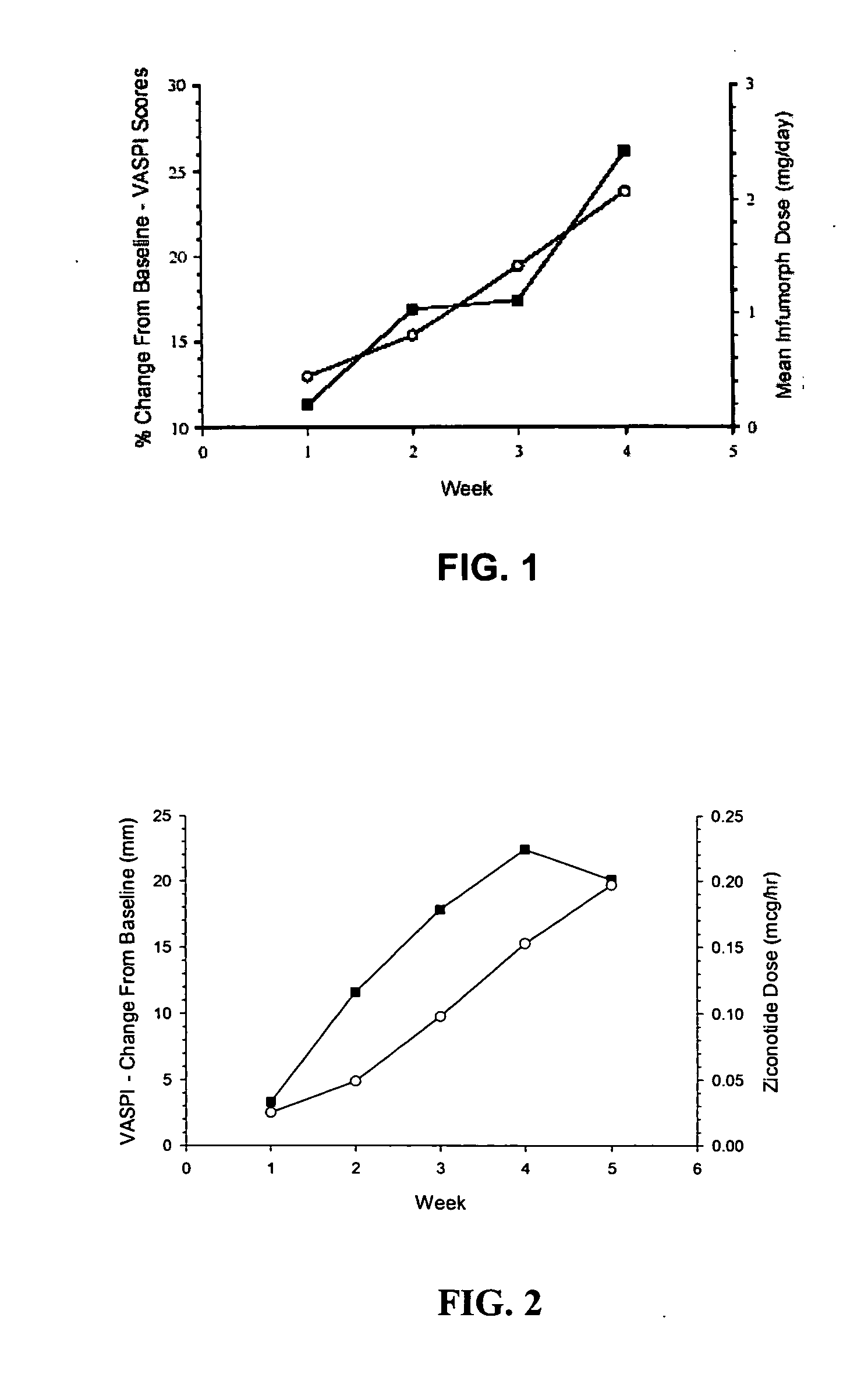
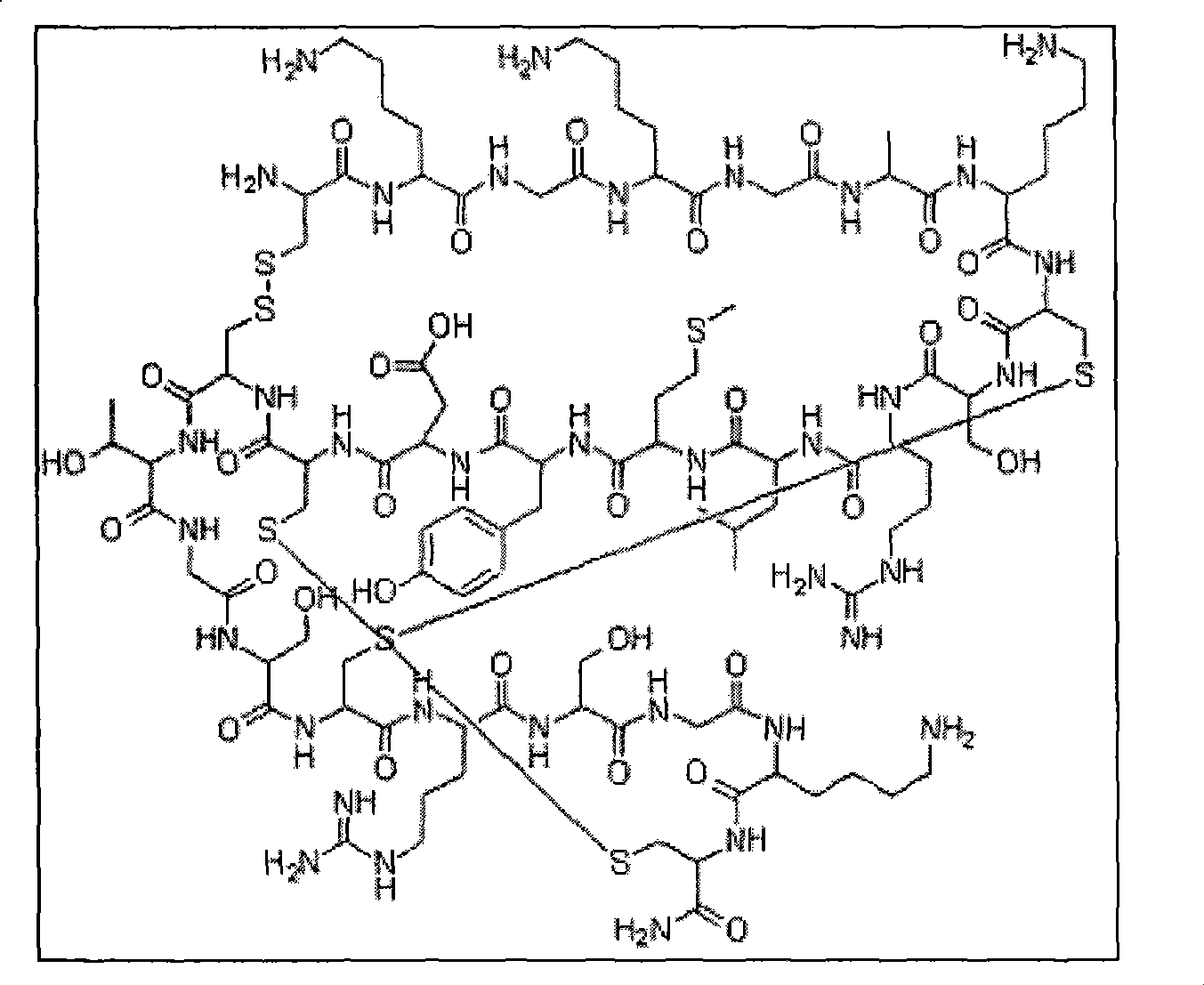
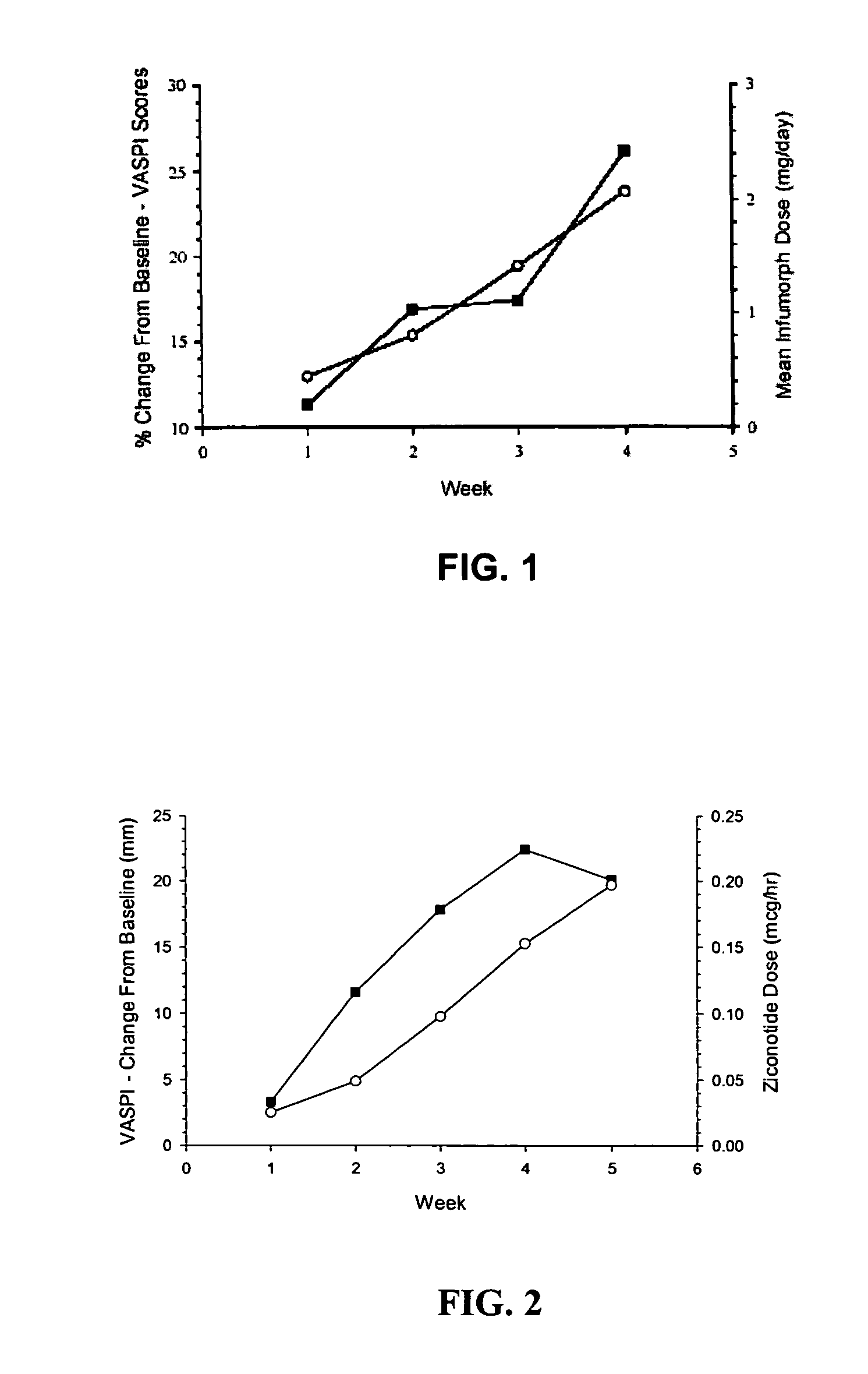
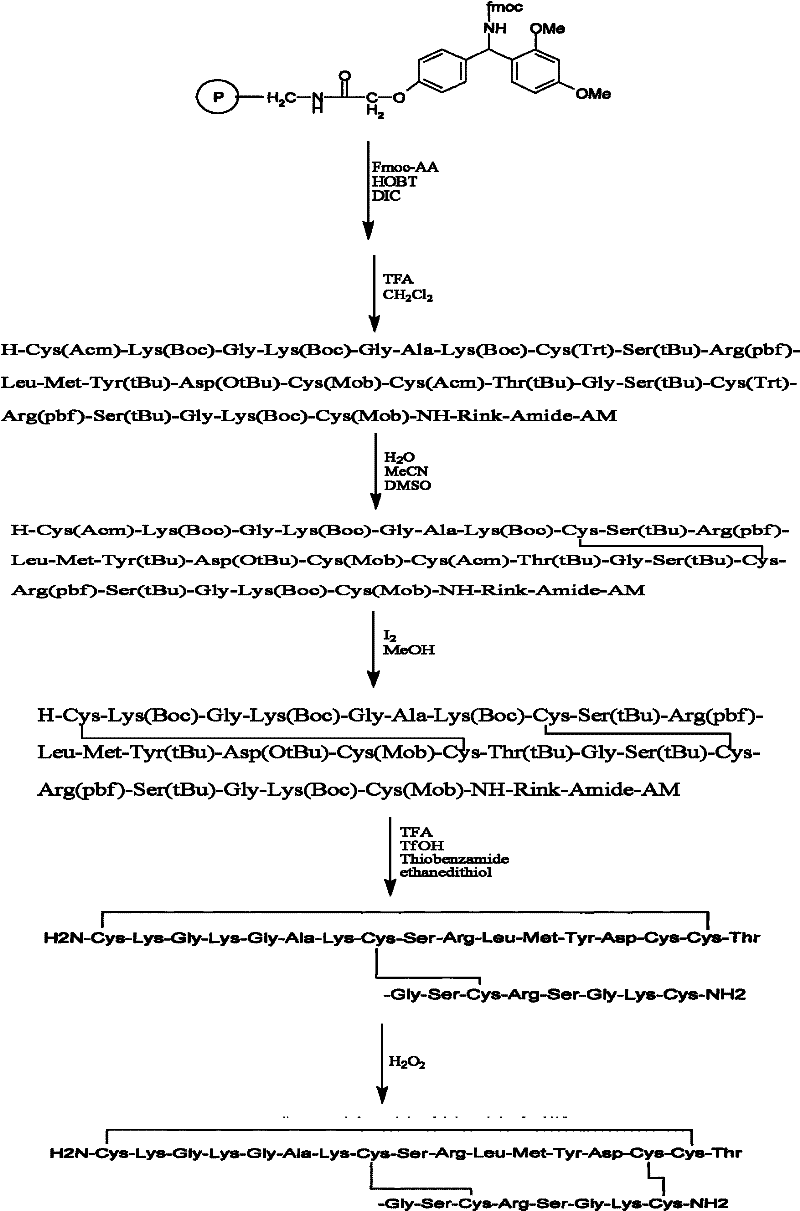
