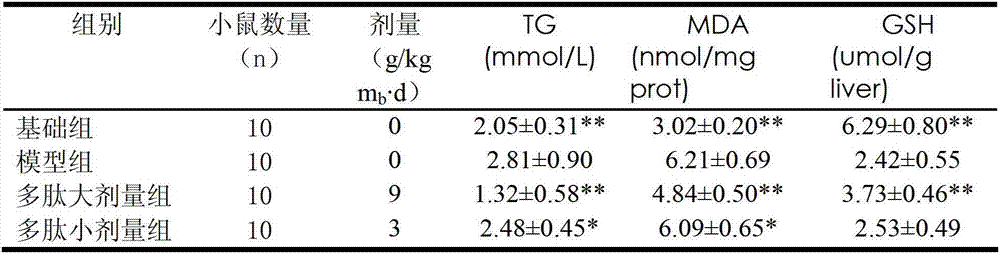
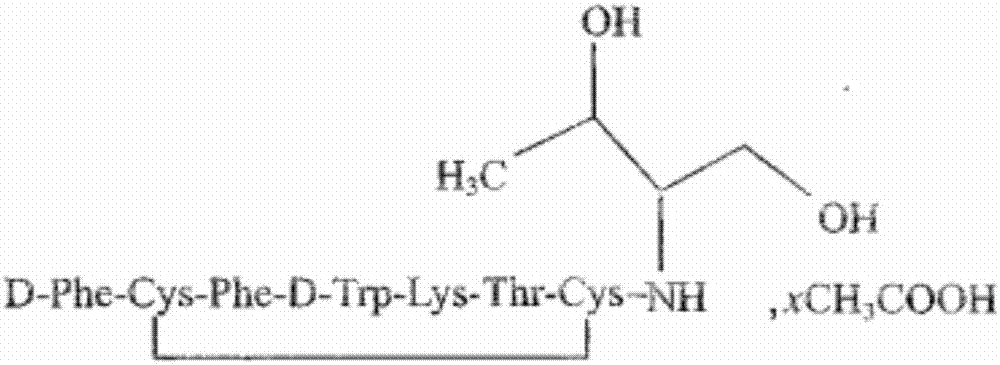
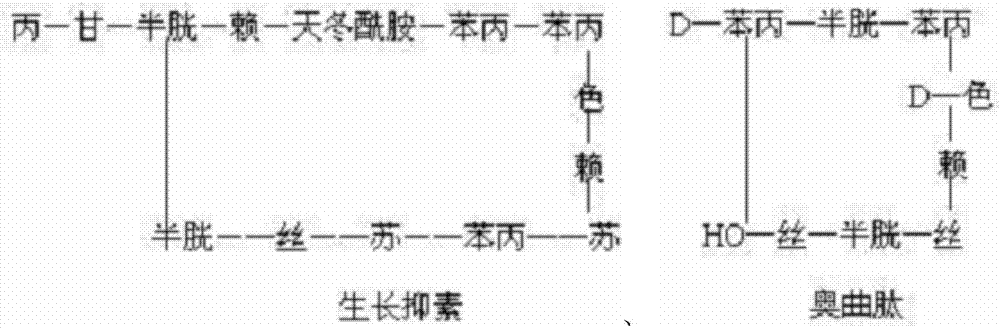
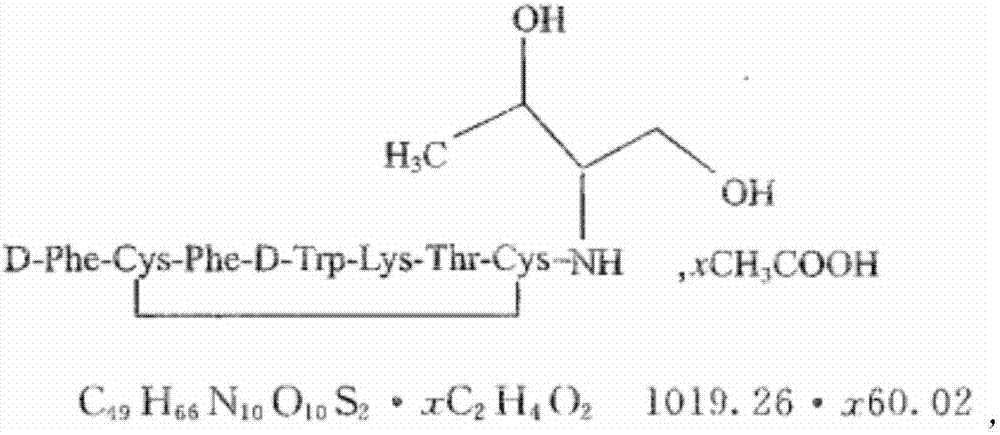
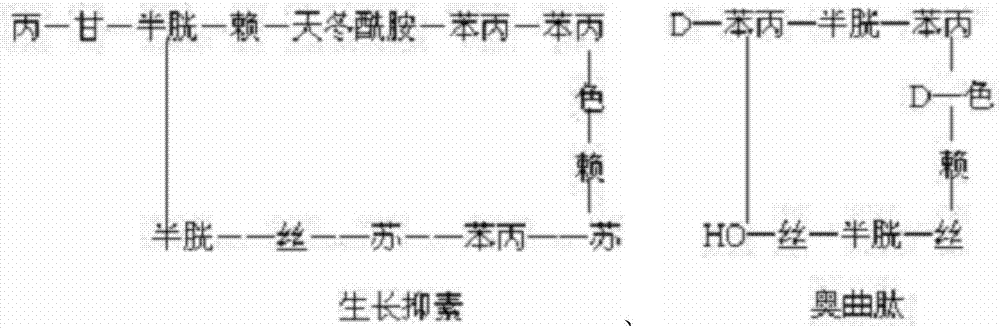
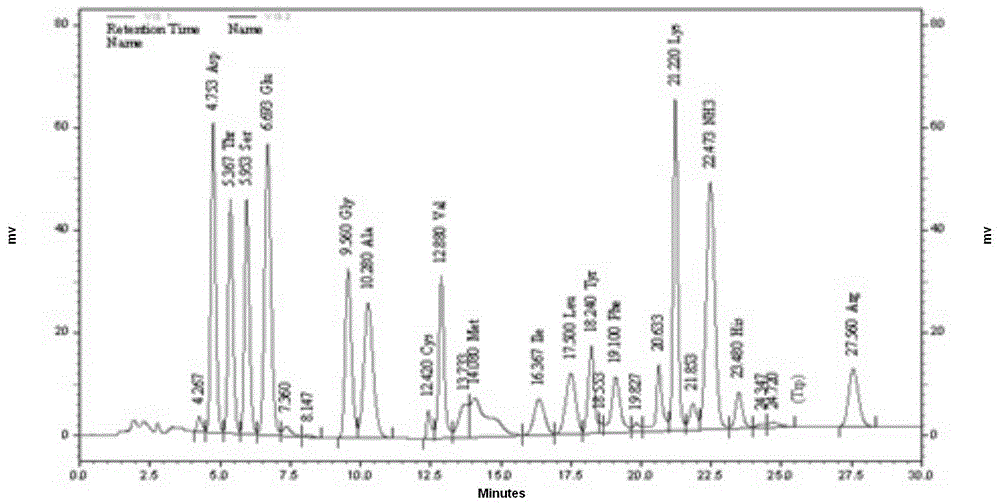
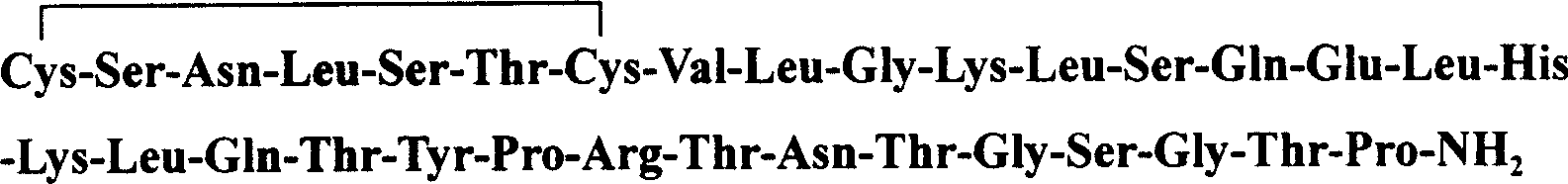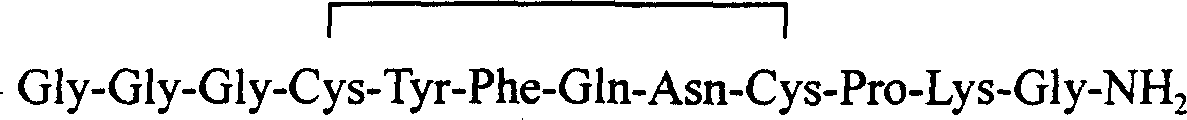Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
77results about How to "High peptide yield" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Holothurian peptide functional food and preparation method thereof
InactiveCN101341978AAvoid residueSave the hassleProtein composition from fishAnimal proteins working-upDiseaseAdditive ingredient
The invention discloses a sea cucumber polypeptide functional food and a preparation method thereof which not only remarkably improves the content of polypeptide, but also has no chemical residues; the contents of salt and arsenic are low; the food is healthier and safer to eat; simultaneously the food is simply operated, easily controlled, is effective and saves energies. The key technical scheme includes: selecting a compound protease of Protamex and needing not to adjust the pH value of materials; carrying out processes of desalting and arsenic removing on an enzymolysis liquid; more than 80 percent of the molecular weight of the product is between 100 to 6000Dalt; wherein, the small polypeptide of 100 to 2100Dalt is more than 70 percent; the product components and the content weight percentages are as follows: 50 to 60 percent of polypeptide, 5 to 10 percent of free amino acids, 2.5 to 7.5 percent of mucoitin as well as containing the inherent nutrition components of a plurality of minerals and vitamins of the sea cucumber. The product has the effects of resisting knub, reducing blood pressure, preventing cardio-cerebrovascular diseases, resisting fatigue, delaying senescence, improving the immunity. The food can be used as a healthy food to eat and can also be used as a food and a medicine additive.
