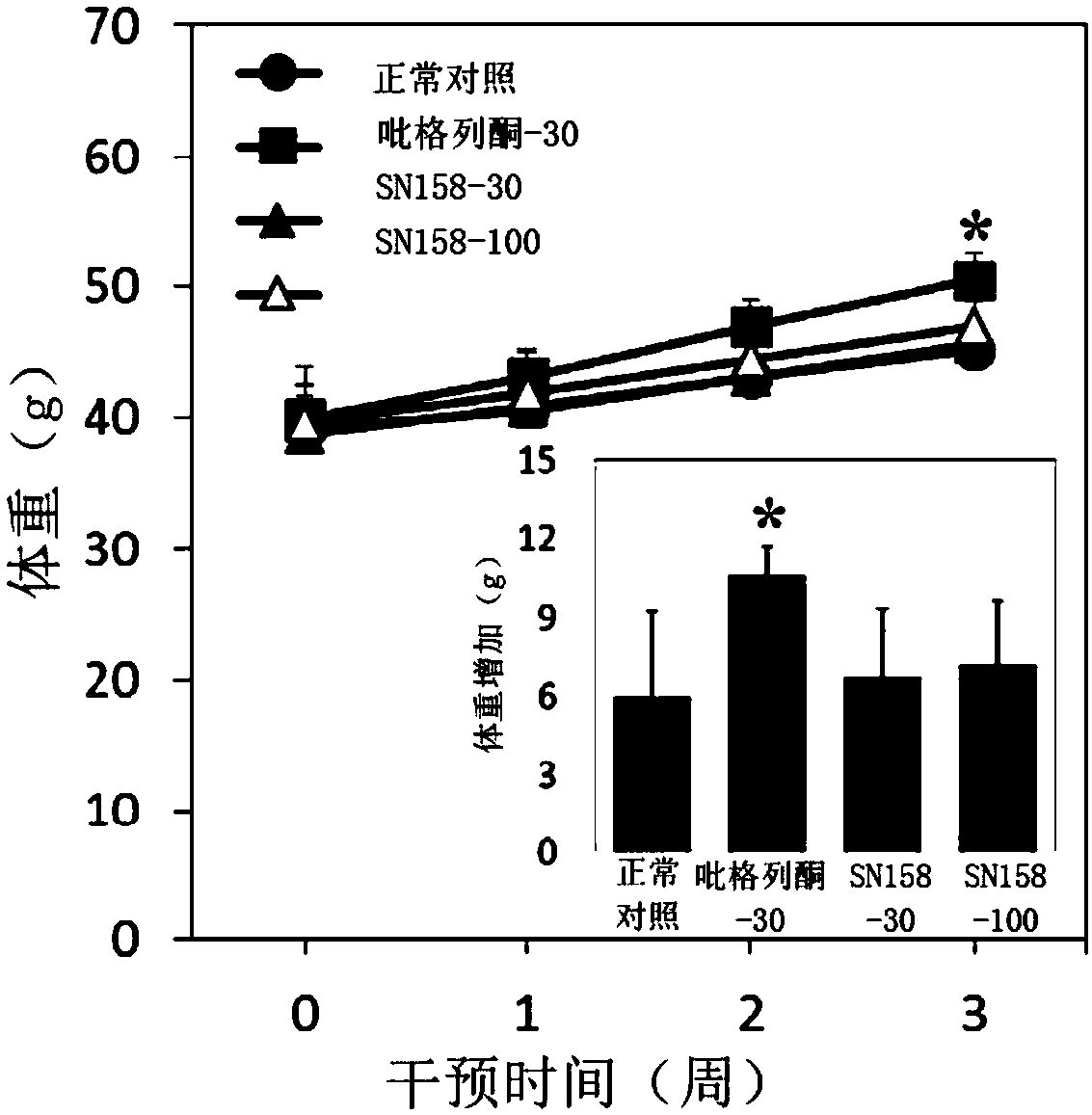
Synthetic route of compound and application thereof in field of preparation of anti-diabetic drugs
ActiveCN110937987ASolve yieldSolve complexityOrganic active ingredientsOrganic compound preparationOrtho positionHATU
The invention belongs to the technical field of drug synthesis, and particularly relates to a synthetic route of a compound and application of the compound in the field of preparation of anti-diabeticdrugs. The invention provides a synthetic route of SN158, and also provides the application of the synthetic route in the field of preparation of anti-diabetic drugs. The synthetic route of the invention comprises the following steps: brominating a raw material, namely 2,4-dihydroxybenzaldehyde; protecting para-hydroxyl groups by using methoxymethyl groups; methylating ortho-hydroxyl groups by using dimethyl sulfate or methyl iodide; and carrying out a Claisen-Schmidt condensation reaction by using a hydrochloric acid ethanol or boron trifluoride diethyl ether solution, with HATU or EDCI andHOBt as coupling agents for an amidation reaction. According to the invention, the yield of the prepared SN158 can reach 60% or above; meanwhile, column chromatographic purification is not needed in the preparation process, and the finally produced compound with a purity of 99% or above can be obtained only through recrystallization; and the method is suitable for large-scale / industrial productionand overcomes the technical defects that SN158 is low in synthesis yield and complex in purification in the prior art.
