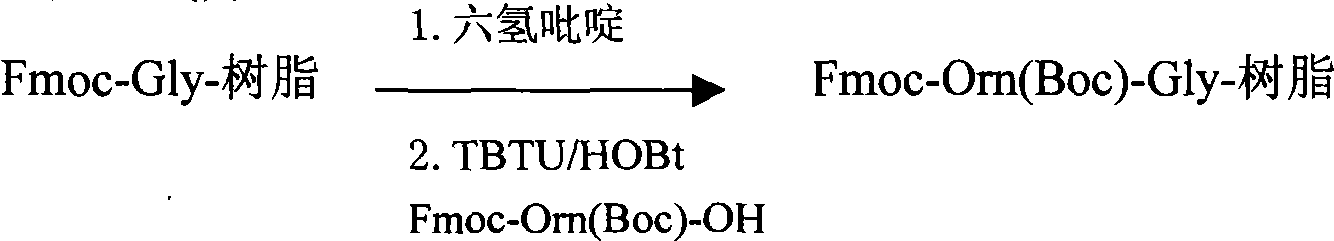
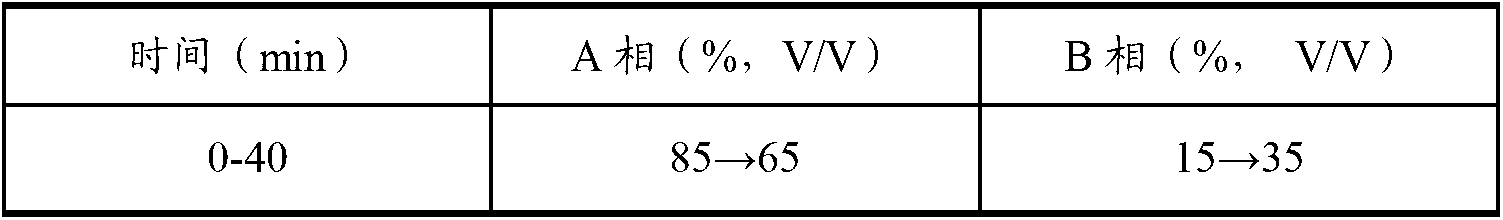
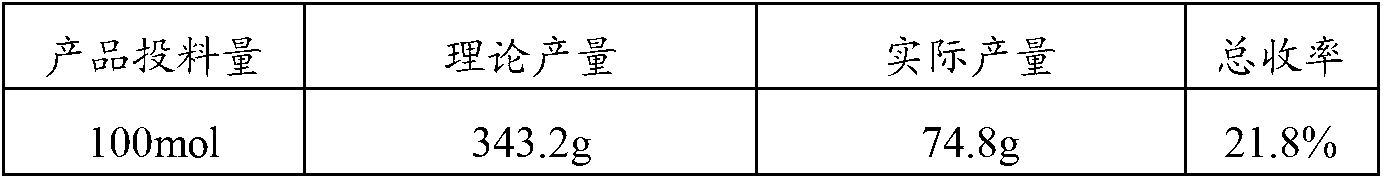
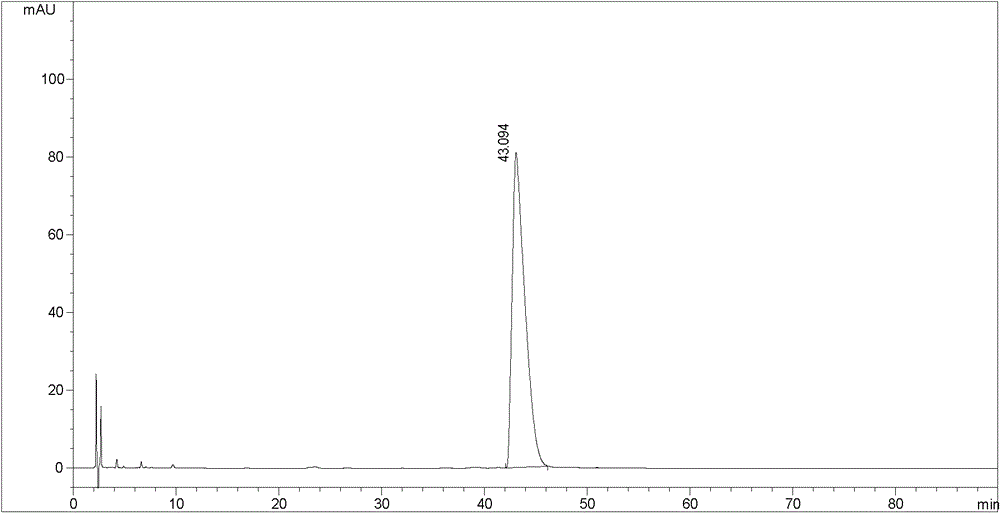
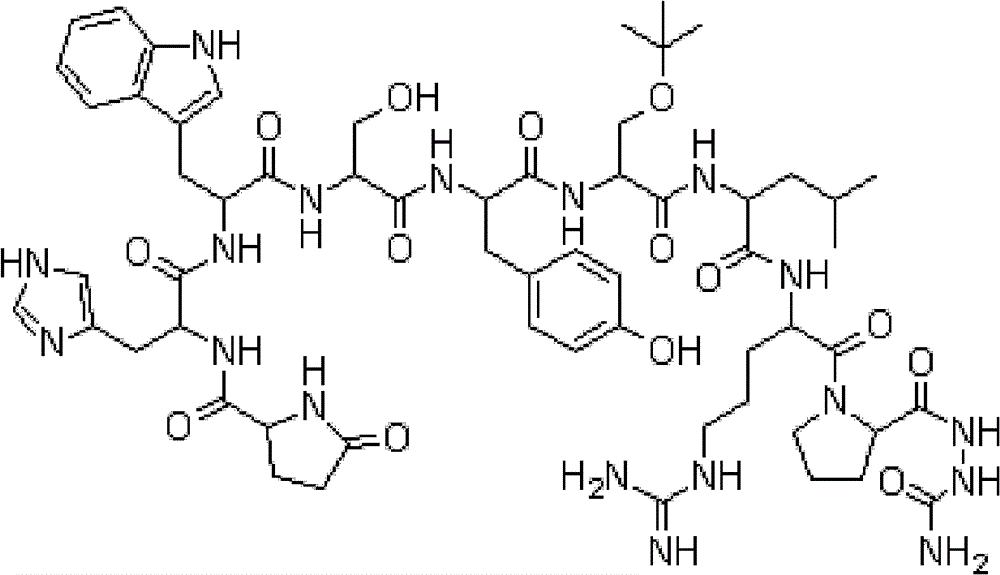
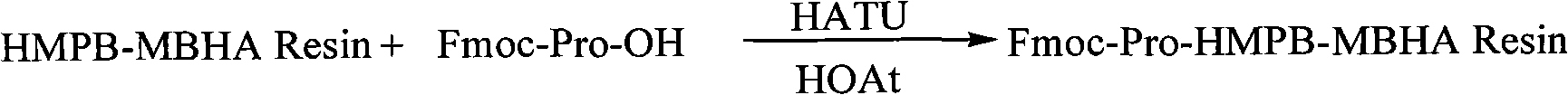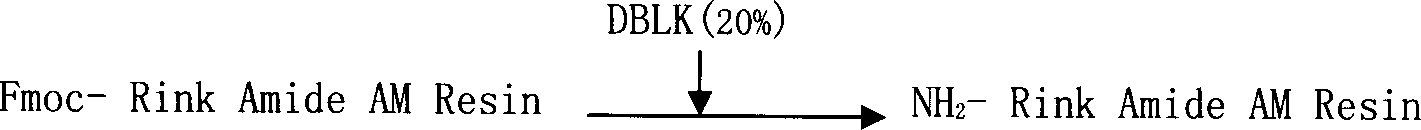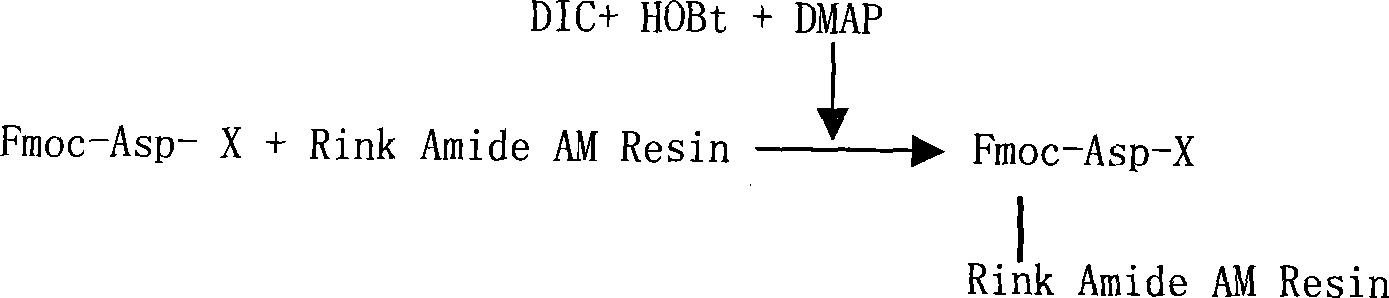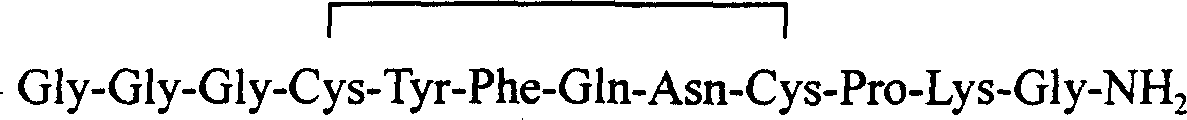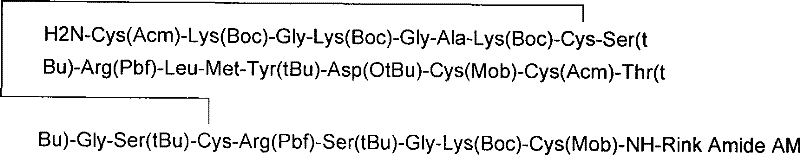Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "MBHA resin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Solid phase synthesis method of ziconotide
InactiveCN101412752AIncrease the speed of cyclizationImprove reaction efficiencyPeptide preparation methodsAnimals/human peptidesSide chainCombinatorial chemistry
The invention relates to a solid phase synthesis method for ziconotide, which comprises the following steps: an Fmoc-Rink Amide-MBHA resin is taken as a solid phase carrier for program reaction; condensation reactions are orderly performed to link 25 amino acids with protecting groups to obtain a linear peptide complete-protection resin of the 25 amino acids,wherein in three groups of Cyses which form a disulfide bond, the Cyses in the same group are simultaneously linked with one of a Trt, Acm or Mob protecting group, and different groups of the Cyses are linked with different protecting groups; the protecting groups of the three groups of the Cyses are orderly removed; a cyclization reaction is performed to form a disulfide ring bond; a side chain protecting group is removed; and the resin is cut to obtain the ziconotide containing three disulfide rings. The method has the main advantages of few side products, high directive efficiency, simplicity, convenience, high yield of products, and favorability for the purification of the products.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Solid-phase preparation method for buserelin
InactiveCN101935339AHigh yieldLow costLuteinising hormone-releasing hormonePeptide preparation methodsPeptide sequenceCombinatorial chemistry