Owner:DALIAN FEIDE BIOIND
Preparation method of synthesizing bivalirudin from solid phase polypeptide
ActiveCN101033249AConvenient sourceReduce usagePeptide-nucleic acidsPeptide preparation methodsSide chainWang resin
This invention discloses a preparation method of solid-phase peptide synthesizing bivalirudin. It includes the following steps: taking any one of triphenyl methyl chloride resin, 4-methyl-triphenyl methyl chloride resin, 4-methoxy-triphenyl methyl chloride resin, 2-chlorine-triphenyl methyl chloride resin, or Wang resin as the starting raw materials, connecting amino acids in turn according to the method of solid-phase synthesis, to get a protective 28-peptide resin, removing Fmoc-protective group in turn, side-chain protecting group and cutting the peptide to get a crude, then purifying the crude through C18 (or C8) high-pressure column to get bivalirudin exquisite article. In this invention, the peptide yield of every step is more than 99%, and the total yield is 14%.
Owner:SHANGHAI SOHO YIMING PHARMA
Method for synthesizing triptorelin from solid phase polypeptide
ActiveCN101357936AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionSide chainFreeze-drying
The invention discloses a preparation method of solid phase peptide synthesis triptorelin, which includes the following steps: with Rink Amide AM resins or Rink Amide MBHA resins as starting materials, amino acids with protective groups are sequentially connected according to solid phase synthesis, so as to obtain protective decapeptide resins, and meanwhile crude products are obtained by sequentially removing Fmoc-protective groups and synchronously removing side-chain protective groups and cutting peptides, and triptorelin elaborate products are prepared after the crude products are separated and purified by C18 (or C8 ) column and freeze-dried. The preparation method is stable in technology, convenient in raw and auxiliary material sources, short in production cycle, high in yield, stable in quality, low in