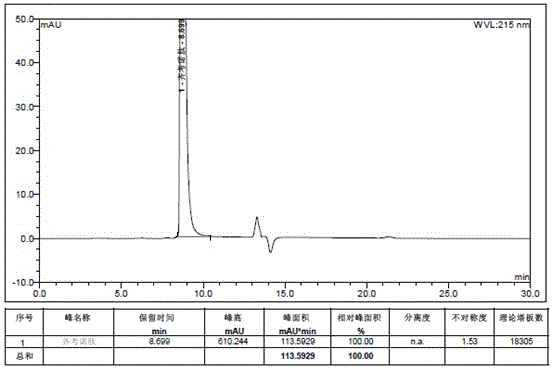
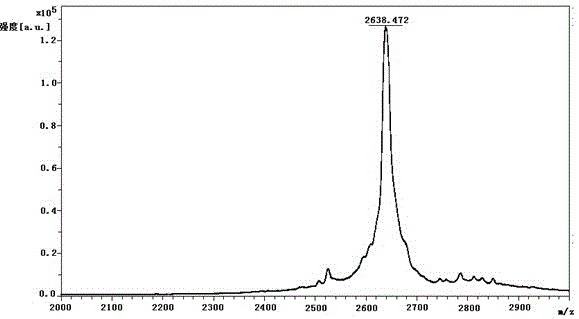
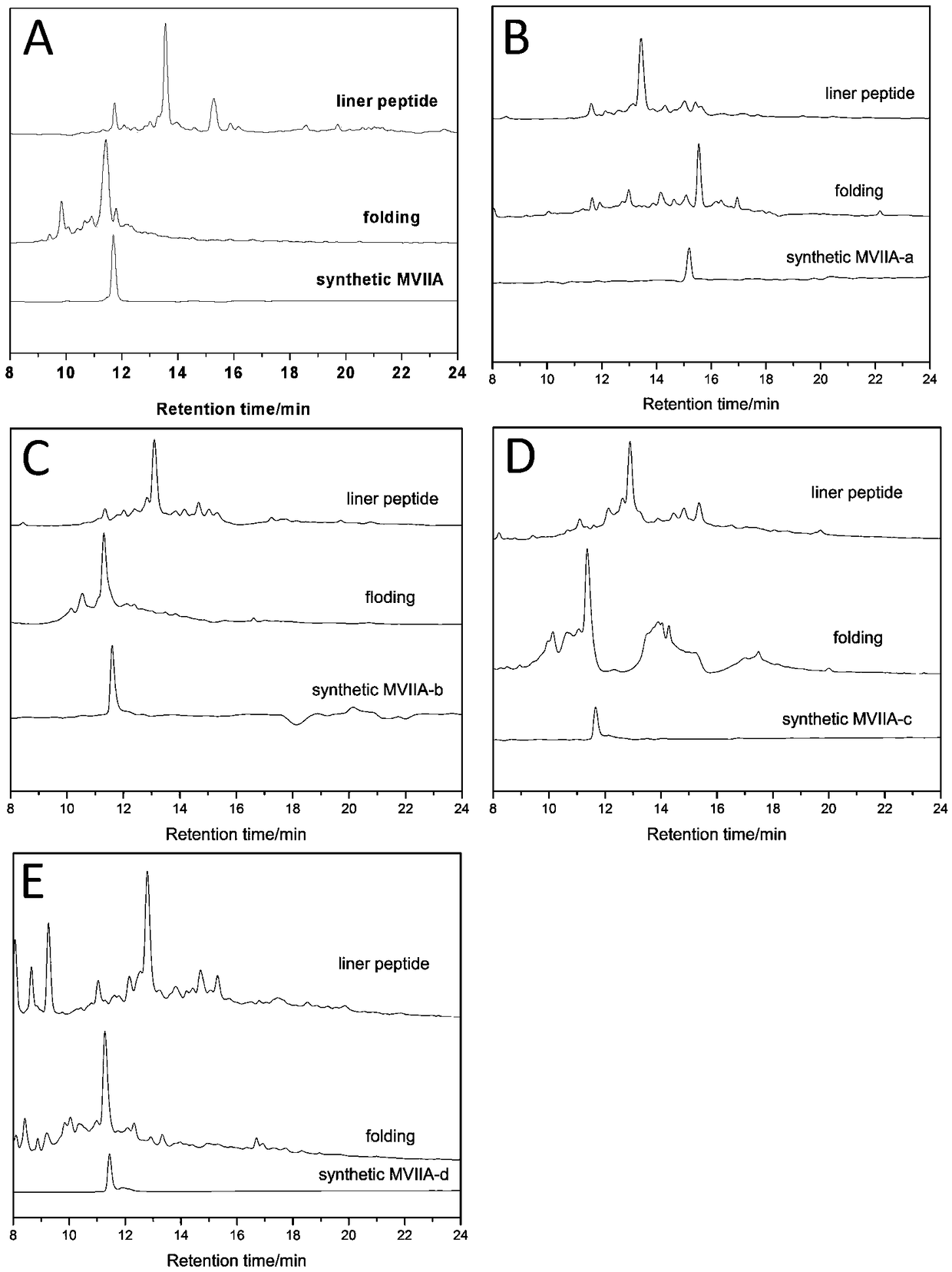