The invention provides a solid-phase preparation method for buserelin. The method comprises the following steps of: 1) preparing a Fmoc-Pro-HMPB-MBHA resin with degree of substitution of between 0.15 and 0.80mmol / g from Fmoc-Pro-OH and a HMPB-MBHA resin with degree of substitution of between 0.2 and 0.9mmol / g by a solid-phase synthesis method; 2) gradually coupling remaining protected amino acid of the Fmoc-Pro-HMPB-MBHA resin according to a peptide sequence to obtain buserelin-HMPB-MBHA resin; 3) cracking the buserelin-HMPB-MBHA resin to obtain fully-protected peptide; and 4) performing ethyl amination, deprotection and purification on the obtained fully-protected peptide to obtain buserelin. The invention aims to provide a buserelin solid-phase synthesis method which has the advantages of high yield, low cost, mild reaction conditions, small environmental pollution and contribution to realizing industrialization.
Owner:HYBIO PHARMA
Method for synthesizing triptorelin from solid phase polypeptide
ActiveCN101357936AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionSide chainFreeze-drying
The invention discloses a preparation method of solid phase peptide synthesis triptorelin, which includes the following steps: with Rink Amide AM resins or Rink Amide MBHA resins as starting materials, amino acids with protective groups are sequentially connected according to solid phase synthesis, so as to obtain protective decapeptide resins, and meanwhile crude products are obtained by sequentially removing Fmoc-protective groups and synchronously removing side-chain protective groups and cutting peptides, and triptorelin elaborate products are prepared after the crude products are separated and purified by C18 (or C8 ) column and freeze-dried. The preparation method is stable in technology, convenient in raw and auxiliary material sources, short in production cycle, high in yield, stable in quality, low in production cost and high in transpeptidase yield. Besides, as the preparation method avoids using poisonous reagents, such as hydrogen fluoride, and the like, the pollution of three wastes is low, purification yield is over 25 percent and each step of transpeptidase yield is above 98 percent; the yield after cutting peptides is 78.8 percent and the total yield is 25.4 percent.