production cost and high in transpeptidase yield. Besides, as the preparation method avoids using poisonous reagents, such as hydrogen fluoride, and the like, the pollution of three wastes is low, purification yield is over 25 percent and each step of transpeptidase yield is above 98 percent; the yield after cutting peptides is 78.8 percent and the total yield is 25.4 percent.
Owner:SHANGHAI SOHO YIMING PHARMA
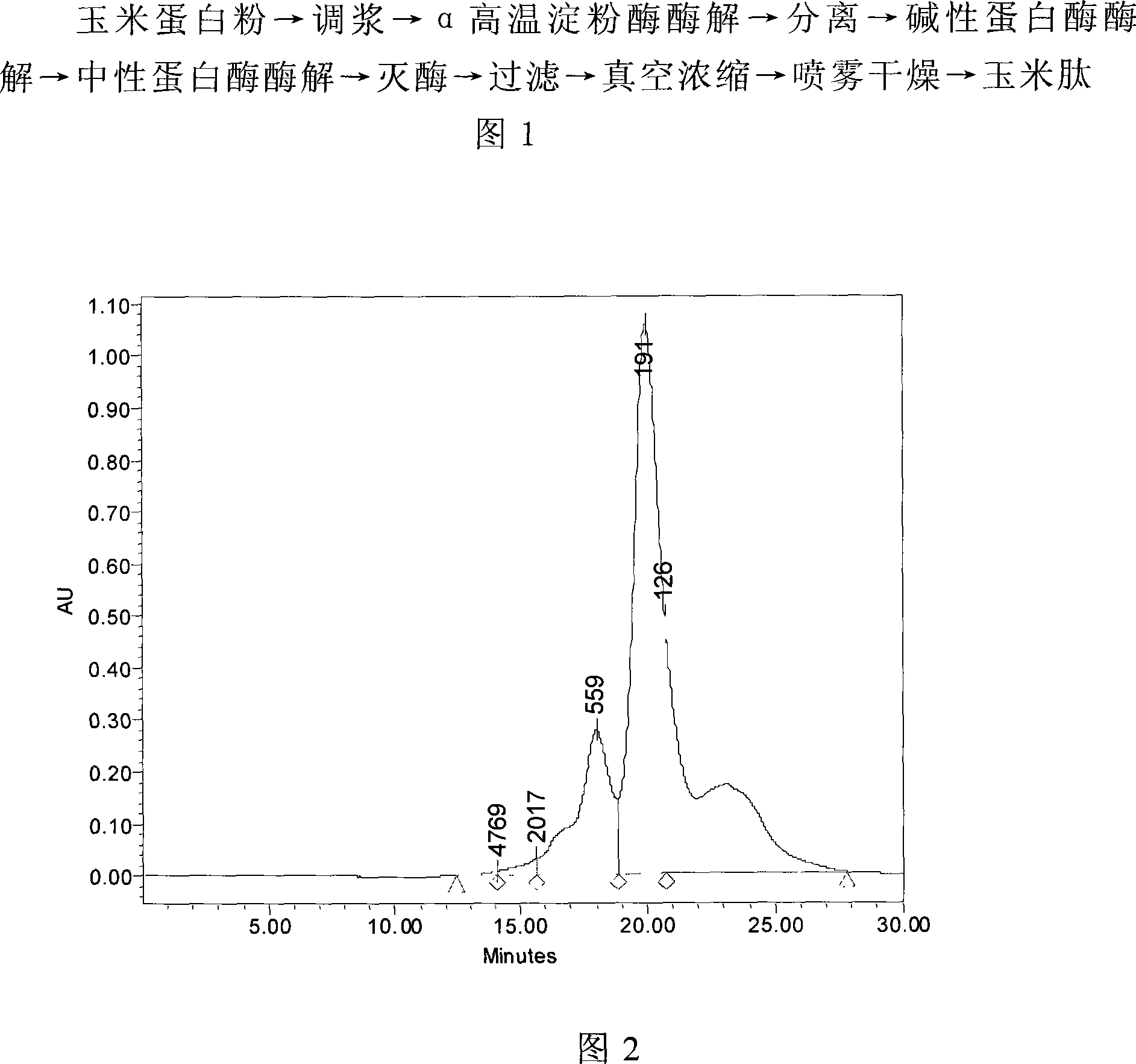
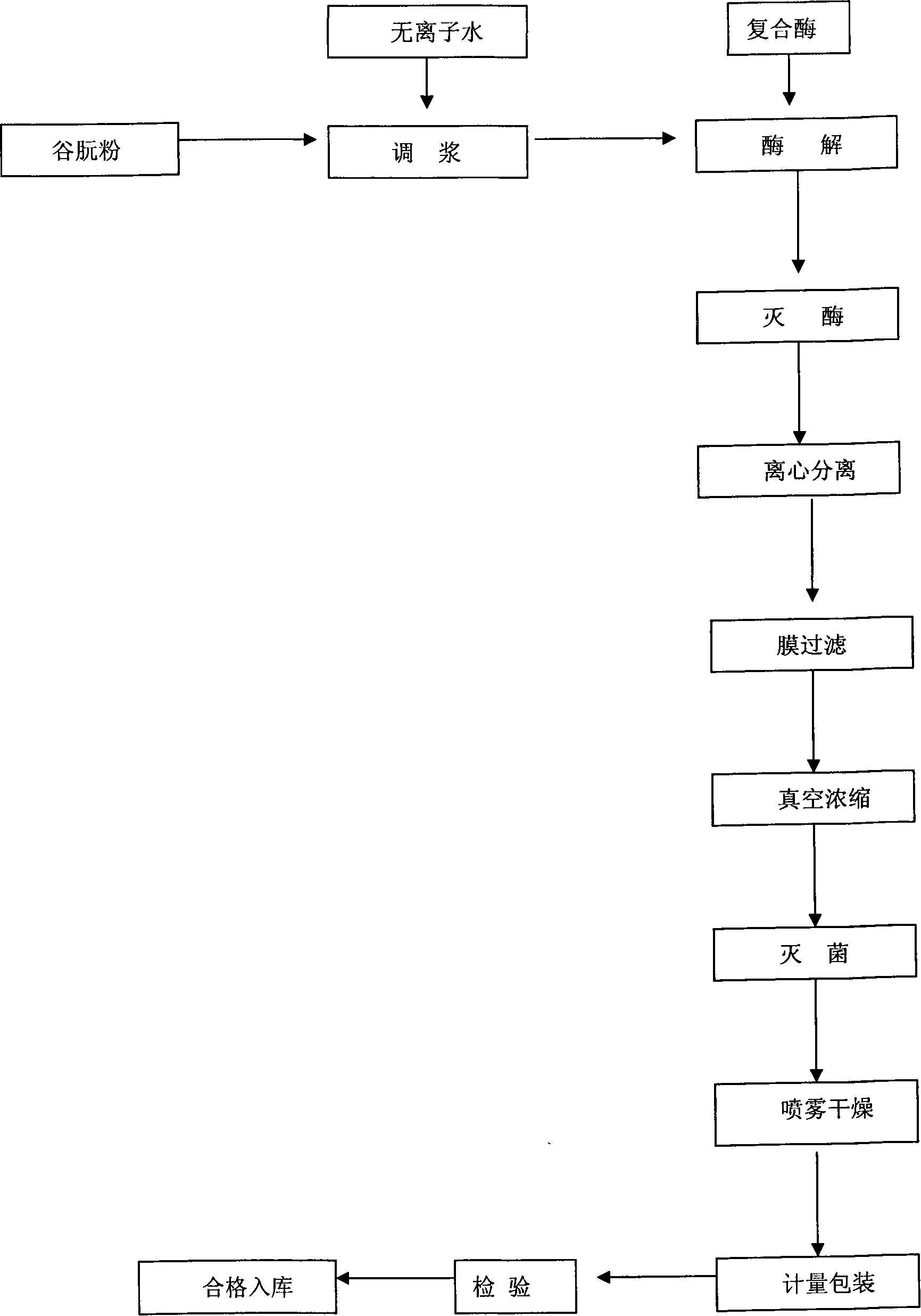
Industrial production method of corn protein polypeptide from corn protein powder by enzymatical process
ActiveCN101096696AHigh peptide yieldComplete enzymatic hydrolysisFermentationBiotechnologyClinical nutrition
The invention discloses a unique extracting method of maize albumen powder from maize, which comprises the following steps: using composite proteinase to enzymolyze zein; separating; hyperfiltering; condensing; spraying; drying; obtaining the white powder shaped product. The invention simplifies the technique and shortens the manufacturing period with good taste and small molecular weight of hybrid peptide, which can be clinical nutrition, hygienic food, sports food and cosmetics.
Owner:中食都庆(山东)生物技术有限公司
Preparation method of synthesizing octriotide from solid phase polypeptide
ActiveCN1923849AConvenient sourceReduce usagePeptidesBulk chemical productionHydrogen fluorideSide chain
The invention discloses an aoqu-peptide preparing method of solid-phase polypeptide, which comprises the following steps: adopting 2-chloride-trityl resin, 4-methyl trityl resin or 4-methoxyl trityl resin as raw material; connecting amino acid with protective group according to solid-phase synthetic method; obtaining protected octapeptide resin; removing Fmoc-protective group sequently; stripping side-chain protective group; cutting peptide to obtain reduced aoqu-peptide; oxidizing through air under pH 7-11 condition; separating and purifying rought product through C18 (C8) column to produce exquisite.