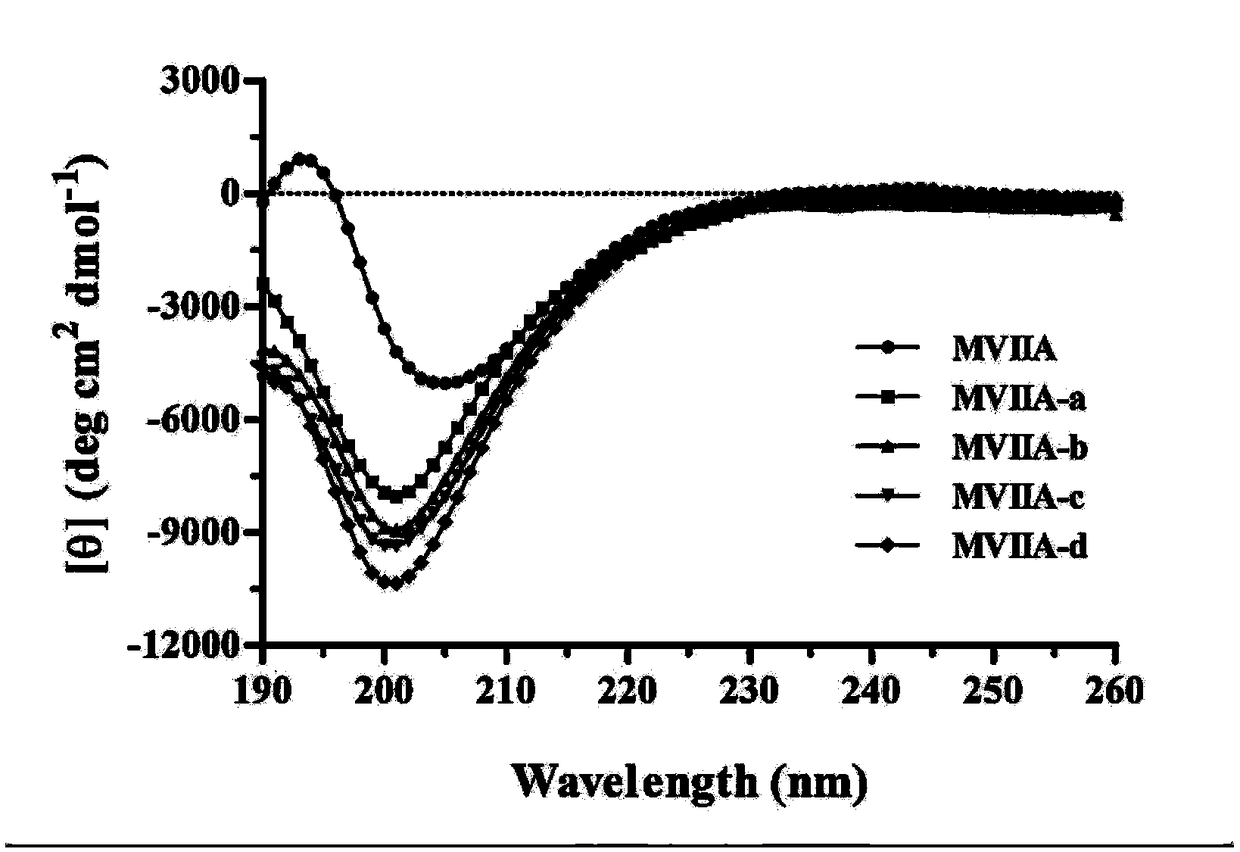
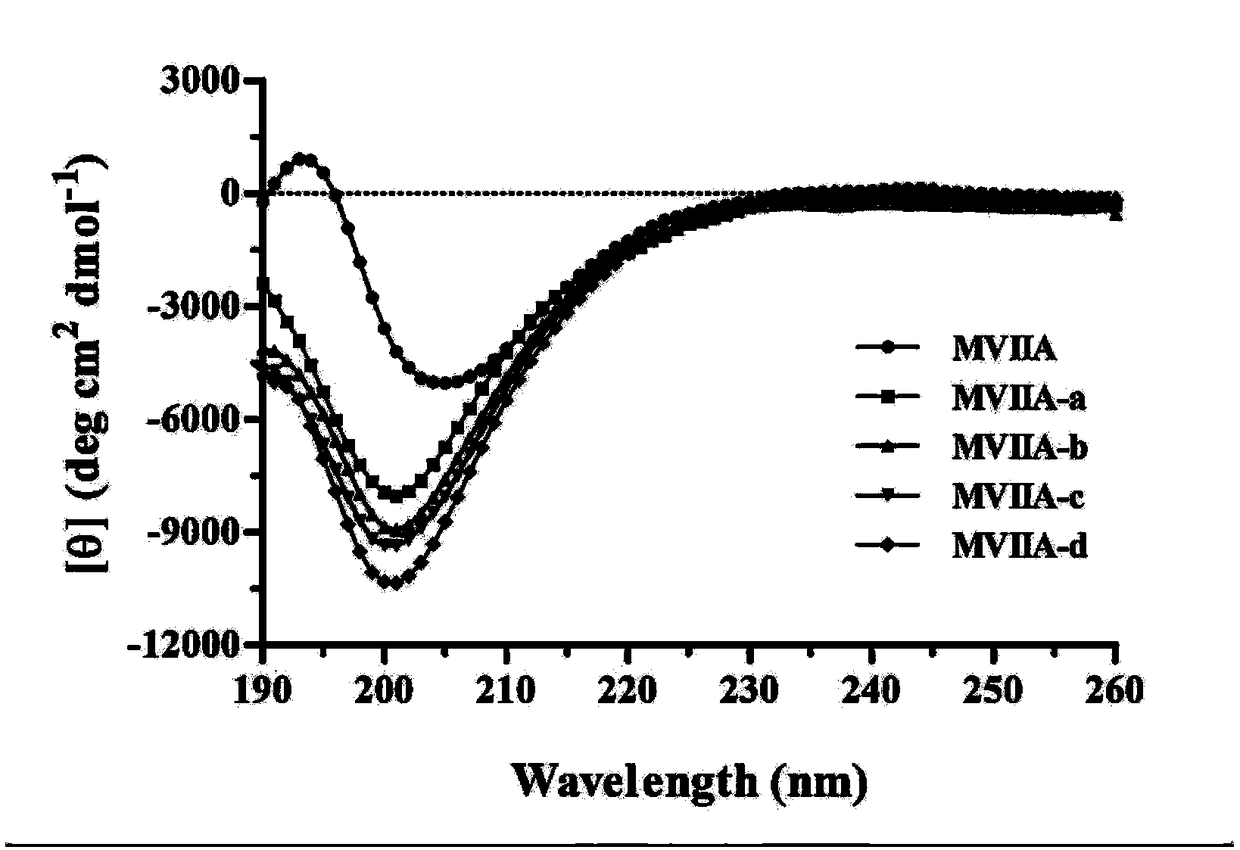
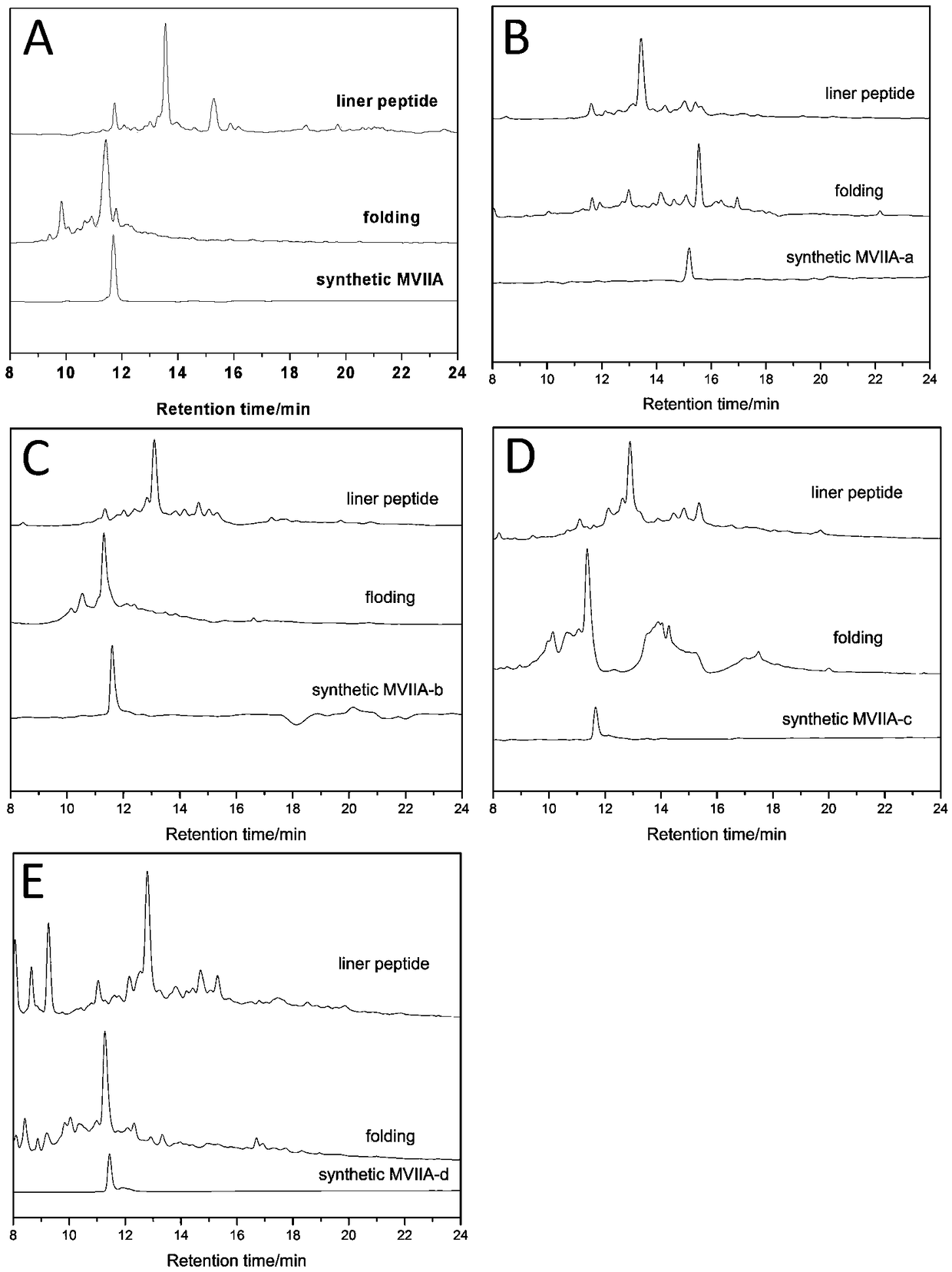
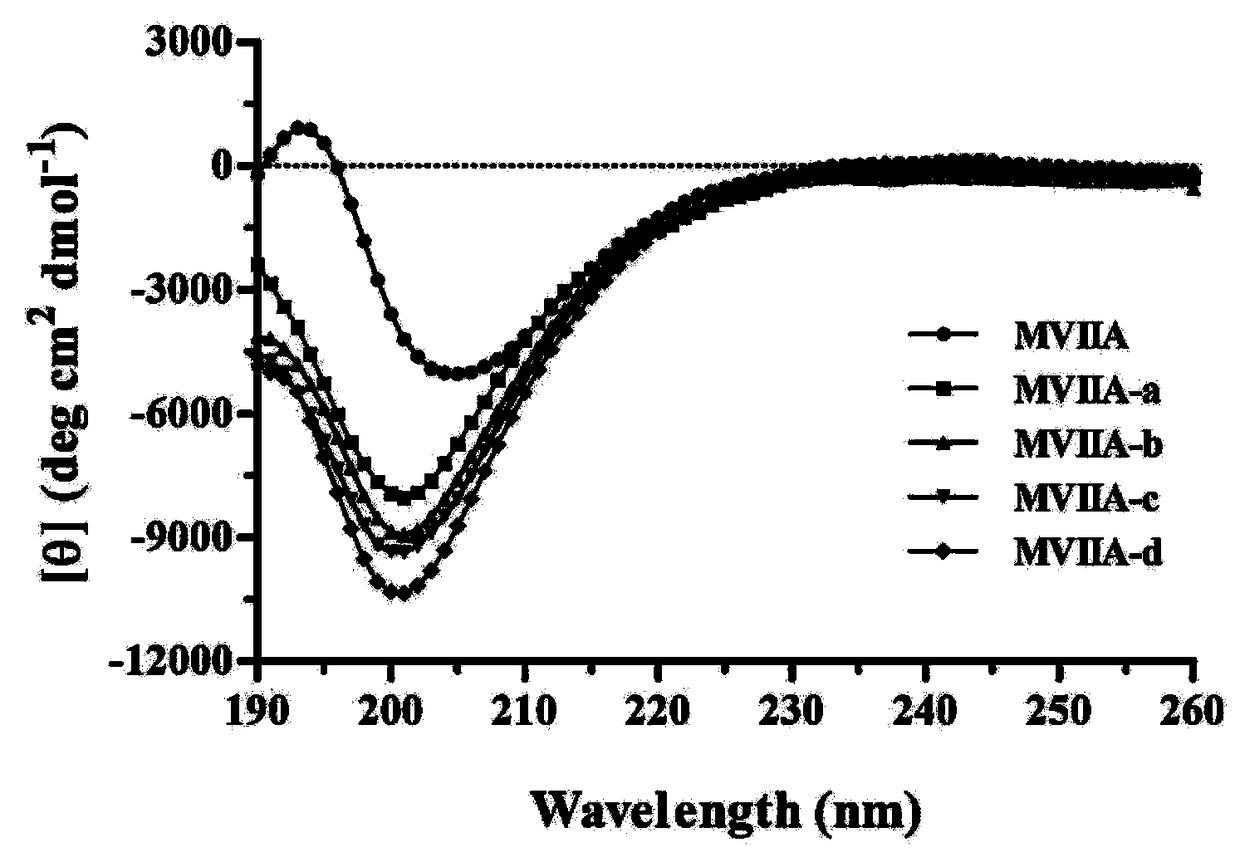