Owner:SHANGHAI SOHO YIMING PHARMA
Preparation method of solid phase synthesis cetrorelix
ActiveCN101284863AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionFreeze-dryingSide chain
A method for realizing the solid phase synthesis of cetrorelix comprises the following steps that: Fmoc-Linker-MBHA-Resin is used as a starting raw material and is connected in turn with amino acid with Fmoc-protecting groups according to a solid phase synthesis method, so as to obtain protective decapeptide resin; meanwhile, the Fmoc-protecting groups are divested in turn; one sort of HBTU / HOBT or DIC / HOBT is used as condensing agent to carry out synthesized peptide, thereby obtaining protective decapeptide resin; acetylation reaction is carried out, and then protecting group side chain divesting and peptide cutting are carried out synchronously to obtain cetrorelix acetate which is separated and purified through C18 or C8 chromatographic column; finally, cetrorelix acetate acetate or trifluoroacetate is obtained after freeze drying.
Owner:滨海吉尔多肽有限公司
Method for synthesizing Exenatide from solid phase polypeptide
ActiveCN101357938AConvenient sourceReduce usagePeptide preparation methodsDepsipeptidesSide chainFreeze-drying
The invention discloses a preparation method of solid phase peptide synthesis Exenatide which includes the following steps: taking Rink Amide resins, Rink Amide AM resins or Rink Amide MBHA resins as starting materials, amino acids with Fmoc protective groups are sequentially connected, so as to obtain protective thirty-nine peptide resins; and meanwhile, after thirty-nine peptide resins are obtained by sequentially removing Fmoc-protective groups and transpeptidase reactions by condensing agents, acellular side-chain protective groups and cutting peptides are carried out sychronously to obtain Exenatide crude products, and then products (comprising medical salts and free alkali, such as acetates, trifluoroacetate, etc.) are obtained after Exenatide crude products are separated and purified by C18 or C8 column and freeze-dried. The preparation method has the advantages of stable technology, conventient raw and auxiliary material sources, short production cycle, low production cost, few three wastes, high yield, stable yield, stable quality, low production cost and high yield.
Owner:SHANGHAI SOHO YIMING PHARMA
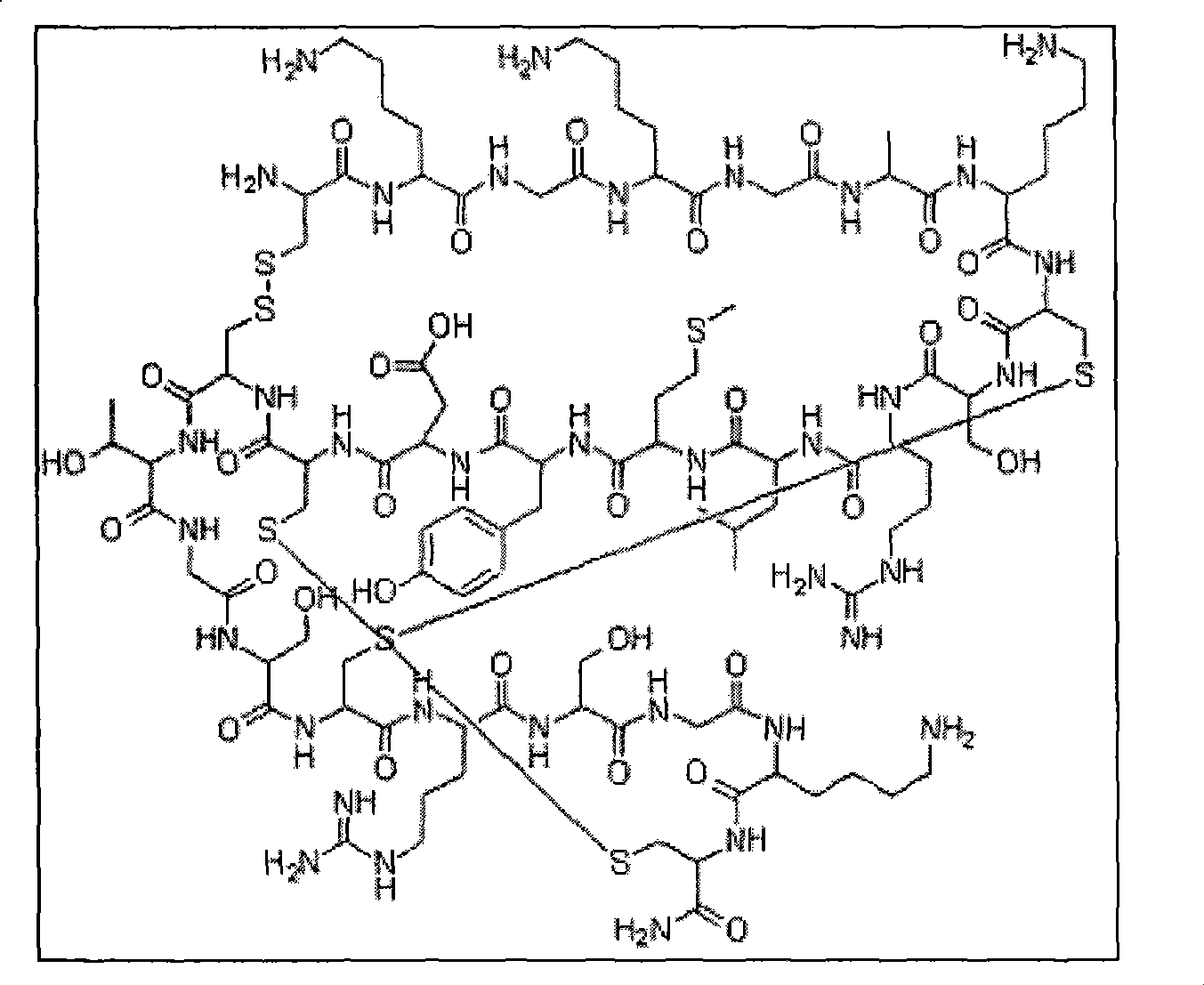
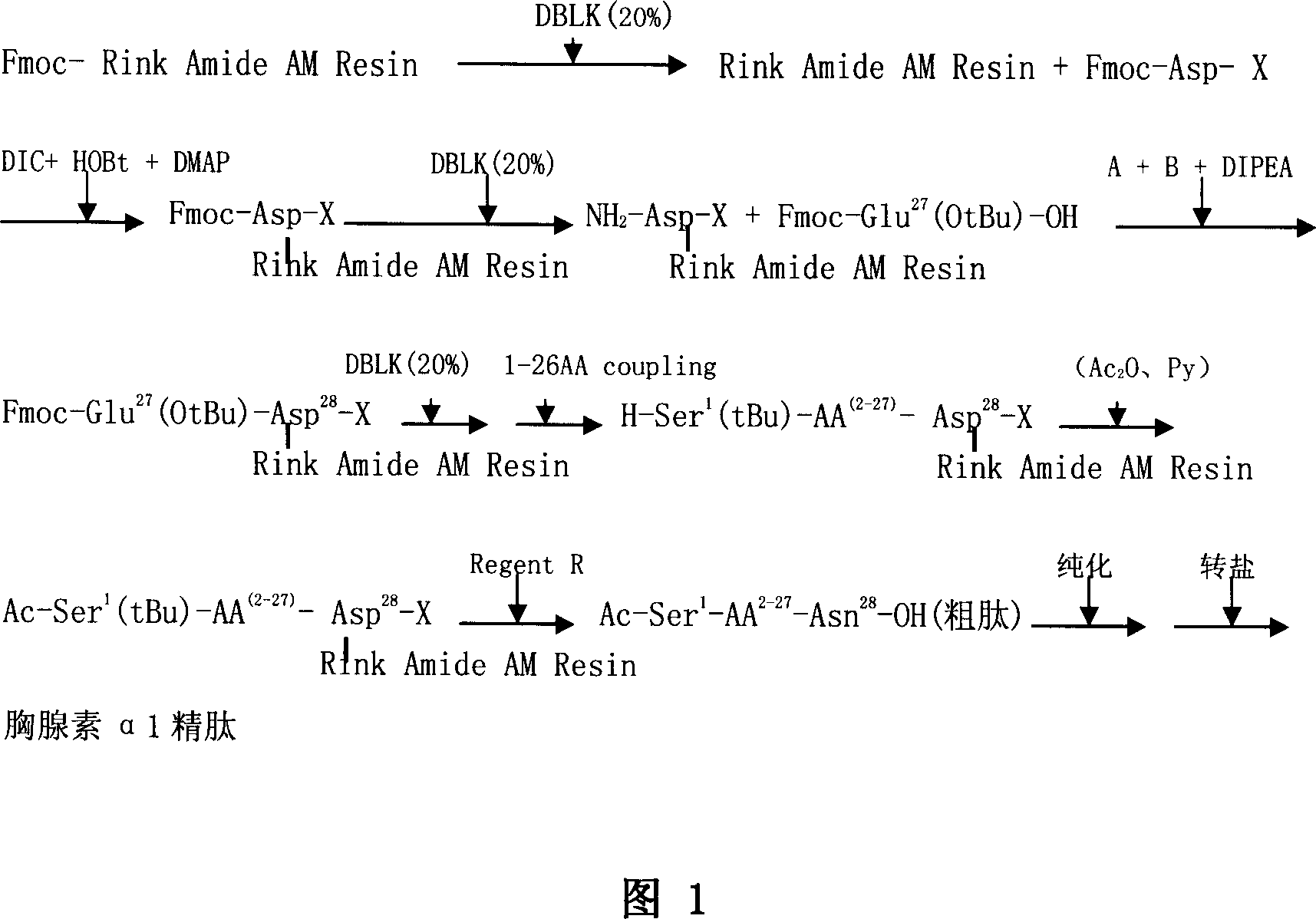
Solid phase synthetic technique for thymosin alpha1