Owner:SHENZHEN LINGLAN BIO PHARMA TECH CO LTD
A method for synthesizing drug polypeptide nafarelin by microwave solid-phase synthesis
ActiveCN105153286BConvenient sourceReduce usageLuteinising hormone-releasing hormonePeptide preparation methodsFreeze-dryingSide chain
The invention relates to a method for synthesizing drug polypeptide nafarelin with a microwave solid-phase synthesis method. According to the solid-phase synthesis method, a raw material is sequentially connected with Fmoc (fluorenylmethoxy carbony) protected amino acids through a microwave reaction instrument, protective decapeptide resin is obtained, meanwhile, Fmos protecting groups are removed sequentially, one of DIC / HOBt, DIC / HOAt, BOP / HOBt, BOP / HOAt, HBTU / HOBt, HBTU / HOAt, HATU / HOBt, HATU / HOAt and HCTU / HOBt is taken as a condensing agent for a peptide synthesis reaction, side chain protecting group removing and peptide cutting are performed synchronously after the protective decapeptide resin is obtained, a crude product of nafarelin is obtained, the crude product is separated and purified by a C18 chromatographic column, the nafarelin is obtained and then subjected to freeze drying, and accordingly, a nafarelin acetate or trifluoroacetate product is obtained.
Owner:苏州强耀生物科技有限公司
A kind of metal-organic framework composite material grafted with ionic liquid and its preparation method and application