Owner:SHANGHAI SOHO YIMING PHARMA
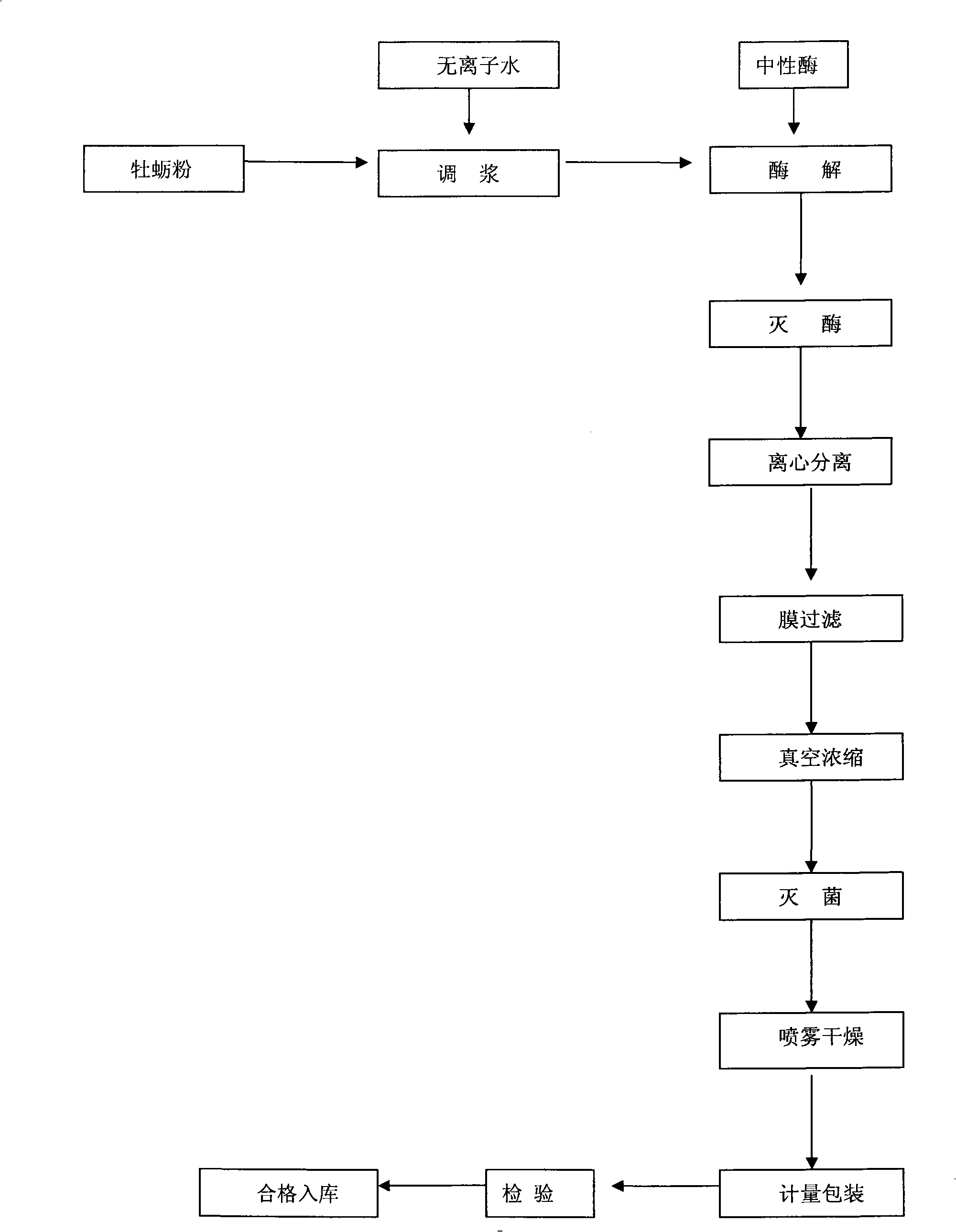
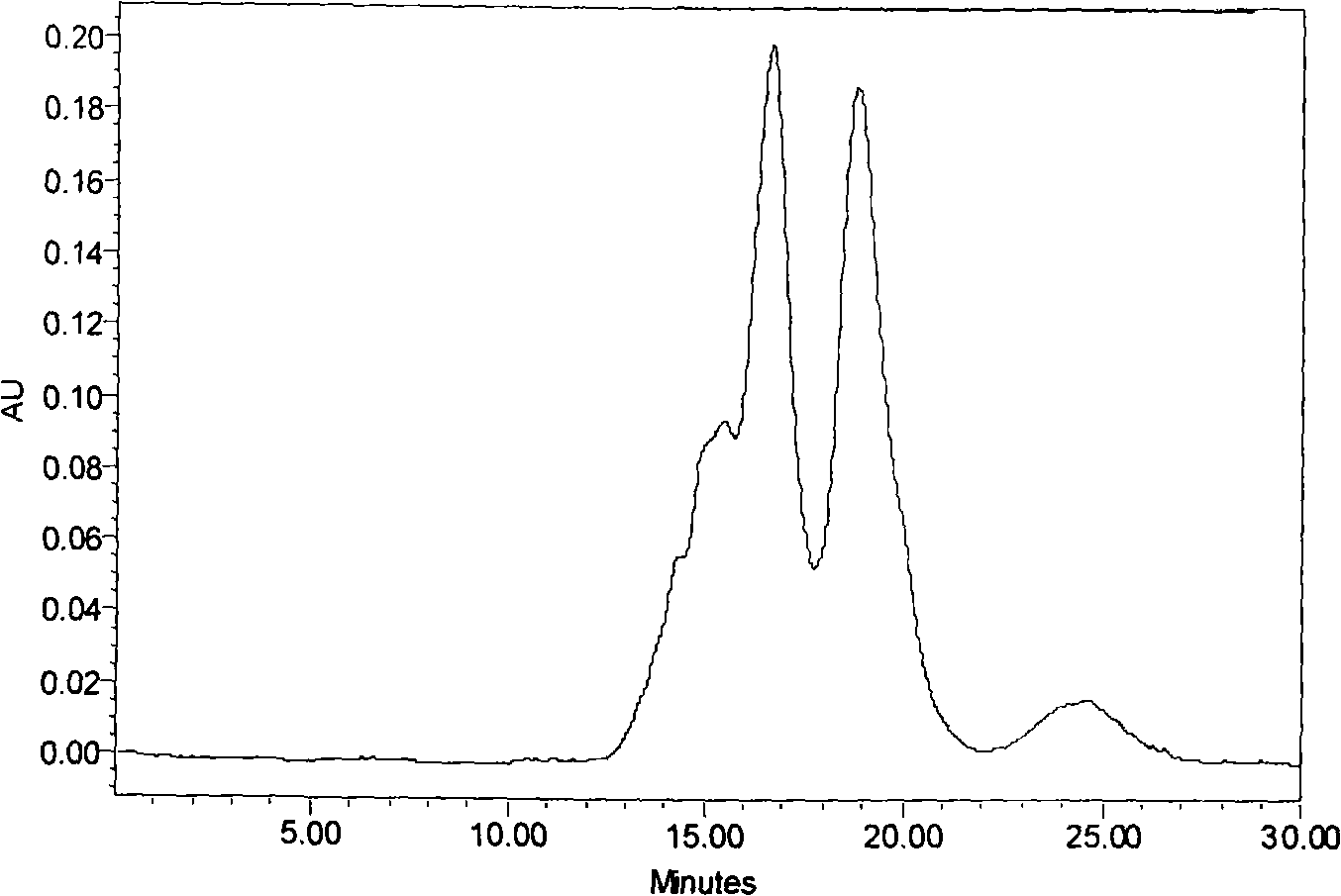
Industrial production method for making peptide of oyster from oyster powder by enzyme method
ActiveCN101263860AHigh peptide yieldHigh technology contentAnimal proteins working-upFood preparationNeutral proteaseEmulsion
The invention discloses an industrial production method for preparing oyster bioactive peptides from oyster powder through enzymatic method, which is characterized in that: the special of the production method lies in comprising the following steps: (1) inputting the oyster powder of 1kg, adding deionized water, carrying out shear through the emulsion shearing machine on the bottom of a tank after finishing feeding and reflowing into the tank; (2) regulating the PH of the feed liquid to 7.0 to 7.5, warming up to 48 to 52 DEG C, adding neutral protease of 10 to 20g and beginning enzymolysis for 6 hours; (3) warming up to 80 DEG C and carrying out killing enzyme for 10 minutes; (4) filtering with ceramic membrane, carrying out spray drying and passing through 60 mesh sieve for the feed liquid when the concentration of vacuum concentration is 20% and packaging with compound bag. The industrial production method has the advantages that: the technology is simple; the generating period is short; the produced oyster bioactive peptides are white, not fishy smell and good taste; and the molecular weight of the contained mixing peptide is small.