ActiveCN101104638AAdvantages of solid phase synthesis processEasy to purifyThymopoietinsPeptide preparation methodsFluoroacetic acidAcetic anhydride
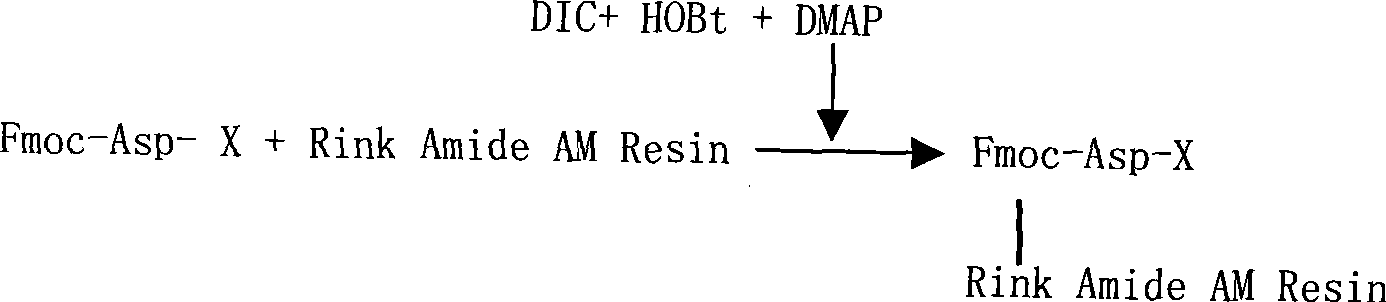
The invention relates to a solid-phase synthesis process of a thymosin alpha 1, belonging to the polypeptide solid-phase synthesis technical field. The invention comprises the following steps: a. a Fmoc-Rink Amide AM resin or a Fmoc-Rink Amide MBHA resin is used as carrier, an H2N-Rink Amide AM resin or an H2N-Rink Amide MBHA resin is obtained after deprotection of the Fmoc; b. side chain carboxyl group of Fmoc-Asp-X is connected with resin amino by the method of solid-phase synthesis to obtain the Fmoc-Asp (resin)-X; c. the left amino acid in the sequence is synthesized in solid-phase with the Fmoc strategy; d. after the amino protection group Fmoc of N terminal amino acid is removed, the N terminal amino acid is acetylated by acetic anhydride and pyridine; e. then the acetylated N terminal amino acid is cut by a cracking agent (tri fluoroacetic acid / benzoylate sulfide / 1, 2- dithioglycol / Anisole) to obtain the thymosin alpha 1; f. crude product of the thymosin alpha 1 is prepared and separated by HPLC to obtain the pure thymosin alpha 1. The invention can increase significantly the yield of the thymosin alpha 1 and decrease the production cost, which is helpful for scale production and has better industrialization prospect.
Owner:苏州天马医药集团天吉生物制药有限公司
Preparing process for synthesizing oxytocin from solid-phase polypeptide
ActiveCN1990501AReduce usageSimple processOxytocins/vasopressinsPeptide preparation methodsProduction rateRink amide resin
The invention discloses a method for preparing oxytocin through solid phase polypeptide synthesis, comprising following steps: taking Rink Amide resin (comprising Rink Amide MBHA resin, Rink Amide Am resin) as raw material, taking amino acid protected by Fmoc, TBTU or HBTU / HOBt as condensing agent, making up amino acid in sequence; adding peptide cutting agent for peptide cutting, adding for precipitation and getting reduced coarse product; adding basic matter, feeding air for oxidation or oxiding with H2O2 with pH being 7.5- 10.0, getting oxidized coarse product; separating and purifying by using C18 or C 8 column and getting final product. The method is characterized by low production cost, simple process, little pollution, high production rate and convenience for industrial production.