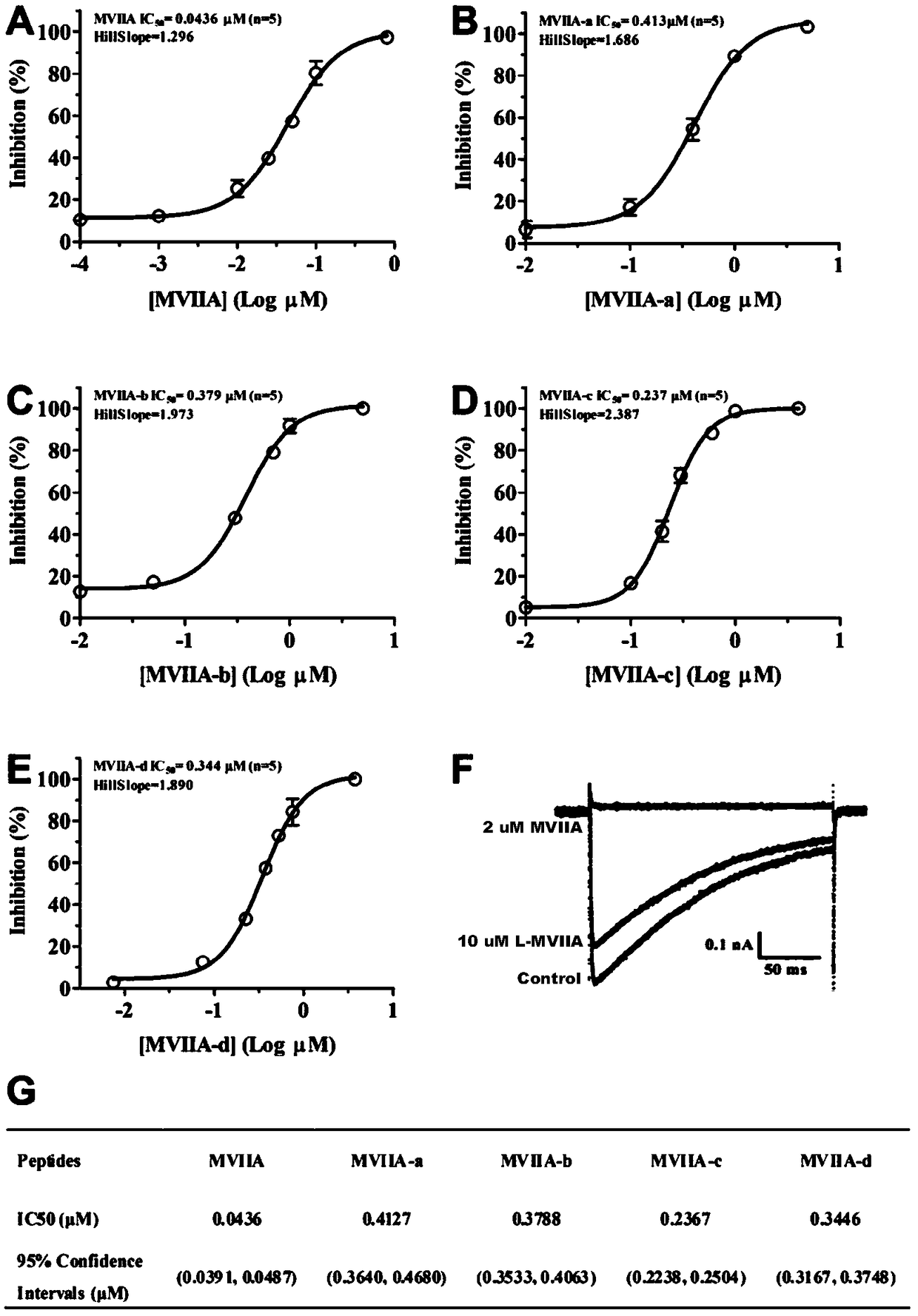
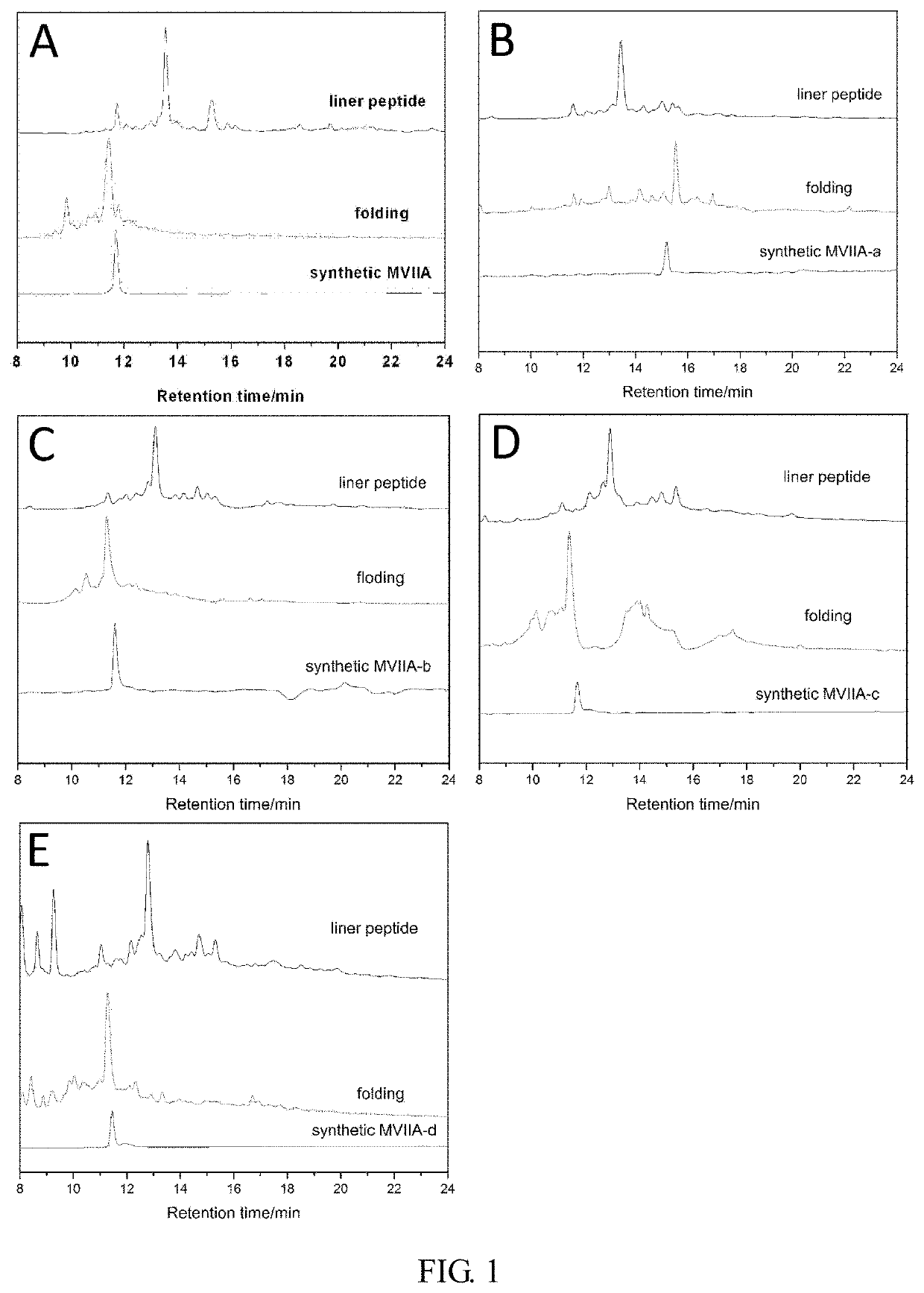
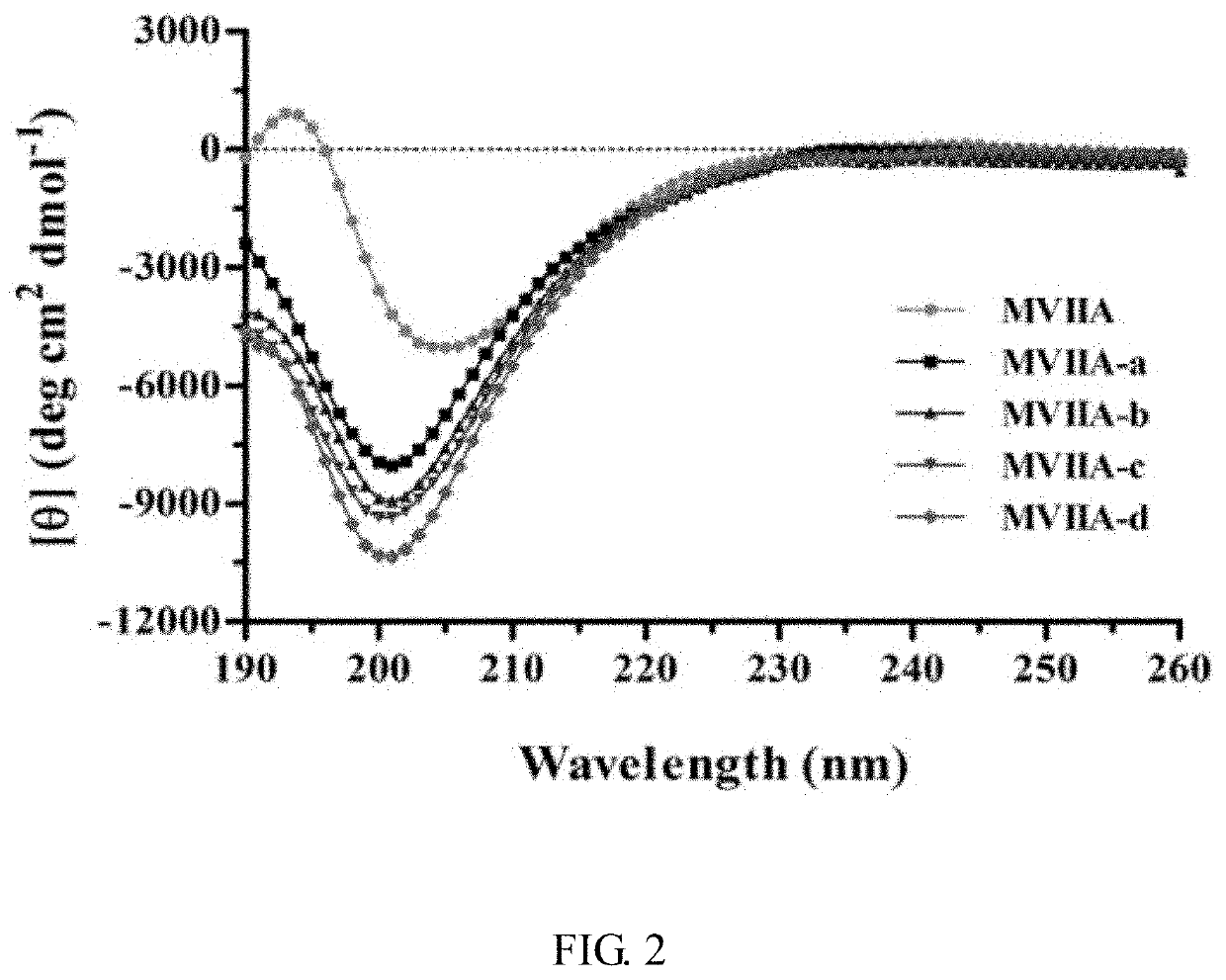
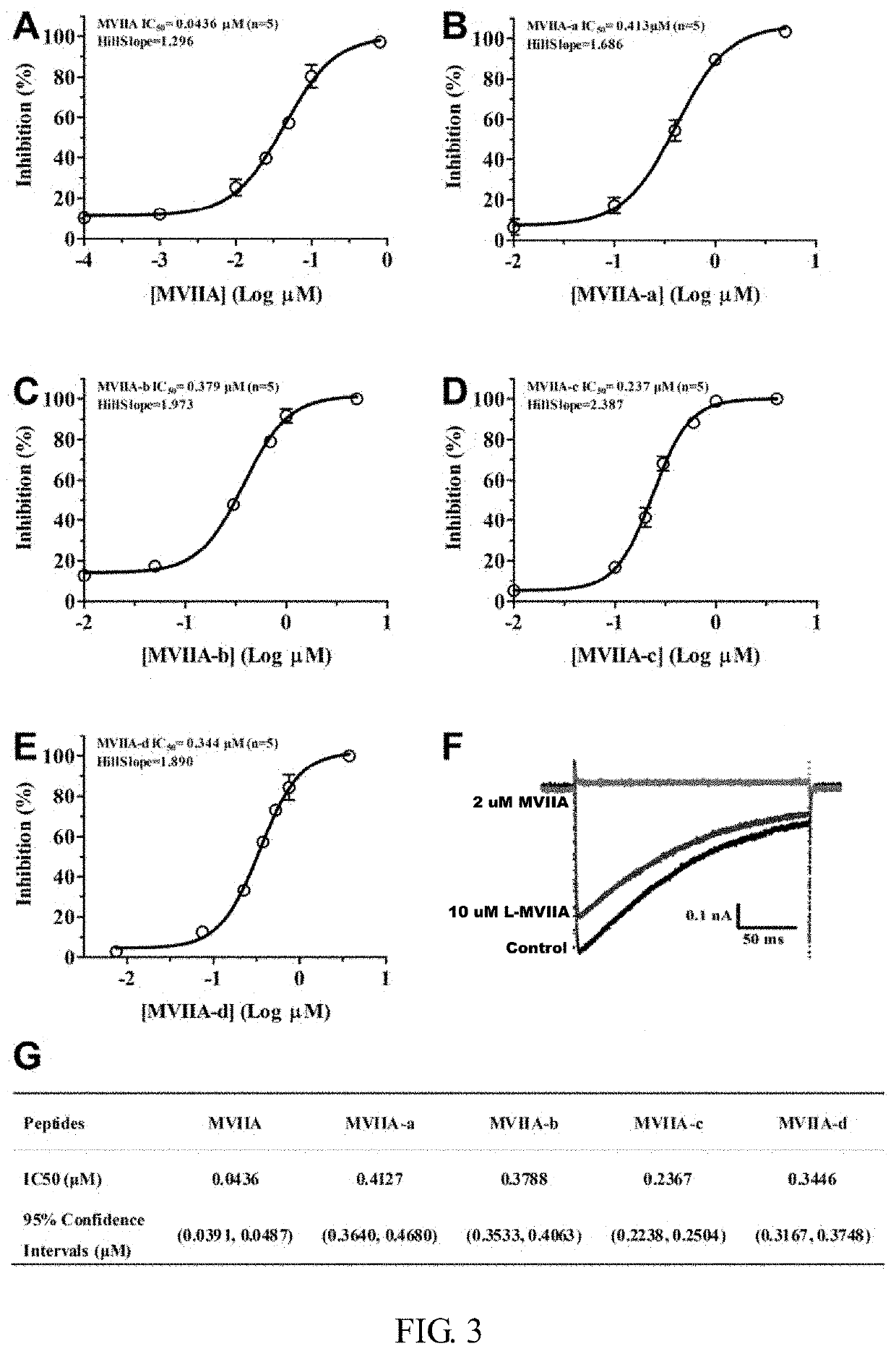