Owner:中食都庆(山东)生物技术有限公司
Industrial process for producing wheat peptide from glutelin powder by enzymic method
ActiveCN101297675AHigh peptide yieldHigh technology contentVegetable proteins working-upFood preparationChemistryProtein formation
The invention discloses an industrial production method for enzymatic preparing wheat peptide from wheat gluten. The production method is characterized by comprising the following steps: (1) putting wheat gluten of 1kg, adding deionized water, and reflowing to a pot; (2) heating up to 50 to 55 DEG C, pH of feed fluid is 7.5 to 8.0, then adding protamex of 1 to 2g, and carrying out enzymolysis for 8 hours; (3) heating up to 80 DEG C, and extinguishing enzyme for 10min; and (4) carrying out filtration by a ceramic membrane and vacuum concentration, treating the feed fluid with spray drying, sieving by a sieve with 60 meshes, and packing by compound bags. The wheat peptide prepared by the method of the invention has the protein content of above 50 percent, molecular weight of wheat peptide of 90 percent is below 1000Dalton. The method of the invention has simple technique and short production cycle, the wheat peptide product produced by the method is white and has good mouthfeel.
Owner:中食都庆(山东)生物技术有限公司
Preparing process for synthesizing oxytocin from solid-phase polypeptide
ActiveCN1990501AReduce usageSimple processOxytocins/vasopressinsPeptide preparation methodsProduction rateRink amide resin
The invention discloses a method for preparing oxytocin through solid phase polypeptide synthesis, comprising following steps: taking Rink Amide resin (comprising Rink Amide MBHA resin, Rink Amide Am resin) as raw material, taking amino acid protected by Fmoc, TBTU or HBTU / HOBt as condensing agent, making up amino acid in sequence; adding peptide cutting agent for peptide cutting, adding for precipitation and getting reduced coarse product; adding basic matter, feeding air for oxidation or oxiding with H2O2 with pH being 7.5- 10.0, getting oxidized coarse product; separating and purifying by using C18 or C 8 column and getting final product. The method is characterized by low production cost, simple process, little pollution, high production rate and convenience for industrial production.
Owner:SHANGHAI SOHO YIMING PHARMA
Preparation method of synthesizing growth chalone from solid phase polypeptide
InactiveCN1923851AConvenient sourceReduce usageSomatostatinsPeptide preparation methodsHydrogen fluorideSomatomedin
The invention discloses a chalone preparing method of solid-phase polypeptide, which comprises the following steps: adopting 2-chloride-trityl resin, 4-methyl trityl resin or 4-methoxyl trityl resin as raw material; connecting amino acid with protective group according to solid-phase synthetic method; obtaining protected octapeptide resin; removing Fmoc-protective group sequently; stripping side-chain protective group; cutting peptide to obtain reduced aoqu-peptide; oxidizing through air under pH 7-11 condition; separating and purifying rought product through C18 (C8) column to produce exquisite.
Owner:SHANGHAI SOHO YIMING PHARMA +1
Process for preparing solid phase polypeptide synthetic eptifibatide
ActiveCN1858060AConvenient sourceHigh peptide yieldPeptide preparation methodsPropanoic acidRink amide resin
The preparation process of solid phase polypeptide synthesized eptifibatide includes the following steps: (1) connecting amino acids one by one with Rink Amide resin, Rink Amide MBHA resin or Rink Amide AM resin as initial material, and Fmoc protected amino aids as monomer, with the last peptide chain being S-benzyl mercapto propionic acid Map(SBzl); (2) adding peptide cutting agent (TFA / HBr / HAc / TIS / EDT) to cut peptide; (3) precipitating and collecting coarse reductant eptifibatide product in ether solvent; (4) dissolving coarse reductant eptifibatide product in water, regulating pH value with ammonia water to 7.5-10.0, introducing air for oxidation, and collecting coarse eptifibatide product; and (5) separating and purifying the coarse product in C18 column to obtain the target product. The present invention has high yield, and is suitable for industrial production.
Owner:SHANGHAI SOHO YIMING PHARMA
Preparation method and use of sunflower seed polypeptide
InactiveCN101904406ARaise the ratio contentHigh preparation enzymatic efficiencyCosmetic preparationsProtein composition from vegetable seedsProtein isolateNitrosamine
The invention provides a preparation method of sunflower seed polypeptide. The method comprises the following steps of: dissolving sunflower seed proteins isolate in water; hydrolyzing the sunflower seed proteins isolate with two-stage protease: alkaline protease and composite acid protease, to obtain the polypeptide, wherein during the hydrolysis, ultrasonic intermittent frequency conversion is performed to assist enzymolysis; and performing microfiltration and ultrafiltration on the enzymolysis liquid to obtain the sunflower seed polypeptide. The method has the advantages of adopting different ultrasonic frequencies and processing times for different proteases, effectively improving the hydrolysis degree and polypeptide yield of the sunflower seed proteins and obviously improving the production efficiency compared with the conventional process. A product prepared by the method has multiple health care functions of eliminating free radicals, removing nitrites, blocking the synthesis of nitrosamines, inhibiting the oxidization of red blood cells, beautifying and caring skins, preventing hepatic injury and the like and can be used for preparing functional foods and cosmetics.
Owner:SHANXI UNIV
Process for preparing corn peptides by taking corn starch sugar residues as raw materials
ActiveCN103305576AIncrease profitOvercome expensiveNatural dyesFermentationResource utilizationBy-product
The invention relates to a process for preparing corn peptides by taking corn starch sugar residues as raw materials. The process comprises the following steps of: crushing corn starch sugar residues, adding absolute ethyl alcohol to extract a yellow pigment, drying the materials subjected to complete pigment extraction, crushing and sieving, adding water, adjusting the pH value, and respectively adding alkaline protease and flavourzyme to hydrolyze; adjusting the pH value by using hydrochloric acid and adding active carbon to decolor and remove bitter; desalting the obtained feed liquid by using a membrane system, and freezing and drying in vacuum to prepare corn peptides powder. According to the process, the corn starch sugar residues are used as the materials to prepare the corn peptides, and the process is low in cost, material consumption and energy consumption, and is beneficial to the improvement of resource utilization of by-products.