Owner:SHANGHAI SOHO YIMING PHARMA
Process for preparing solid phase polypeptide synthetic eptifibatide
ActiveCN1858060AConvenient sourceHigh peptide yieldPeptide preparation methodsPropanoic acidRink amide resin
The preparation process of solid phase polypeptide synthesized eptifibatide includes the following steps: (1) connecting amino acids one by one with Rink Amide resin, Rink Amide MBHA resin or Rink Amide AM resin as initial material, and Fmoc protected amino aids as monomer, with the last peptide chain being S-benzyl mercapto propionic acid Map(SBzl); (2) adding peptide cutting agent (TFA / HBr / HAc / TIS / EDT) to cut peptide; (3) precipitating and collecting coarse reductant eptifibatide product in ether solvent; (4) dissolving coarse reductant eptifibatide product in water, regulating pH value with ammonia water to 7.5-10.0, introducing air for oxidation, and collecting coarse eptifibatide product; and (5) separating and purifying the coarse product in C18 column to obtain the target product. The present invention has high yield, and is suitable for industrial production.
Owner:SHANGHAI SOHO YIMING PHARMA
Method for synthesizing atosiban acetate from solid phase polypeptide
ActiveCN101357937AConvenient sourceHigh peptide yieldPeptide preparation methodsBulk chemical productionPreparative hplcSide chain
The invention discloses a preparation method of solid phase peptide synthesis atosiban which includes the following steps: taking Rink Amide resins, Rink Amide MBHA resins or Rink Amide AM resins as starting materials and taking Fmoc amino acids as monomers, amino acids are grafted one by one and mercaptopropionic acids (Map(SX)) are protected by the last peptide chain; after protected nonapeptide resins are obtained, the acellular side-chain protective groups and cutting peptides are synchronized; then cutting peptides is carried out, and reduced crude atosiban is collected; the pH value is adjusted to 7.5 to 10.0, and oxidized crude atosiban is collected; target products are obtained by the separation and purification by preparative HPLC(C18 or C8 column). The preparation method is convenient in material source, simplifies technology and reduces cost; and the preparation method is low in the pollution of three wastes and is high in yield, and the preparation method is convenient for being industrialized and has good industrialization prospect.
Owner:SHANGHAI SOHO YIMING PHARMA
Preparation method of acetic acid redfish calcitonin
InactiveCN103254305AImprove coupling efficiencyHigh yieldCalcitoninsPeptide preparation methodsFreeze-dryingRedfish
The invention discloses a preparation method of acetic acid redfish calcitonin. The preparation method comprises the following steps of: deprotecting Rink Amide MBHA resin by using a deprotection reagent and removing a Fmoc protecting group; sequentially coupling the deprotected Rink Amide MBHA resin serving as a starting material with Fmoc-protected amino acids serving as monomers to obtain acetic acid redfish calcitonin peptide resin, wherein a condensing agent is N,N-diisopropyl carbodiimide (DIC) / 1-hydroxybenzotriazole (HOBt) or benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) / HOBt; splitting the acetic acid redfish calcitonin peptide resin, adding diethyl ether and precipitating the acetic acid redfish calcitonin peptide resin to obtain reduction type acetic acid redfish calcitonin crude peptide; cyclizing the reduction type acetic acid redfish calcitonin crude peptide to obtain oxidation type acetic acid redfish calcitonin crude peptide; and performing purification, salt conversion, concentration and freeze drying on the oxidation type acetic acid redfish calcitonin crude peptide to obtain the acetic acid redfish calcitonin. According to the preparation method, the yield of the acetic acid redfish calcitonin reaches over 20 percent.
Owner:QINGDAO GUODA BIOLOGICAL PHARMA
Process of preparing calf thymus alphal
ActiveCN101033248AReduce usageWith large-scale production capacityPeptide preparation methodsAnimals/human peptidesAcetic anhydrideSide chain
The invention discloses a new technology to prepare calf thymus alpha 1 (natural existing in thymus cells of calf and other animals), which uses Fmoc-strategy solid phase method, including the following steps: a. taking any one of Rink Amide PEGA resin, Rink Amide AM resin, Rink Amide MBHA resin or Rink Amide PEGA as the starting material, using the amino acid protected by Fmoc as the monomer to hang resin, connecting amino acids in turn to get a protective 28-peptide resin according to the method of solid-phase synthesis, b. using acetic anhydride to close heads during the process in turn, c. removing Fmoc-protective group in turn, d. synchronizing the removal of side-chain protecting group and the cut of peptide to get a crude, e. purifying the crude with antiphase HPLC to get thymosin alpha 1.
Owner:SHANGHAI SOHO YIMING PHARMA
Solid phase polypeptide synthesis preparation method for terlipressin
ActiveCN1865282AReduce usageSimple processOxytocins/vasopressinsPeptide preparation methodsIndustrial constructionRink amide resin
The invention discloses a Telis-vasophysin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Rink Amide resin (concluding Rink Amide MBHA resin, Rink AmideAM resin) as original material, Fmoc protective amino acid as monomer, TBTU or HBTU-to-HOBt as condensing agent to connect amino sequently; using Boc-Gly-OH for the last peptide chain; adding peptide cutting agent to cut peptide; adding ether to deposit to obtain crude product; adding alkali material to oxidate at 7.5-10.0 pH value to generate oxide crude product; proceeding separation and purifying through C18 (or C8) pillar to produce object product. The method possesses low manufacturing cost, simple technology, high obtaining rate and low environmental pollution, which is convenient to do industrial construction.
Owner:SHANGHAI SOHO YIMING PHARMA
Synthesis method of goserelin
InactiveCN102746383AHigh reactivityEasy to synthesizeLuteinising hormone-releasing hormonePeptide preparation methodsSynthesis methodsTrifluoroacetic acid
The invention relates to a solid phase synthesis method of goserelin. The method consists of: first reacting RinkAmide MBHA Resin with carbonyldiimidazole (CDI) to obtain an intermediate product, which then reacts with Fmoc-NH-NH2 to obtain Fmoc-NH-NH-CO-NH-Resin, then conducting a programmed reaction, carrying out a condensation reaction in order to connect corresponding amino acids, thus obtaining goserelin resin; performing cutting with a low concentration trifluoroacetic acid solution so as to obtain a crude peptide solution, conducting purification to obtain the goserelin. According to the invention, by reacting a high-activity reactant carbonyldiimidazole with Fmoc-NH-NH2 and Rink Amide MBHA Resin to obtain Fmoc-NH-NH-CO-NH-Resin, the reaction efficiency is greatly improved, so that the reaction can be completed rapidly and efficiently, thus completely solving the problem of difficult synthesis of Fmoc-NH-NH-CO-NH-Resin, and improving the reaction efficiency and yield effectively.
