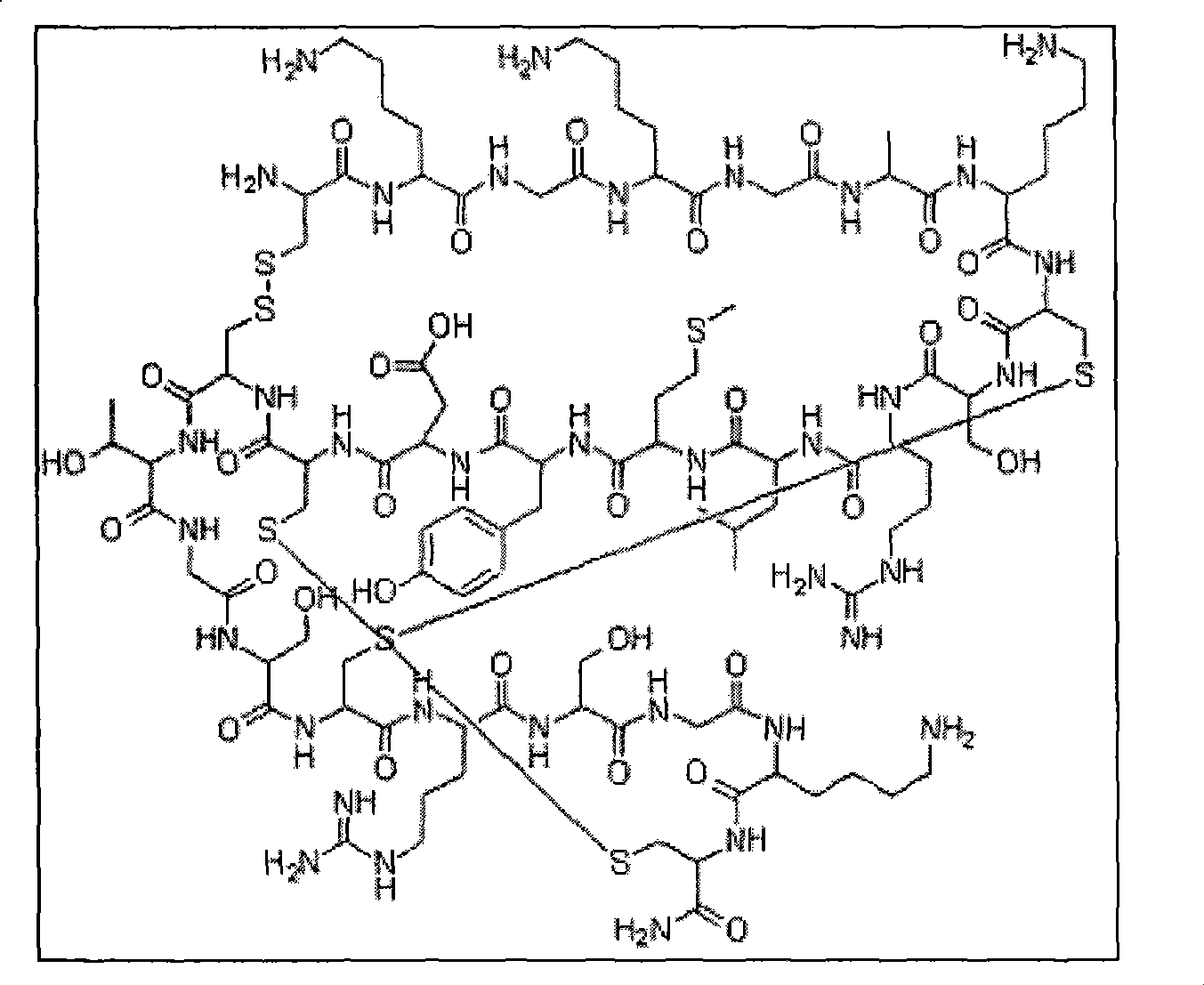
Solid phase synthesis method of ziconotide
A technology of solid-phase synthesis and ziconotide, which is applied in the field of biochemistry, can solve the problems of difficult to obtain high-purity products, cumbersome extraction process, and complicated process control, so as to facilitate product purification, high product yield, and improve The effect of cyclization speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Preparation of Ziconotide Linear Peptide Resin
[0044] Operation: (1) Put 50g (25mmol) of Fmoc-Rink Amide-AM resin in the polypeptide synthesis reactor, alternately wash twice with 300ml of dichloromethane and 300ml of ethanol, 2 minutes each time, and drain. Add 500 ml of 20% (v / v) piperidine / DMF solution, stir at room temperature for 20 minutes, and drain. The resin was alternately washed 3 times with dichloromethane and 300ml ethanol, 2 minutes each time, and drained.
[0045] (2) After dissolving 16.9g of Fmoc-protected amino acid and HOBt in 500ml of dichloromethane / DMF (volume ratio 1:1), add it to the resin, and then add 19ml of N,N-diisopropylcarbodiimide dropwise, After the drop was completed, it was stirred at room temperature for 3 hours.
[0046] (3) Drain. The resin was alternately washed 3 times with 300ml of dichloromethane and 300ml of ethanol, each time for 2 minutes, and drained.
[0047] (4) Steps (1)-(3) are repeated until all amin...
Embodiment 2
[0052] Embodiment 2: the preparation of ziconotide one cyclic peptide resin
[0053] Operation: Add the ziconotide linear peptide resin obtained in Example 1 into a 2000ml round-bottomed flask, add 1% (v / v) trifluoroacetic acid / dichloromethane solution 1300ml, stir and react at room temperature for 20 minutes, filter, and use After the resin was washed alternately with dichloromethane and methanol, 1200ml of water / acetonitrile (volume ratio 6:4) and 48ml of DMSO (dimethyl sulfoxide) were added, the pH was adjusted to 8 with dilute ammonia, and the reaction was stirred at room temperature for 4 hours. A cyclic peptide resin of ziconotide was obtained.
Embodiment 3
[0054] Embodiment 3: the preparation of ziconotide bicyclic peptide resin
[0055] Operation: Add the ziconotide monocyclic peptide resin obtained in Example 2 into a 2000ml round bottom flask, add 1300ml of iodine / methanol solution (128g iodine is dissolved in 1300ml methanol), stir and react at room temperature for 2 hours, filter, and use DMF (N, N-dimethylformamide), methanol, 1M ascorbic acid DMF solution alternately wash the resin, dry to obtain Ziconotide bicyclic peptide resin 126.1g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com