Owner:SHANDONG LUZHOU FOOD GROUP
Method for synthesizing atosiban acetate from solid phase polypeptide
ActiveCN101357937AConvenient sourceHigh peptide yieldPeptide preparation methodsBulk chemical productionPreparative hplcSide chain
The invention discloses a preparation method of solid phase peptide synthesis atosiban which includes the following steps: taking Rink Amide resins, Rink Amide MBHA resins or Rink Amide AM resins as starting materials and taking Fmoc amino acids as monomers, amino acids are grafted one by one and mercaptopropionic acids (Map(SX)) are protected by the last peptide chain; after protected nonapeptide resins are obtained, the acellular side-chain protective groups and cutting peptides are synchronized; then cutting peptides is carried out, and reduced crude atosiban is collected; the pH value is adjusted to 7.5 to 10.0, and oxidized crude atosiban is collected; target products are obtained by the separation and purification by preparative HPLC(C18 or C8 column). The preparation method is convenient in material source, simplifies technology and reduces cost; and the preparation method is low in the pollution of three wastes and is high in yield, and the preparation method is convenient for being industrialized and has good industrialization prospect.
Owner:SHANGHAI SOHO YIMING PHARMA
Polypeptide synthesis method for octreotide acetate
ActiveCN103351426AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionFluoroacetic acidAceric acid
The invention relates to a polypeptide synthesis method for octreotide acetate. The method comprises the following steps of: taking chloromethyl resin as a starting raw material, preparing a cesium salt from Boc-Thr(tBu)-OH, sequentially connecting amino acids with protecting groups according to a solid-phase synthesis method so as to obtain protected octapeptide resin, meanwhile, removing Boc protecting groups by sequentially using HCl / isopropyl alcohol, carrying out peptide connecting reaction in a manner of taking DIC and HOBT as condensing agents, carrying out reduction by using palladium carbon / hydrogen gas, meanwhile, cutting off peptide chains so as to obtain reduced octreotide, introducing air at the Ph of 7.8-9 so as to cyclize disulfide linkages, then, obtaining a crude octreotide product, and carrying out separation and purification through a C18 column, thereby preparing a fine octreotide acetate product. The method disclosed by the invention has the advantages that threoninol and Fmoc-threoninol are not adopted, the production cost is very low, the method has large-scale production capacity, the process is stable, the raw and auxiliary materials are convenient to obtain, the production cycle is short, the yield of connected peptide is high, the quality is stable, the use of highly-toxic reagents, such as hydrogen fluoride, trifluoroacetic acid and the like, is avoided, and the pollution caused by waste gas, waste water and waste residues is little.
Owner:SHANGHAI SOHO YIMING PHARMA
Industrial production method of ovum protein polypeptide from fowl ovum by enzymatical process
The invention discloses a unique extracting method of egg albumin from eggs, which comprises the following steps: using composite proteinase to enzymolyze egg; separating; hyperfiltering; condensing; spraying; drying; obtaining the white powder shaped product. The invention simplifies the technique and shortens the manufacturing period with good taste and small molecular weight of hybrid peptide, which can be clinical nutrition, hygienic food, sports food and cosmetics.
Owner:中食都庆(山东)生物技术有限公司
Solid phase polypeptide synthesis preparation method for salcatonin
ActiveCN1865283AReduce usageWith large-scale production capacityCalcitoninsPeptide preparation methodsSolid phasesSide chain
The invention discloses a new salmon calcimar preparing method of Fmoc-strategy solid-phase polypeptide, which comprises the following steps: a. adopting Fmoc-Rink Amide MBHA or Rink Amide AM resin to connect the 32-piptide resin protected by kinds of amino acid after eluting Fmoc-protection sequently; b. eluting Fmoc-protective group sequently; c. removing side-chain protective group and cutting peptide synchronously to obtain reduced crude product; d. making crude product to do oxidation reaction through (7.5-10.0 pH) air; proceeding inverse-phase HPLC separation and purifying to produce fine salmon calcimar. The invention possesses scale producing capacity, which improves the quality, obtaining rate, and total obtaining rate.
Owner:SHANGHAI SOHO YIMING PHARMA
Preparation method of soybean whey polypeptides with liver protection and antioxidation effects
The invention provides a preparation method of soybean whey polypeptides with liver protection and antioxidation effects. The preparation method comprises the following steps: carrying out flocculation treatment and impurity removal on soybean whey wastewater, adding 0.5-1.5 grams of cysteine to each liter of whey water and carry out hydrolysis for 5-7 hours under the conditions that 1000-2500 activity unit of acid protease is added to each gram of proteins, the pH value is 2-3 and the temperature is 40-55 DEG C; and carrying out hydrolysis for 3-5 hours under the conditions that 1000-2500U / g of pepsase is added, the temperature is 35-45 DEG C and pH value is 2-3; and carrying out enzyme killing, centrifugation, filtration, concentration and drying on hydrolysate to obtain the soybean whey polypeptides. Animal experiments prove that the soybean whey polypeptides have strong antioxidation and acute liver injury resistance effects. The polypeptides contain more than 80% of small molecular oligopeptides below 1000Da. Through human test, the polypeptides have good taste and do not have side effects. The polypeptides can serve as the raw materials of common nutritional and health food.
Owner:LINYI SHANSONG BIOLOGICAL PRODS
Preparation of octreotide acetate and octreotide acetate injection pharmaceutical composition
ActiveCN106866788AConvenient sourceReduce usagePeptide/protein ingredientsDigestive systemDisulfide bondSolid-phase synthesis
The invention relates to preparation of octreotide acetate and an octreotide acetate injection pharmaceutical composition. Specifically, the invention relates to a method for preparing the octreotide acetate; the method comprises the following steps: taking merrifield resin as a starting raw material and preparing Boc-Thr(tBu)-OH into cesium salt; sequentially connecting amino acid with protecting groups according to a solid-phase synthesis method to obtain protected octapeptide resin; removing Boc-protecting groups in sequence by utilizing HCl / isopropyl alcohol and carrying out peptide linking reaction by utilizing a condensing agent; reducing with palladium-carbon / hydrogen; meanwhile, chopping off a peptide chain to obtain reduced octreotide; ventilating air under the condition that the pH (Potential of Hydrogen) is 8 to 9 to form a ring by a disulfide bond, so as to obtain an octreotide crude product; separating and purifying the octreotide crude product through a C18 column to prepare refined octreotide. The invention further relates to the octreotide acetate injection pharmaceutical composition. The method for preparing the octreotide acetate and the octreotide acetate injection pharmaceutical composition, provided by the invention, have the excellent technical effects shown in the description.
Owner:CHENGDU TIANTAISHAN PHARMA
Extraction method of tuna polypeptide
ActiveCN105154503AShorten heating timeSave energyPeptide preparation methodsFermentationNeutral proteaseProteinase activity
The invention discloses an extraction method of tuna polypeptide and aims to mainly solve the problem that tuna polypeptide extracted by an existing method has fishy smell. The method includes: extracting protein in tuna processing leftovers via a thin salt solution, removing the fishy smell from the extracted tuna protein via modified bentonite, adjusting pH of tuna protein liquid before fishy smell removal according to the principle isoelectric precipitation of protein, precipitating the extracted tuna protein, thereby avoiding adsorption of the modified bentonite for the protein, enzymatically hydrolyzing the protein via neutral protease and compound protease to obtain the tuna polypeptide. The tuna polypeptide extracted by the method has no fishy smell and is high in yield.