Owner:HANGZHOU JIUYUAN GENE ENG
Preparation method for synthesizing proteidin with solid phase polypeptide
ActiveCN102336813AReduce pollutionReduce manufacturing costPeptide preparation methodsDiethyl etherDisulfide bond
The invention discloses a preparation method for synthesizing proteidin with a solid phase peptide. The technical scheme of the invention comprises the following steps of: connecting amino acids with Fmoc-protection groups in sequence by taking Rink Amide MBHA resin as an initial raw material and taking HBTU / HOBt as a concentration agent according to a solid phase synthesis method to obtain protected heptadeca-peptide resin, wherein Boc-Arg-OH.HCl is taken as a last amino acid; adding a peptide cutting reagent for cutting peptides; adding diethyl ether for precipitating to obtain a reduced crude product; protecting the -SH groups of 5-bit and 14-bit Cys with Trt; oxidizing with hydrogen peroxide at the pH 8.5-10.0; protecting the -SH groups of 7-bit and 12-bit Cys with Acm; oxidizing with iodine in a methanol solution to obtain a dual disulfide bond Cys5-14Cys7-12 crude product; and separating by adopting a C8 high-efficiency liquid phase column for purifying to obtain a target product, wherein the purity of the target product is 99.21 percent, and the total yield is 23.68 percent. The method has the advantages of low production cost, simple process, low environmental pollution, high yield and convenience for industrial implementation.
Owner:SHANGHAI SOHO YIMING PHARMA
Solid phase polypeptide synthesis preparation method for terlipressin
ActiveCN1865282BReduce usageSimple processOxytocins/vasopressinsPeptide preparation methodsIndustrial constructionRink amide resin
The invention discloses a Telis-vasophysin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Rink Amide resin (concluding Rink Amide MBHA resin, Rink AmideAM resin) as original material, Fmoc protective amino acid as monomer, TBTU or HBTU-to-HOBt as condensing agent to connect amino sequently; using Boc-Gly-OH for the last peptide chain; adding peptide cutting agent to cut peptide; adding ether to deposit to obtain crude product; adding alkali material to oxidate at 7.5-10.0 pH value to generate oxide crude product; proceeding separation and purifying through C18 (or C8) pillar to produce object product. The method possesses low manufacturing cost, simple technology, high obtaining rate and low environmental pollution, which is convenient to do industrial construction.
Owner:SHANGHAI SOHO YIMING PHARMA
Preparation method of solid phase synthesis cetrorelix
ActiveCN101284863BConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionFreeze-dryingSide chain
Owner:滨海吉尔多肽有限公司
Method for synthesizing tesamorelin through solid phases
InactiveCN102702343AConvenient sourceReduce usagePeptide preparation methodsDepsipeptidesSide chainSynthesis methods
The invention provides a method for synthesizing tesamorelin through solid phases and mainly solves the technical problems of long reaction time and high raw material cost of the existing synthesis method. According to the method, RinkAmideAM resin or RinkAmide MBHA resin is used as initial raw materials, trans-hexenoic acid and amino acid with Fmoc protecting groups are sequentially connected, side-chain full-protection tesamorelin resin is synthesized and obtained, the side-chain group protection removal and the peptide cutting are synchronously carried out, the tesamorelin crude peptide is obtained, the separation purification is carried out through C18 or C4 columns, and products are obtained through freezing and drying. The method has the characteristics that the process is stable, resources of raw and auxiliary materials are convenient to obtain, the reaction operation is simple, the post treatment is easy, the yield is high, the cost is low, the production smell is low, and the like.
Owner:GL BIOCHEM SHANGHAI +2
Preparation method of salmon calcitonin acetate
InactiveCN103254305BImprove coupling efficiencyHigh yieldCalcitoninsPeptide preparation methodsFreeze-dryingDiethyl ether
Owner:QINGDAO GUODA BIOLOGICAL PHARMA
Preparation method and product of exenatide
ActiveCN106749611AReduce accumulationAddressing Synthetic Scale-upPeptide preparation methodsVasoactive intestinal peptideCombinatorial chemistryExenatide
The invention discloses a preparation method of exenatide. The exenatide is prepared from protection fragments of 13 fragments in a synthesis mode: His-Gly-COOH, Glu-Gly-Thr-COOH, Phe-Thr-Ser-COOH, Asp-Leu-Ser-COOH, Lys-Gln-COOH, Met-Glu-Glu-COOH, Glu-Ala-Val-COOH, Arg-Leu-Phe-Ile-COOH, Glu-Trp-Leu-COOH, Lys-Asn-Gly-COOH, Gly-Pro-Ser-COOH, Ser-Gly-Ala-COOH and Pro-Pro-Pro-Ser-Rink Amide MBHA Resin. By adopting the preparation method, the synthesis efficiency is improved, impurity accumulation is reduced, and the purification difficulties can be alleviated.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Synthetic method of carbetocin acetate
PendingCN111217892AReduce pollutionFew reaction stepsOxytocins/vasopressinsPeptide preparation methodsButyrateCombinatorial chemistry
The invention belongs to the technical field of amino acids, and discloses a synthetic method of carbetocin acetate. The synthetic method comprises the following steps of using Fmoc-Gly-OH and amino resin Rink Amide-MBHA Resin as starting raw materials, firstly, removing Fmoc protecting groups with a 20% DBLK solution, and performing condensation to obtain Fmoc-Gly-Rink Amide-MBHA Resin; sequentially enabling Fmoc protected amino acids to be connected, after connection, removing the Fmoc protecting groups, and performing condensation with 4-bromo butyric acid; removing Mtt protecting groups oncysteine with a weak acid system, and performing cyclization to obtain carbetocin peptide resin; and cutting the peptide resin, and purifying obtained crude peptide, to obtain pure peptide of the carbetocin. The method is simple and feasible, convenient to operate and suitable for large-scale production.
Owner:XIANGYU PHARMA
Preparation method of plecanatide
The invention provides a preparation method of plecanatide, and relates to the technical field of polypeptide synthesis. AM resin or MBHA resin is modified through FMPB, H-Leu-OtBu serves as first amino acid to be coupled to the modified resin, amino acid at sites difficult to connect is modified and then is gradually coupled to the modified resin according to the sequence of plecanatide main chain peptide, so that the synthesis difficulty of plecanatide linear peptide is greatly reduced, and the problems of many impurities, low purity, low yield, amino acid racemization and the like in the existing synthesis are solved. The Cys at sites of 4 and 12 in the sequence are subjected to first oxidation bonding and then subjected to column chromatography coarse purification, so that the purity of the obtained plecanatide is improved; the purification liquid is diluted, and the Cys at the 7-site and the 15-site are subjected to the second oxidation bonding, such that the concentration of thepre-oxidized plecanatide line peptide can be reduced so as to reduce the intermolecular disulfide bond during the second oxidation bonding process, and improve the yield of plecanatide; and further, the operation is simple and convenient, and the method is suitable for industrial production.