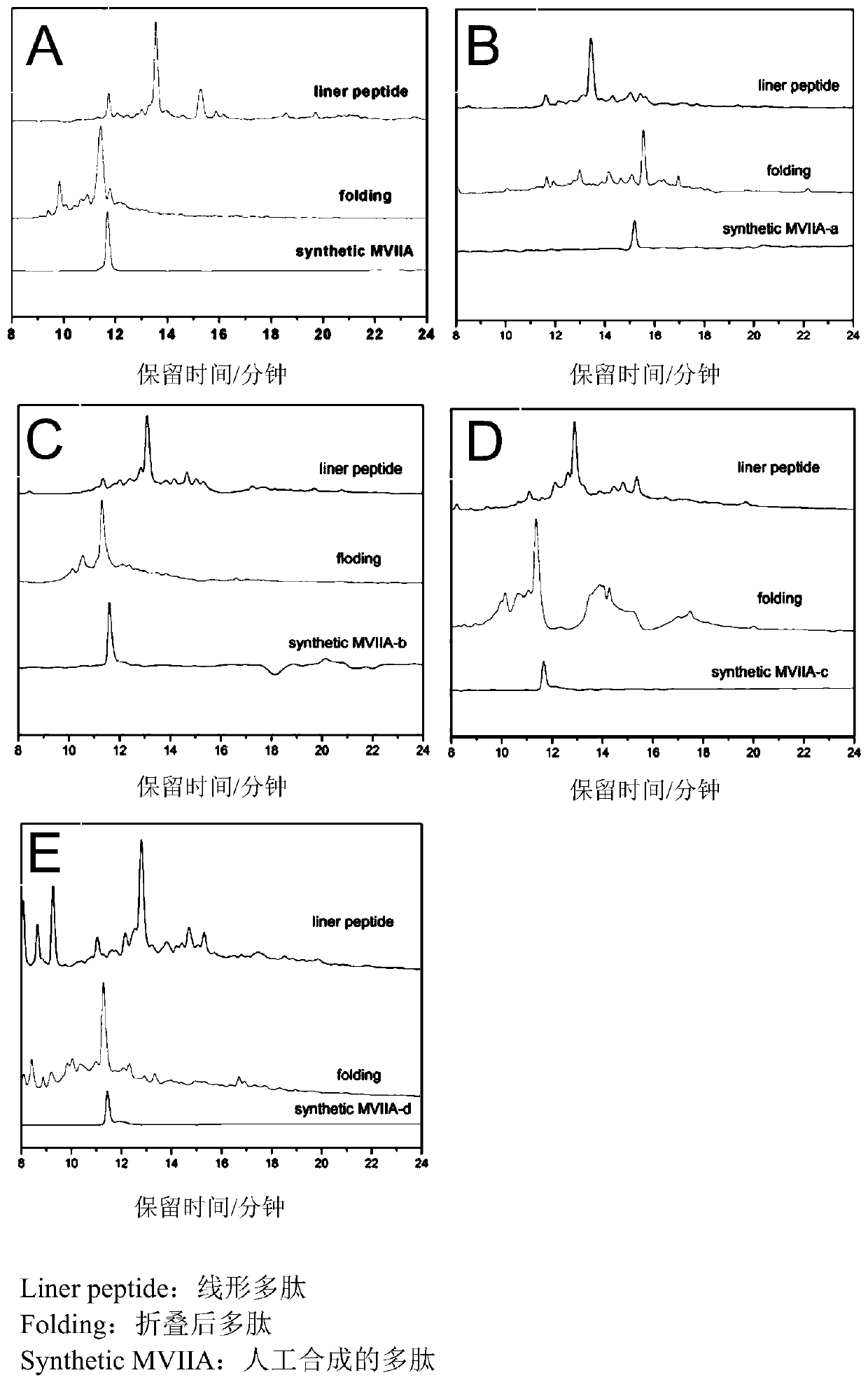
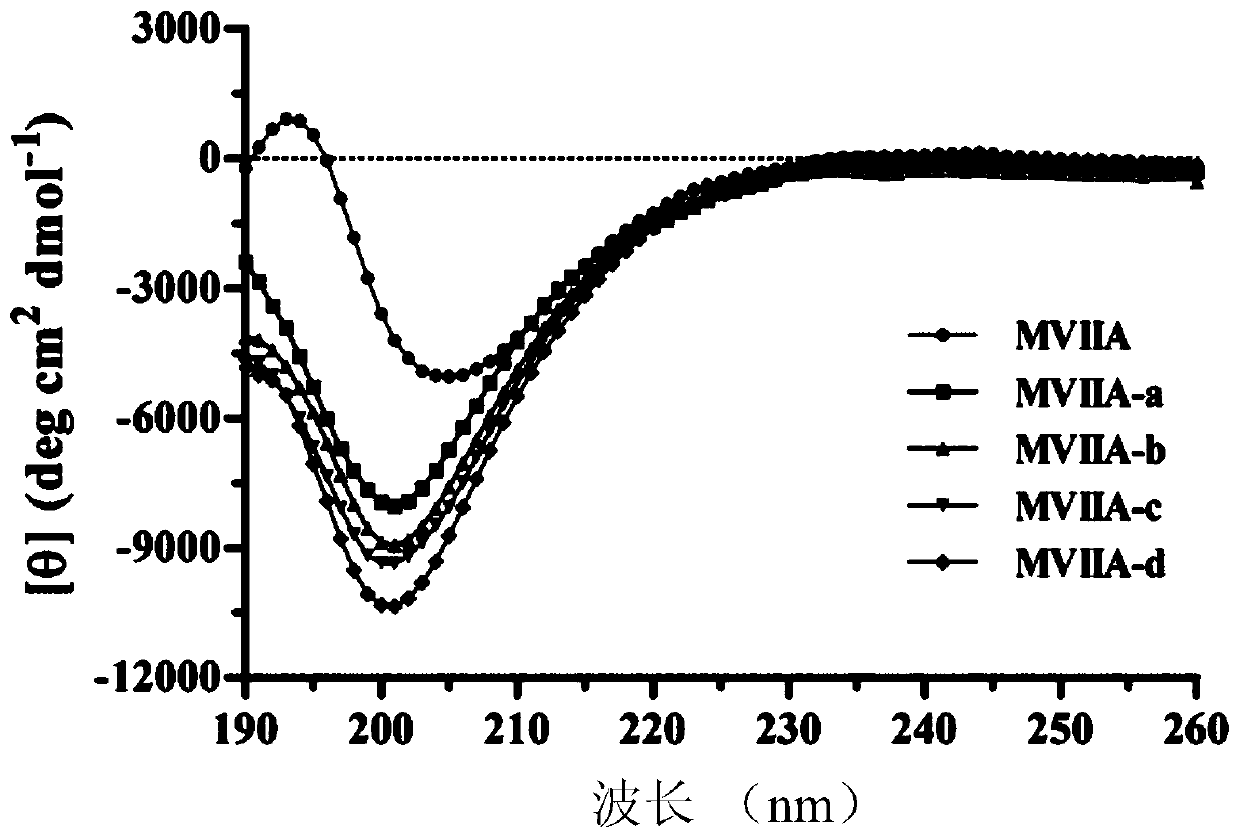
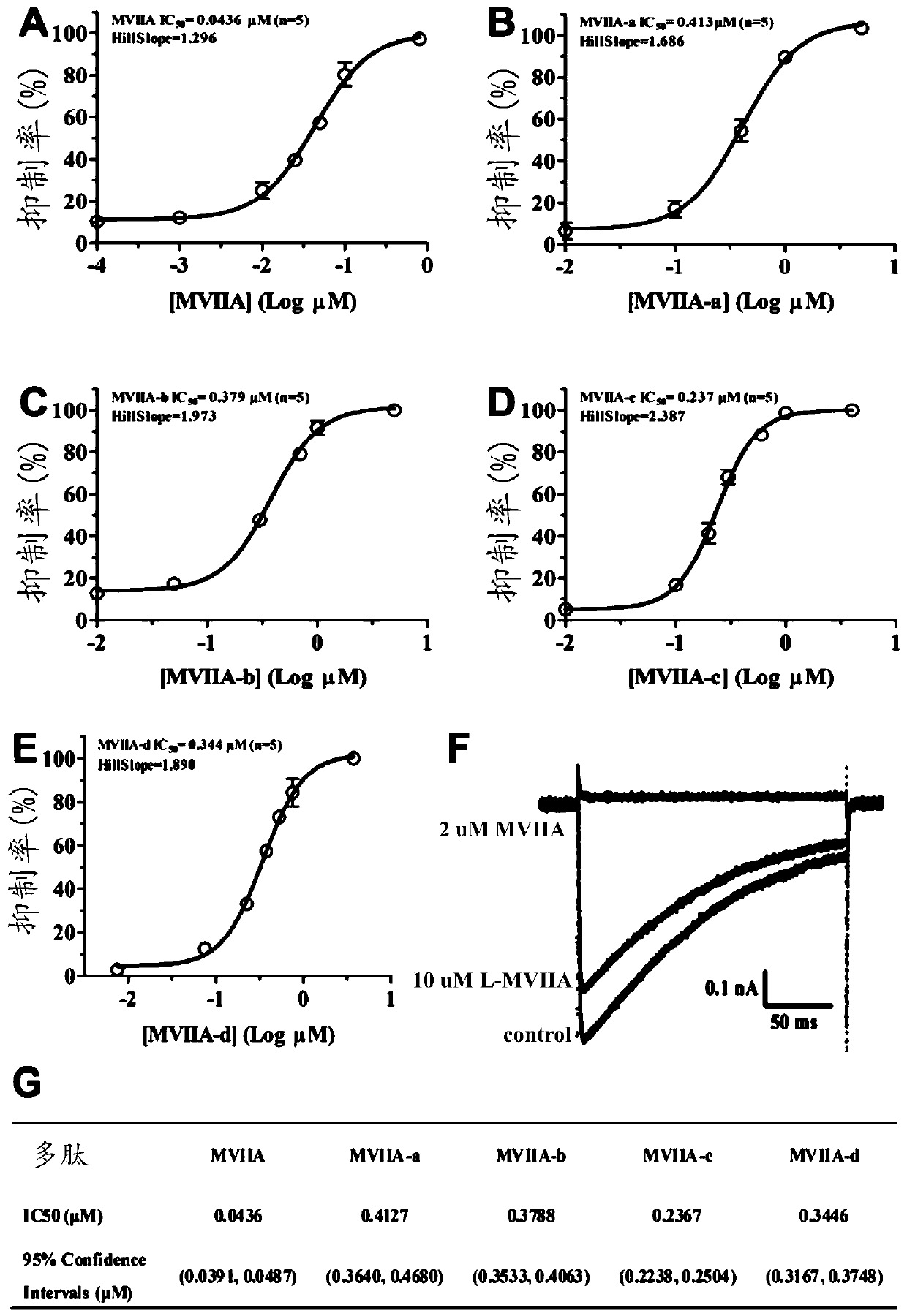
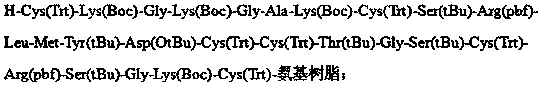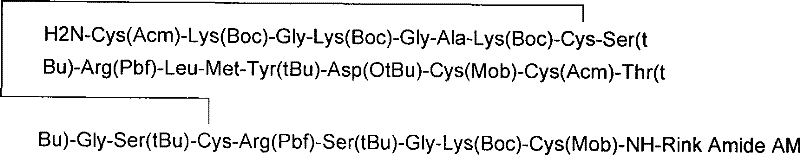Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Ziconotide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is a non-narcotic pain reliever that is used to treat ongoing pain when other treatments or medications cannot control your pain.