Owner:山东思科生物科技有限公司
Placenta polypeptide preparation method
ActiveCN105420325AEasy to prepareReduce manufacturing costHydrolasesPeptide preparation methodsUltrafiltrationPROTEASE M
The invention relates to a bioactivator preparation method, in particular to a placenta polypeptide preparation method. The placenta polypeptide preparation method comprises the steps of cutting a placenta into pieces, and adding normal saline into the cut placenta for tissue homogenate; mixing the obtained product with protease for enzymatic hydrolysis, and performing centrifugation and collecting centrifugate; performing extraction through reverse micelles to produce placental polypeptides and crude protease extract, subjecting the crude protease extract to ultrafiltration, collecting ultrafiltrate, and drying the ultrafiltrate to produce the placenta polypeptides. The preparation method is simple, production cost is low, equipment investment is little, and large-scale production is facilitated.
Owner:GUIZHOU TAIBANG BIOLOGICAL PROD
Process of preparing calf thymus alphal
ActiveCN101033248AReduce usageWith large-scale production capacityPeptide preparation methodsAnimals/human peptidesAcetic anhydrideSide chain
The invention discloses a new technology to prepare calf thymus alpha 1 (natural existing in thymus cells of calf and other animals), which uses Fmoc-strategy solid phase method, including the following steps: a. taking any one of Rink Amide PEGA resin, Rink Amide AM resin, Rink Amide MBHA resin or Rink Amide PEGA as the starting material, using the amino acid protected by Fmoc as the monomer to hang resin, connecting amino acids in turn to get a protective 28-peptide resin according to the method of solid-phase synthesis, b. using acetic anhydride to close heads during the process in turn, c. removing Fmoc-protective group in turn, d. synchronizing the removal of side-chain protecting group and the cut of peptide to get a crude, e. purifying the crude with antiphase HPLC to get thymosin alpha 1.
Owner:SHANGHAI SOHO YIMING PHARMA
Solid phase polypeptide synthesis preparation method for terlipressin
ActiveCN1865282AReduce usageSimple processOxytocins/vasopressinsPeptide preparation methodsIndustrial constructionRink amide resin
The invention discloses a Telis-vasophysin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Rink Amide resin (concluding Rink Amide MBHA resin, Rink AmideAM resin) as original material, Fmoc protective amino acid as monomer, TBTU or HBTU-to-HOBt as condensing agent to connect amino sequently; using Boc-Gly-OH for the last peptide chain; adding peptide cutting agent to cut peptide; adding ether to deposit to obtain crude product; adding alkali material to oxidate at 7.5-10.0 pH value to generate oxide crude product; proceeding separation and purifying through C18 (or C8) pillar to produce object product. The method possesses low manufacturing cost, simple technology, high obtaining rate and low environmental pollution, which is convenient to do industrial construction.
Owner:SHANGHAI SOHO YIMING PHARMA
Octreotide acetate injection pharmaceutical composition and octreotide acetate
ActiveCN106860854AConvenient sourceReduce usagePeptide/protein ingredientsDigestive systemOctreotide acetateSodium bicarbonate
The invention relates to an octreotide acetate injection pharmaceutical composition and octreotide acetate. Specifically, the octreotide acetate injection pharmaceutical composition provided by the invention is prepared from octreotide acetate containing 0.1g of octreotide, 40g to 50g of mannitol, 3g to 4g of lactic acid, a proper amount of sodium hydrogen carbonate which is added until the pH (Potential of Hydrogen) is 3.7 to 4.7 and a proper amount of injection water which is added until the total amount is 1000ml and the like. The invention further relates to an octreotide acetate product and a preparation method thereof. The octreotide acetate injection pharmaceutical composition and the octreotide acetate, provided by the invention, have the excellent technical effects shown in the description.
Owner:CHENGDU TIANTAISHAN PHARMA
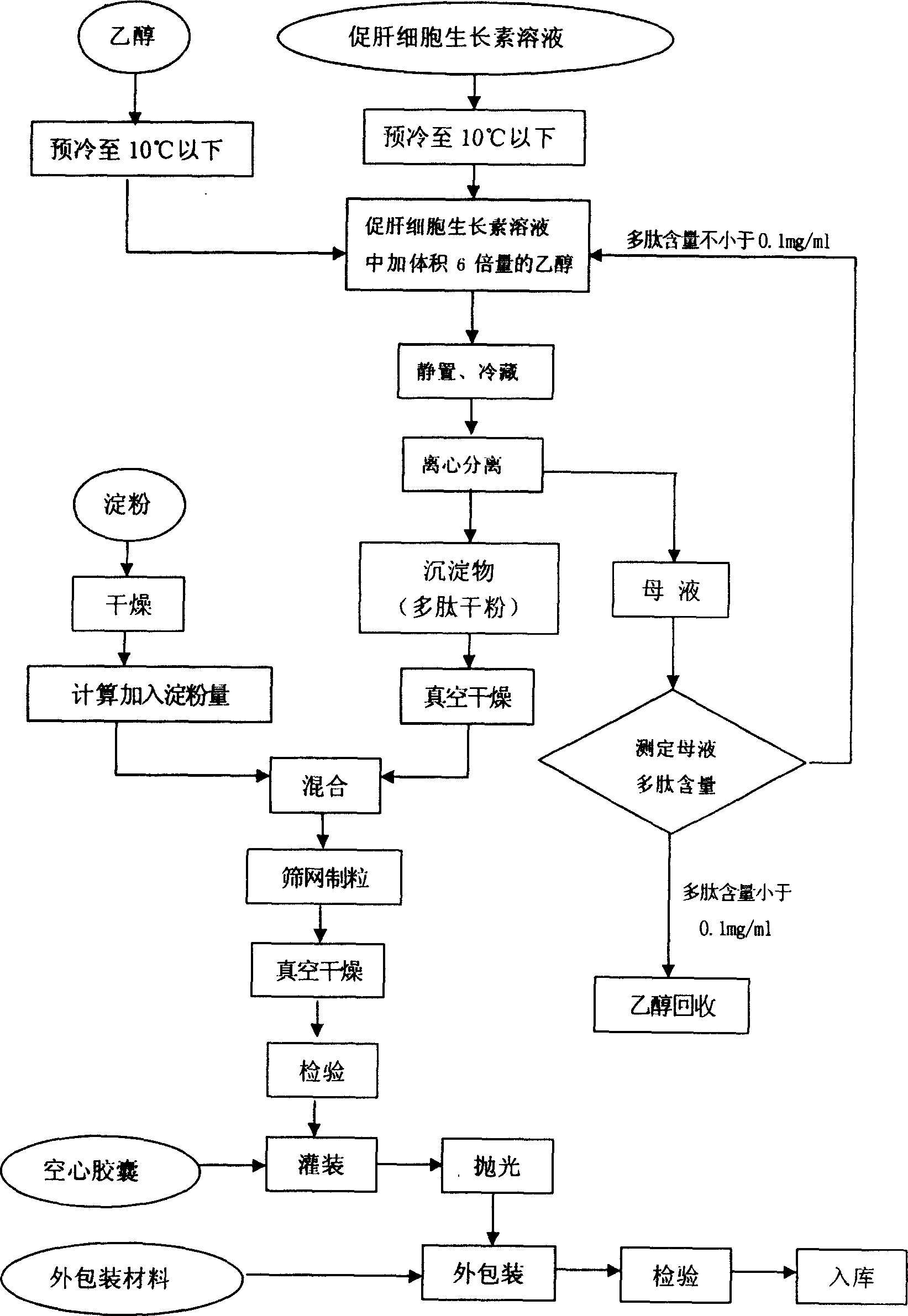
Technology for preparing capsule of liver cell growth promoting factor
ActiveCN1628843ABiological activity is not affectedEase of mass productionPeptide/protein ingredientsDigestive systemAlcoholDiluent
The invention provides a technology for preparing capsule of liver cell growth promoting factor which comprises the steps of, extracting polypeptide dried powder from hepatocyte growth-promoting factors solution, charing diluent, mixing and palletizing, and loading into capsules, wherein the polypeptide dried powder extracting procedure comprises employing alcohol depositing method, charging ethanol into the solution of hepatocyte growth-promoting factors, refrigerating, separating, and collecting precipitation.
