Method for synthesizing atosiban acetate from solid phase polypeptide
A solid-phase peptide synthesis, atosi-like technology, applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of complex operation, high labor cost, and three wastes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Peptide chain preparation:
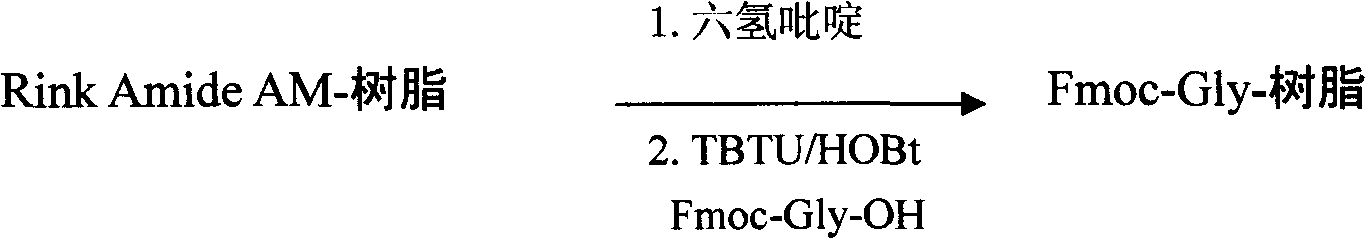
[0161] Weigh the amount of Rink Amide AM resin equivalent to 720mmol (200 mesh, 0.80mmol / g), put it into the SHYM-25 peptide synthesizer, soak it in 6000ml DMF to swell the resin, and drain it. Add 6400 ml of a DMF solution with a volume concentration of 25% hexahydropyridine, and shake at 25° C. for 30 minutes. Drain, wash several times with DMF, respectively, and drain.
[0162] Preparation of Fmoc-Gly-resin:
[0163] Add Fmoc-Gly-OH (MW: 297.3, 1440mmol) 428g, HOBT (MW: 135.1, 1440mmol) 195g, TBTU (MW: 321, 1440mmol) 462g, NMM 320ml (MW = 101.2), 4000ml DMF, shake the mixture at 25°C Shake for 1.5 hours. Drain, wash with DMF several times, and drain.
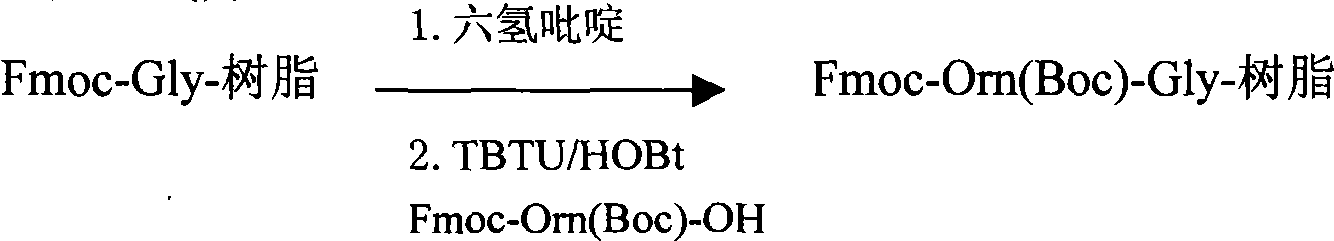
[0164] Preparation of Fmoc-Orn(Boc)-Gly-resin:
[0165] Add 6400 ml of a DMF solution with a volume concentration of 25% hexahydropyridine, and shake at 25° C. for 30 minutes. Drain, wash several times with DMF, and drain.
[0166] Fmoc-Orn (Boc)-OH (MW: 454.5, 1440mmol) 654g, TBTU (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com