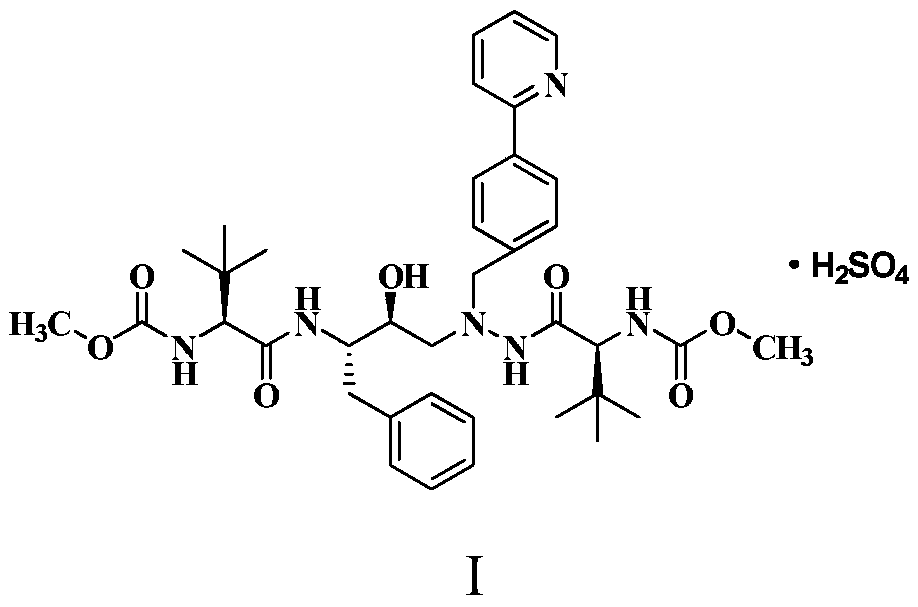
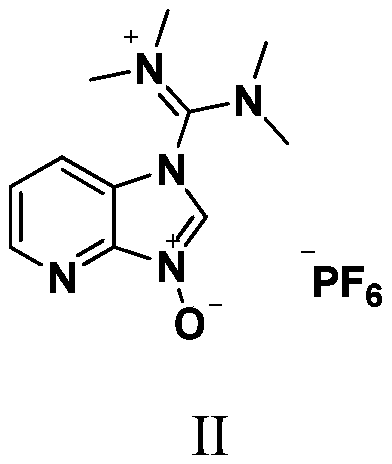
A method for preparing anti-AIDS drug atazanavir monomer
An anti-AIDS and atazanavir technology, applied in the field of preparing atazanavir monomer, can solve the problems of eye and skin irritation, complex separation and purification of products, unsuitable for industrial production, etc., to achieve easy separation and purification, less pollution , Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment one: the preparation of atazanavir monomer
[0023] At 42°C, 1-[4-(pyridin-2-yl)-phenyl]-4(S)-hydroxy-5(S)-2,5-diamino-6-phenyl-2-nitrogen 36.2g (100mmol) of heterohexane, 41.6g (220.0mmol) of N-methoxycarbonyl-L-tert-leucine, 83.6g (220mmol) of HATU and 71.2g of pyridine were placed in a 500mL eggplant-shaped bottle, and 200mL Stir isopropyl acetate for 1.5 h, TLC detection, the reaction is complete, the reaction solution was washed with saturated brine and purified water in turn 200mL×2, the water layer was discarded, and the organic phase was added to anhydrous magnesium sulfate to dry for 6 hours and then filtered, and then the filtrate Heat to 35°C, add 0.3g activated carbon for decolorization, stir for 20min, cool to 20-30°C and filter, concentrate the organic phase in vacuo to obtain oily atazanavir monomer crude product, add 300mL isopropanol to the above oil, and then Heat to reflux to make it completely dissolved, then add 300mL of n-hexane dropwis...
Embodiment 2
[0024] Embodiment two: the preparation of atazanavir monomer
[0025]At 35°C, 1-[4-(pyridin-2-yl)-phenyl]-4(S)-hydroxy-5(S)-2,5-diamino-6-phenyl-2-nitrogen 36.2g (100mmol) of heterohexane, 37.8g (200.0mmol) of N-methoxycarbonyl-L-tert-leucine, 76.0g (200mmol) of HATU and 71.2g of pyridine were placed in a 500mL eggplant-shaped bottle, and 200mL Methyl isobutyl ketone was stirred for 1 hour, and detected by TLC. After the reaction was completed, the reaction solution was washed with 10% potassium dihydrogen phosphate and purified water in turn to 200mL×2, the water layer was discarded, and the organic phase was dried by adding anhydrous sodium sulfate for 6 hours. Filtrate, then heat the filtrate to 50°C, add 0.4g injection carbon 767 to decolorize at the same time, stir for 20min, cool to 20-30°C and filter, concentrate the organic phase in vacuo to obtain oily atazanavir monomer crude product, pour into the above oil Add 300mL of ethanol, then heat to reflux at 80°C to disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com