ActiveCN111229320BRaw materials are easy to getGood catalyticOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCycloadditionMetal-organic framework
Owner:LIAONING UNIVERSITY
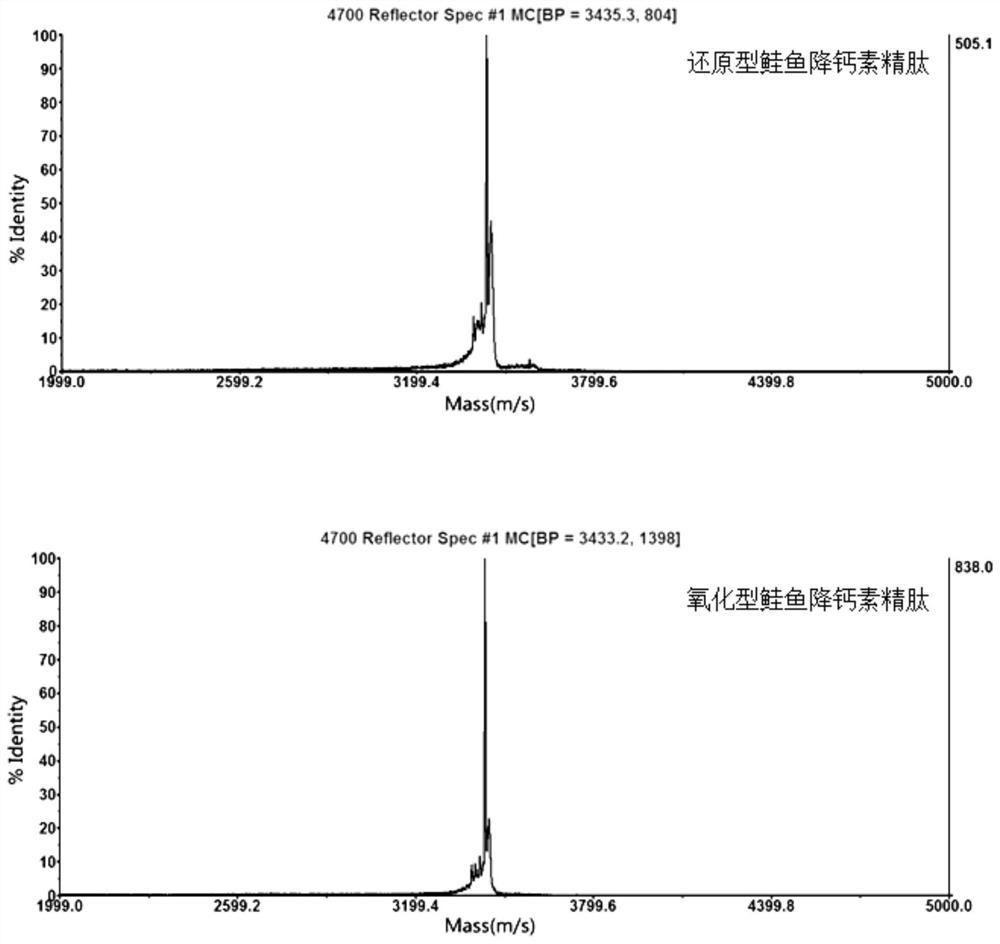
A kind of preparation method of pure solid phase synthesis salmon calcitonin
ActiveCN111793125BHigh yieldShort reaction timeCalcitoninsPeptide preparation methodsAmino acid synthesisRecombinant salmon calcitonin
The invention discloses a preparation method for solid-phase synthesis of salmon calcitonin, which comprises (1) preparing calcitonin deprotected docosopeptide resin, (2) preparing reduced salmon calcitonin crude product, (3) compounding (4) Separation and purification step, characterized in that: said step (1) prepares calcitonin-deprotected docosopeptide resin specifically as follows: amino resin is used as starting material, and amino acid protected by Fmoc is used as monomer , using HOBT / HATU as condensation reagent, take off the Fmoc protecting group sequentially in the polypeptide synthesis column, couple corresponding amino acids one by one, obtain deprotected thirty-docosopeptide resin; wherein, in the amino acid coupling step, according to each amino acid According to the degree of difficulty of synthesis, different coupling times are designed. In the present invention, by analyzing the difficulty of amino acid synthesis and changing the coupling time, the synthesis efficiency can be improved, the product purity can be increased, and the production cost can be reduced.