Owner:浙江华缔药业集团有限责任公司
Method for preparing antioxidative peptide through fermentation of cordyceps militaris nanoparticles
ActiveCN104789621ARich in nutrientsRich in functional active ingredientsMicroorganism based processesFermentationBacillus licheniformisMicroparticle
A method for preparing antioxidative peptide through fermentation of cordyceps militaris nanoparticles is characterized by comprising the steps: drying cordyceps militaris at the temperature of 60-80 DEG C, carrying out coarse crushing, passing through a 40-mesh sieve, then carrying out nano crushing for 3-5 h, and thus obtaining cordyceps militaris nanoparticles; adding the cordyceps militaris nanoparticles to distilled water, shaking evenly, adjusting to a natural pH value, sterilizing for 20-30 min in a high-pressure sterilization pot at the temperature of 121 DEG C, and thus obtaining a seed culture medium; putting a beef extract, peptone and sodium chloride in distilled water, allowing the pH to be 6-7, carrying out high-pressure steam sterilization for 20-30 min at the temperature of 121 DEG C, cooling to obtain an ordinary beef extract-peptone culture medium, inoculating the ordinary beef extract-peptone culture medium with a slant strain of a bacillus licheniformis strain, at the temperature of 30-40 DEG C, culturing for 1-2 d, to obtain inoculated and activated bacillus licheniformis, inoculating the seed culture medium with the activated bacillus licheniformis, and thus obtaining the antioxidative peptide product. The method is high in efficiency, low in cost, short in production cycle, and suitable for large-scale production.
Owner:JILIN SERICULTURE SCI RES INST
A kind of preparation method of placental polypeptide
ActiveCN105420325BIncrease contentThe parameters are economical and reasonableHydrolasesPeptide preparation methodsEnzymatic hydrolysisCentrifugation
The invention relates to a bioactivator preparation method, in particular to a placenta polypeptide preparation method. The placenta polypeptide preparation method comprises the steps of cutting a placenta into pieces, and adding normal saline into the cut placenta for tissue homogenate; mixing the obtained product with protease for enzymatic hydrolysis, and performing centrifugation and collecting centrifugate; performing extraction through reverse micelles to produce placental polypeptides and crude protease extract, subjecting the crude protease extract to ultrafiltration, collecting ultrafiltrate, and drying the ultrafiltrate to produce the placenta polypeptides. The preparation method is simple, production cost is low, equipment investment is little, and large-scale production is facilitated.
Owner:GUIZHOU TAIBANG BIOLOGICAL PROD
Solid phase polypeptide synthesis preparation method for terlipressin
ActiveCN1865282BReduce usageSimple processOxytocins/vasopressinsPeptide preparation methodsIndustrial constructionRink amide resin
The invention discloses a Telis-vasophysin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Rink Amide resin (concluding Rink Amide MBHA resin, Rink AmideAM resin) as original material, Fmoc protective amino acid as monomer, TBTU or HBTU-to-HOBt as condensing agent to connect amino sequently; using Boc-Gly-OH for the last peptide chain; adding peptide cutting agent to cut peptide; adding ether to deposit to obtain crude product; adding alkali material to oxidate at 7.5-10.0 pH value to generate oxide crude product; proceeding separation and purifying through C18 (or C8) pillar to produce object product. The method possesses low manufacturing cost, simple technology, high obtaining rate and low environmental pollution, which is convenient to do industrial construction.
Owner:SHANGHAI SOHO YIMING PHARMA
Industrial process for producing wheat peptide from glutelin powder by enzyme method
ActiveCN101297675BHigh peptide yieldHigh technology contentVegetable proteins working-upFermentationGlutelinCeramic membrane
The invention discloses an industrial production method for enzymatic preparing wheat peptide from wheat gluten. The production method is characterized by comprising the following steps: (1) putting wheat gluten of 1kg, adding deionized water, and reflowing to a pot; (2) heating up to 50 to 55 DEG C, pH of feed fluid is 7.5 to 8.0, then adding protamex of 1 to 2g, and carrying out enzymolysis for8 hours; (3) heating up to 80 DEG C, and extinguishing enzyme for 10min; and (4) carrying out filtration by a ceramic membrane and vacuum concentration, treating the feed fluid with spray drying, sieving by a sieve with 60 meshes, and packing by compound bags. The wheat peptide prepared by the method of the invention has the protein content of above 50 percent, molecular weight of wheat peptide of 90 percent is below 1000Dalton. The method of the invention has simple technique and short production cycle, the wheat peptide product produced by the method is white and has good mouthfeel.
Owner:中食都庆(山东)生物技术有限公司
Solid phase polypeptide synthesis preparation method for leuprorelin
ActiveCN1865280BConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionHydrogen fluorideLeuprorelin
The invention discloses a bright-ala-ruilin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Wang resin or CTC resin as original material to connect amino with protective group to produce protective nonapeptide resin; removing Fmoc-protective group sequently; proceeding side-chain protective group synchronizingly and cutting peptide; connecting ethylaminethrough ethylamine-to-HOBT to produce crude product; proceeding separation and purifying through C18 (or C8) pillar to produce fine bright-ala-ruilin. The invention avoids the utility of poisonous agent, which improves the purifying, peptide connecting and obtaining rate.
Owner:SHANGHAI SOHO YIMING PHARMA
Octreotide acetate injection drug composition and application thereof
ActiveCN109432394AConvenient sourceReduce usagePeptide/protein ingredientsDigestive systemDrugChemistry
The invention relates to an octreotide acetate injection drug composition and application thereof. The octreotide acetate injection drug composition comprises 0.1g of octreotide acetate (by octreotide), 40-50g of mannitol, 3-4g of lactic acid, an appropriate amount of sodium hydrogen carbonate with the pH adjusted to 3.7-4.7, 1000ml of water for injection, and the like. The invention also discloses an octreotide acetate product and a preparation method thereof. The invention further relates to a method of determining impurities, such as oxidatively degraded impurities, in the octreotide acetate or the composition thereof. The octreotide acetate injection drug composition displays excellent technical effects as described in the description, for example, bulk drugs have higher production efficiency in the preparation process than those in the prior art, the drug loss during injection preparation is significantly reduced, and the growth rate of the impurities in long-term stability reserved samples is significantly lower than that in the prior art.
Owner:CHENGDU TIANTAISHAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com