Owner:杭州信海医药科技有限公司
Solid phase synthesis method of ZP120
InactiveCN101407538AEasy to operateLow costPeptide preparation methodsBulk chemical productionSide chainTrifluoroacetic acid
The invention provides a solid phase synthetic method of ZP120: Ac-Arg-Tyr-Tyr-Arg-Trp-Lys-Lys-Lys-Lys-Lys-Lys-Lys-NH2, which uses Fmoc-Rink Amide-MBHA resin as a material to carry out procedure reaction, and the salt of the ZP120 can be obtained by sequentially connecting amino protecting groups and then removing the groups, acidifying, and removing and cutting side chain protecting groups and resin, and then the ZP120 can be obtained further. The beneficial effect of the invention mainly embodies on: (1) the invention that uses the Fmoc Rink Amide-MBHA resin as a solid phase synthetic carrier is low in cost; (2) N,N-diisopropyl carbodiimide is used as a peptide synthesis condensing agent, and the product process is low in racemization, and the product is good in quality and high in yield; (3) trifluoroacetate is used for cutting the ZP120 peptide resin, and the form of peptide amide can be obtained directly after the cutting, and the subsequent treatment is simple; and (4) the technique is simple and systematic, and is beneficial to industrialization production.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Insulin amyloid polypeptide inhibitor, preparation method and application thereof
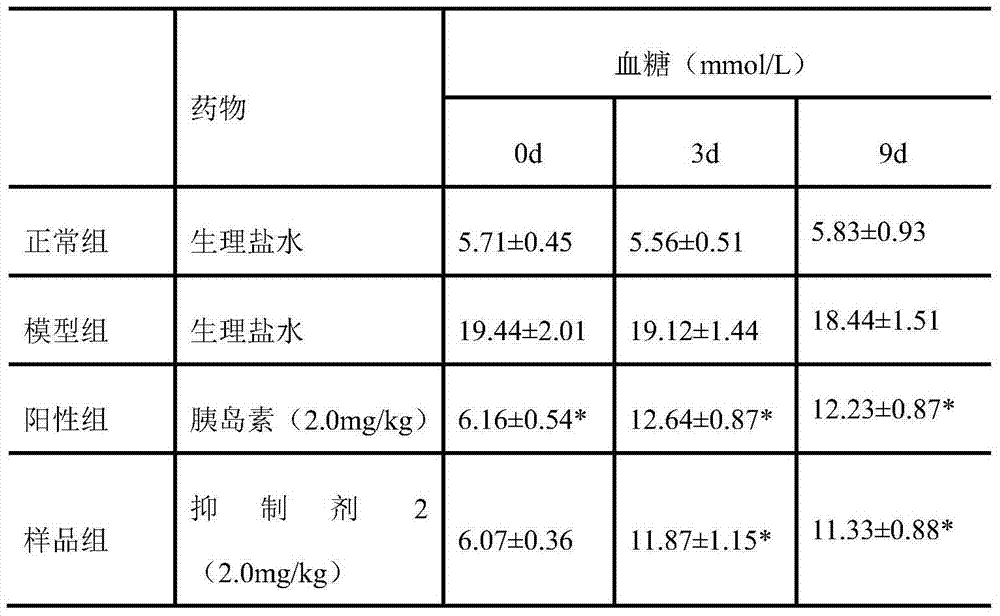
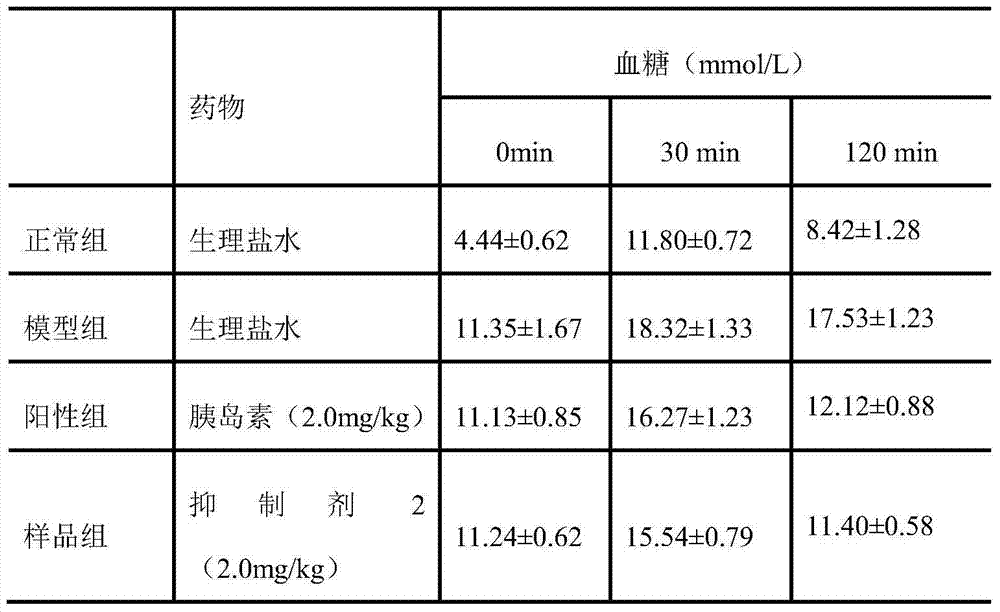
The invention relates to the field of drugs and particularly relates to an inhibitor 1 compound which can treat diabetes. A sequence of the inhibitor 1 is KATPIESHQVJAAEKRKC. A preparation method of the inhibitor 1 is carried out through Fmoc-protected solid-phase synthetic technology, with Rink amide MBHA resin and Fmoc-Gly-Wang resin as supporters, with 6-chloro-1-hydroxylbenzotriazole and N,N-diisopropyl carbodiimide as condensing agents, and with trifluoroacetic acid as a cutting reagent. The invention also provides an application of the inhibitor 1 for preventing or treating diabetes and complications thereof, wherein the complications comprise diabetic nephropathy, diabetic hypertension, diabetic eye diseases and diabetic neurogenic lesion. The inhibitor 1 can be employed for preventing or treating the diabetes and the complications thereof through various dosing methods, such as subcutaneous injection or intramuscular injection, intravenous injection or intravenous drip, and oral medication, such as pills, capsules, nasal spray agent and the like. The islet amyloid polypeptide inhibitor 1 can targetedly inhibit generation of islet amyloid polypeptide, thereby preventing or treating diabetes.