Owner:湖南甲骨文生物医药有限公司
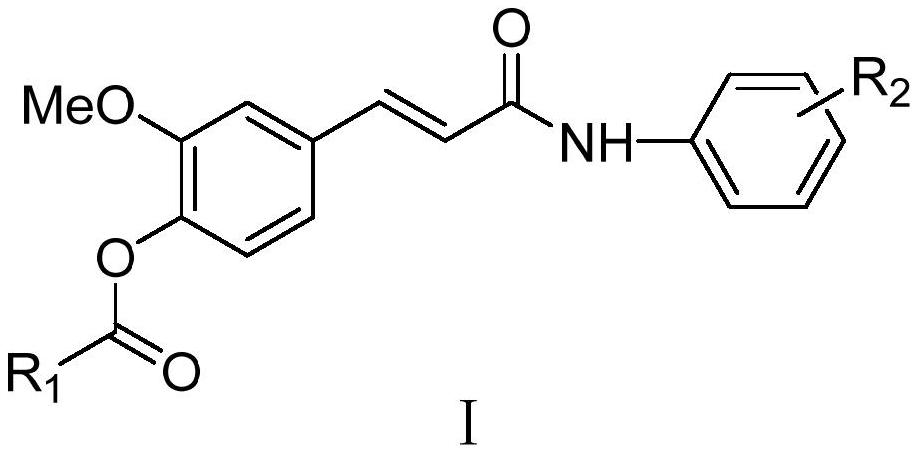
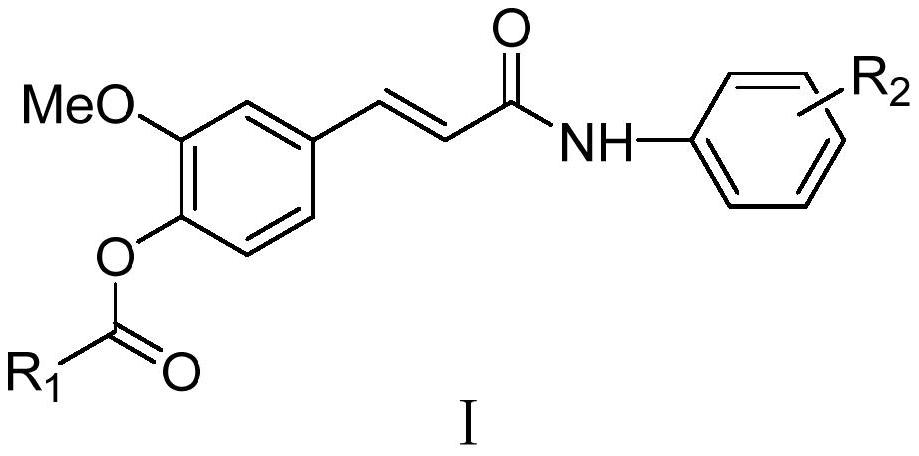
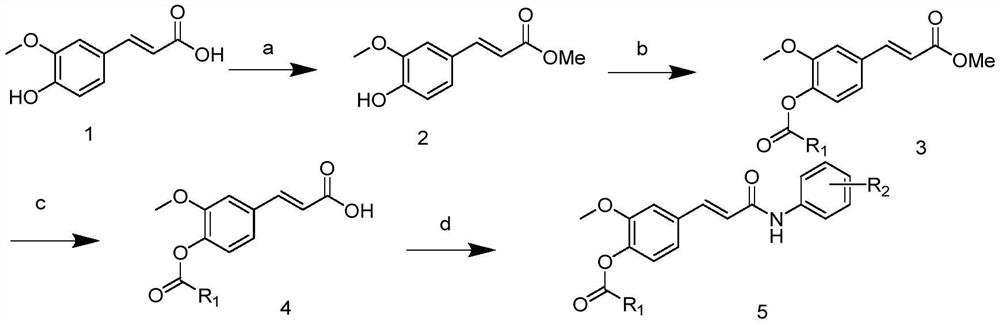
Ferulic acid derivative as well as preparation method and application thereof
PendingCN114591202AMild reaction conditionsEasy to repeatNervous disorderCarbamic acid derivatives preparationCarbamateLithium hydroxide
The invention discloses a ferulic acid derivative as well as a preparation method and application thereof, specifically, ferulic acid is taken as a raw material, ferulic acid and methanol are subjected to reflux under the condition of concentrated sulfuric acid to obtain ferulic acid methyl ester, the ferulic acid methyl ester reacts with different substituted carbamoyl chloride to generate a ferulic acid carbamate substituted derivative, and the ferulic acid carbamate substituted derivative is obtained. Further removing a methyl protecting group under the action of lithium hydroxide to expose a carboxyl terminal, and finally reacting with different substituted aniline under the actions of HATU and DIPEA to generate the target ferulic acid derivative. In-vitro cholinesterase activity tests are carried out on the synthesized ferulic acid derivative, most of the compounds have inhibitory activity on cholinesterase, and the compound is an ideal cholinesterase-resistant chemical entity and has important significance in treatment of Alzheimer's disease.
Owner:XIAN MEDICAL UNIV
Oat bran phenolic amide alkaloid, preparation method thereof and application of oat bran phenolic amide alkaloid in preparation of antipruritic products
PendingCN114539092AInhibition of hypersecretionLess scratchesOrganic active ingredientsCosmetic preparationsCaffeic acidHATU
The invention relates to the technical field of medicinal chemistry, and particularly discloses oat bran phenolic amide alkaloid, a preparation method thereof and application of the oat bran phenolic amide alkaloid in preparation of itching relieving products. The oat bran phenolic amide alkaloid has a structure as shown in a formula I or a formula II. The preparation method of the oat bran phenolic amide alkaloid specifically comprises the following steps: taking a reaction substrate caffeic acid analogue, adding an organic solvent, adding 4-(2-aminoethyl) phenol and triethylamine while stirring, and adding HATU after 3-6 minutes; after charging is finished, continuously reacting for 3-6 hours at room temperature, and monitoring disappearance of the reaction raw materials by TLC (thin layer chromatography); and stopping stirring, carrying out reduced pressure distillation on the reaction system to remove the solvent, and carrying out silica gel column chromatography purification on residues to obtain the oat bran phenolic amide alkaloid. Researches show that the oat bran phenolic amide alkaloid or the composition thereof disclosed by the invention has an excellent itching relieving effect; therefore, the oat bran phenolic amide alkaloid or the composition thereof can be used for developing antipruritic products.
Owner:深圳海创生物科技有限公司
ADC linker preparation method
ActiveCN113527418AReduce generationHigh yieldPeptide preparation methodsPeptides with abnormal peptide linkPtru catalystHydrogen atmosphere
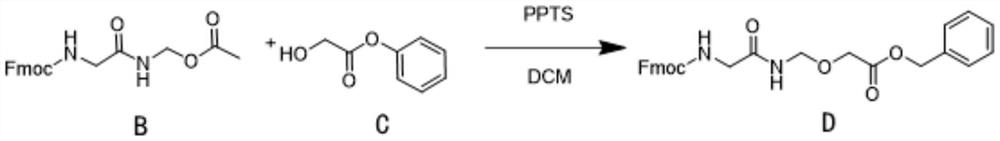
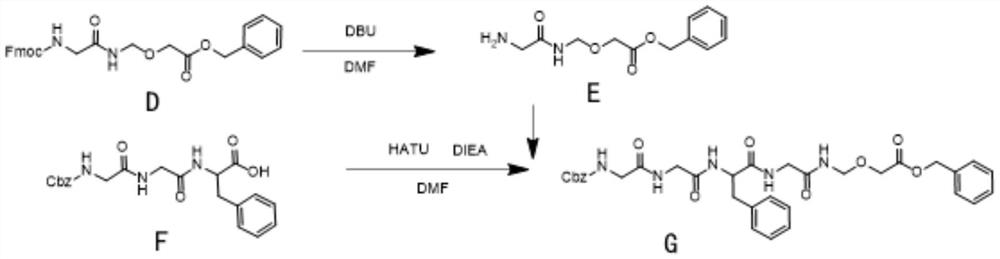
The invention relates to an ADC linker preparation method, which comprises: 1) using A as a raw material, using copper acetate as a catalyst, carrying out N2 replacement, adding Pb (OAc) 4, and carrying out a heating reaction to obtain B; 2) adding a compound B, a compound C and PPTS into DCM, and performing reflux overnight to obtain D; (3) adding a compound D into DMF (Dimethyl Formamide), stirring and dissolving, adding DBU, stirring and removing Fmoc to obtain a solution containing E; adding F, DIEA and HATU into DMF, adding the solution containing E after stirring, continuing the reaction, and obtaining G; 4) adding DMF (Dimethyl Formamide) and Pd / C into the compound G, carrying out hydrogenolysis in a hydrogen atmosphere, and sequentially carrying out operations such as crystallization and the like on a product in the DMF to obtain H; 5) adding DMF, H, I and DIEA at the same time, adjusting pH after stirring reaction is completed, and obtaining ADC linker through purification. The preparation method is simple and easy to implement and has few impurities, and a high-purity product can be obtained by crystallizing the product finally.
Owner:成都普康唯新生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com