Owner:SUZHOU PULUODA BIOLOGICAL SCI & TECH
Solid phase synthetic technique for thymosin alpha1
ActiveCN101104638BEasy to purifyHigh purityThymopoietinsPeptide preparation methodsAcetic anhydrideFluoroacetic acid
Owner:苏州天马医药集团天吉生物制药有限公司
Insulin amyloid polypeptide inhibitor, preparation method and application thereof
Insulin amyloid polypeptide inhibitor, preparation method and application thereof. The invention relates to the field of drugs and particularly relates to an inhibitor 1 compound which can treat diabetes. A sequence of the inhibitor 2 is VLSVAALNHLDKATPIESH. A preparation method of the inhibitor 2 is carried out through Fmoc-protected solid-phase synthetic technology, with Rink amide MBHA resin and Fmoc-Gly-Wang resin as supporters, with 6-chloro-1-hydroxylbenzotriazole and N,N-diisopropyl carbodiimide as condensing agents, and with trifluoroacetic acid as a cutting reagent. The invention also provides an application of the inhibitor 2 for preventing or treating diabetes and complications thereof, wherein the complications comprise diabetic nephropathy, diabetic hypertension, diabetic eye diseases and diabetic neurogenic lesion. The inhibitor 2 can be employed for preventing or treating the diabetes and the complications thereof through various dosing methods, such as subcutaneous injection or intramuscular injection, intravenous injection or intravenous drip, and oral medication, such as pills, capsules, nasal spray agents and the like. The islet amyloid polypeptide inhibitor 2 can targetedly inhibit generation of islet amyloid polypeptide, thereby preventing or treating diabetes.
Owner:刘旭
Synthesis method of carbetocin
ActiveCN112094324AImprove the efficiency of the cyclization reactionHigh purityOxytocins/vasopressinsPeptide preparation methodsPharmaceutical drugDrugs preparations
The invention belongs to the field of polypeptide drug preparation methods, and relates to a synthesis method of carbetocin. The synthesis method of carbetocin comprises the following steps of (1) using Rink Amide MBHA Resin as a carrier, performing swelling treatment, and then, sequentially coupling protected Gly, protected Leu, protected Pro, protected Cys, protected Asn, protected Gln, protected Ile and protected Tys to swell Rink Amide-MBHA Resin through a condensation reaction, and performing drying to obtain peptide resin 1; (2) performing reaction on the peptide resin 1 with vinylaceticacid to obtain peptide resin 2; (3) cracking the peptide resin 2 to obtain a carbetocin intermediate I; and (4) performing cyclization reaction on the carbetocin intermediate I to obtain the carbetocin. The synthesis method has the advantages of high reaction selectivity, quick reaction, few by-products and impurities, high yield, safety, environment protection, simplicity and easy operation.
Owner:NINGBO SANSHENG BIOLOGICAL TECH CO LTD
Antibacterial active peptide
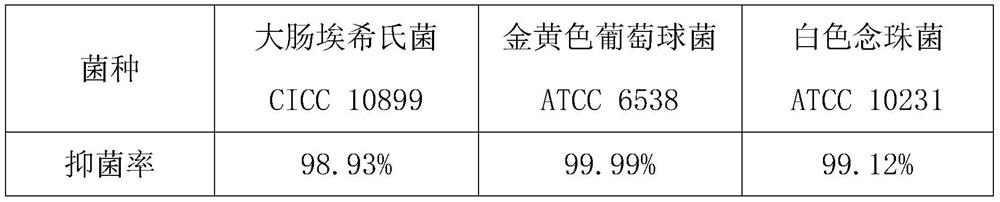
ActiveCN111961118AHigh antibacterial activityGood antibacterial effectPeptide preparation methodsStaphylococcusStaphyloccocus aureus
The invention discloses an antibacterial active peptide. An amino acid sequence of a polypeptide molecule of the antibacterial active peptide is Ac-RRPVPIIYCNRRKGRCQKM-NH2; a preparation method of theantibacterial active peptide comprises the following steps: by taking Rink-MBHA resin as a solid phase carrier, and adding the Rink-MBHA resin into a main reaction kettle and carrying out treatment;carrying out amino acid condensation by adopting a linear synthesis mode, and performing drying in a vacuum dryer after synthesis to obtain peptide resin; and cracking the peptide resin to obtain a polypeptide crude product, and purifying the polypeptide crude product to obtain the antibacterial active peptide. The antibacterial active peptide disclosed by the invention is high in antibacterial activity, has a remarkable antibacterial effect when the concentration is 10mu g / ml, is wide in antibacterial spectrum, and has a remarkable antibacterial effect on escherichia coli, staphylococcus aureus, candida albicans and the like; the antibacterial active peptide destroys cell membranes of bacteria through a physical effect so as to achieve the antibacterial purpose, drug resistance is not generated, and the antibacterial active peptide raw material is low in price, easy to obtain, easy to prepare and produce and capable of bringing a better use prospect.
Owner:合肥瑞克生物科技有限公司
Solid-phase synthesis of ATL peptides
The invention disclose a solid phase synthesis process for a ATL peptide. The process comprises using MBHA resin as a solid phase carrier, combines protective lysyl Boc-Lys(2-Cl-Z) on the resin, completing the synthesis of ATL by stepwise peptide combining method for protecting aminophenol with Boc, modifying N end with acetic anhydride by acetylizing to obtain all-protected peptide resin; depriving the side chain protecting group of the formacyl of Trp with piperidine, and at last, depriving side chain protection group with fluoroform sulfonic acids and incising the resin to obtain ATL coarse peptide; and then purifying the crude product with the highly effective liquid phase of reversed phase C18 filler, separating and purifying with methanol-water gradient eluting process to obtain ATL peptide refining product. The process of the invention with single-step condensation reaction, has high yield and low by-product, and the final crude product has high purity and small impurity content, the operation is simple, and the total yield is high.
Owner:济南环肽医药科技有限公司
Solid-phase synthesis of ziconotide
InactiveCN101412752BImprove orientation efficiencyHigh yieldPeptide preparation methodsAnimals/human peptidesSide chainCombinatorial chemistry
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Solid-phase synthesis of ATL peptides
The invention discloses a solid phase synthesis process for a ATL peptide. The process comprises using MBHA resin as a solid phase carrier, combines protective lysyl Boc-Lys(2-Cl-Z) on the resin, completing the synthesis of ATL by stepwise peptide combining method for protecting aminophenol with Boc, modifying N end with acetic anhydride by acetylizing to obtain all-protected peptide resin; depriving the side chain protecting group of the formacyl of Trp with piperidine, and at last, depriving side chain protection group with fluoroform sulfonic acids and incising the resin to obtain ATL coarse peptide; and then purifying the crude product with the highly effective liquid phase of reversed phase C18 filler, separating and purifying with methanol-water gradient eluting process to obtain ATLpeptide refining product. The process of the invention with single-step condensation reaction has high yield and low by-product, and the final crude product has high purity and small impurity content, the operation is simple, and the total yield is high.
Owner:济南环肽医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com