Method for reducing pain
ActiveUS20050192218A1Relieve painRetain potencyBiocideNervous disorderIntrathecal usePharmaceutical formulation
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
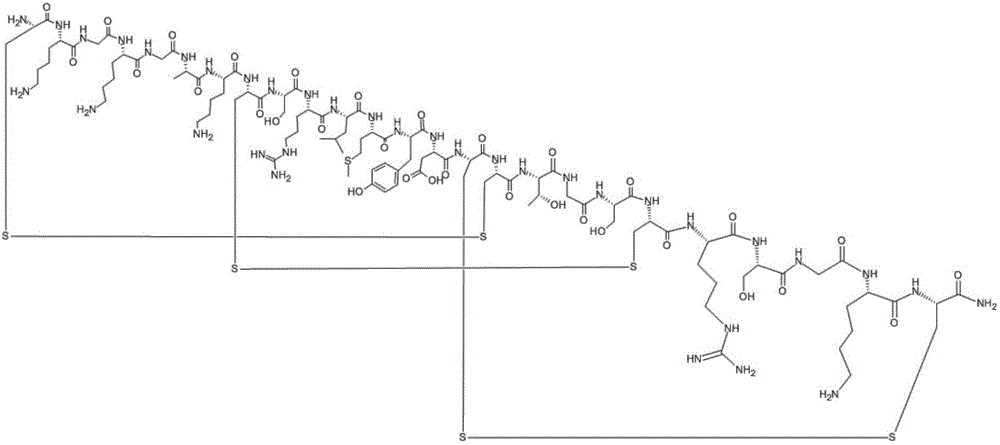
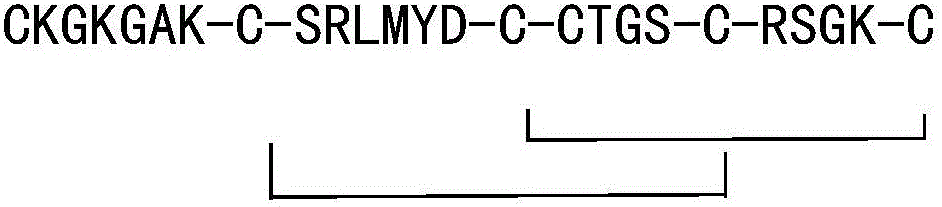
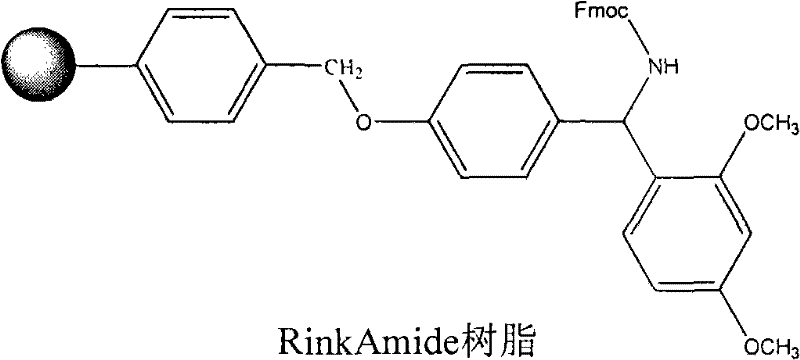
Solid phase synthesis method of ziconotide
InactiveCN101412752AIncrease the speed of cyclizationImprove reaction efficiencyPeptide preparation methodsAnimals/human peptidesSide chainCombinatorial chemistry
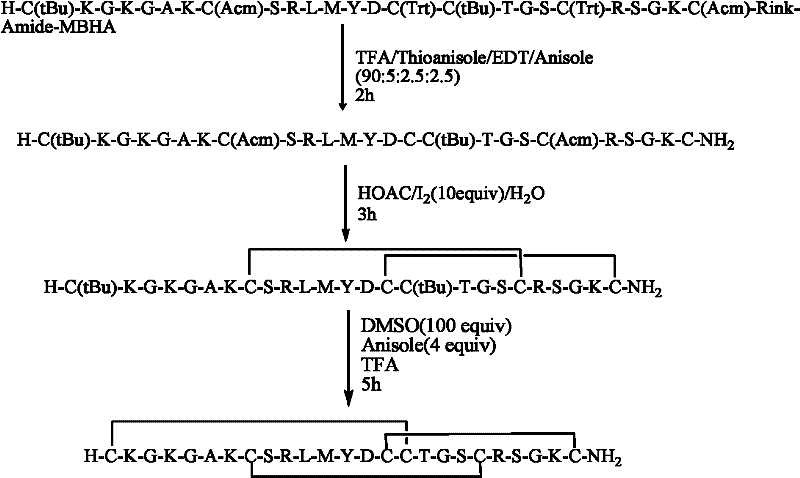
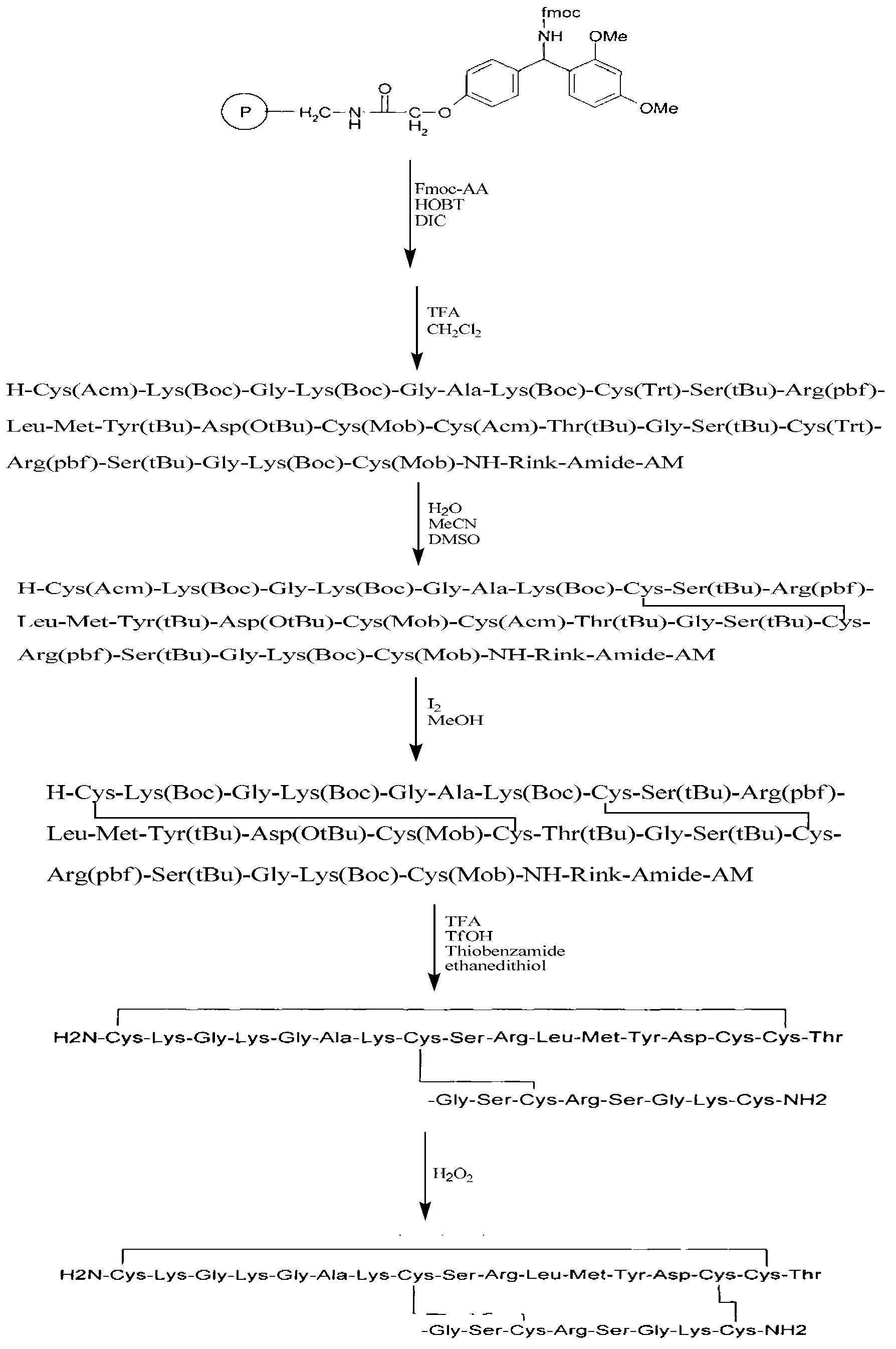
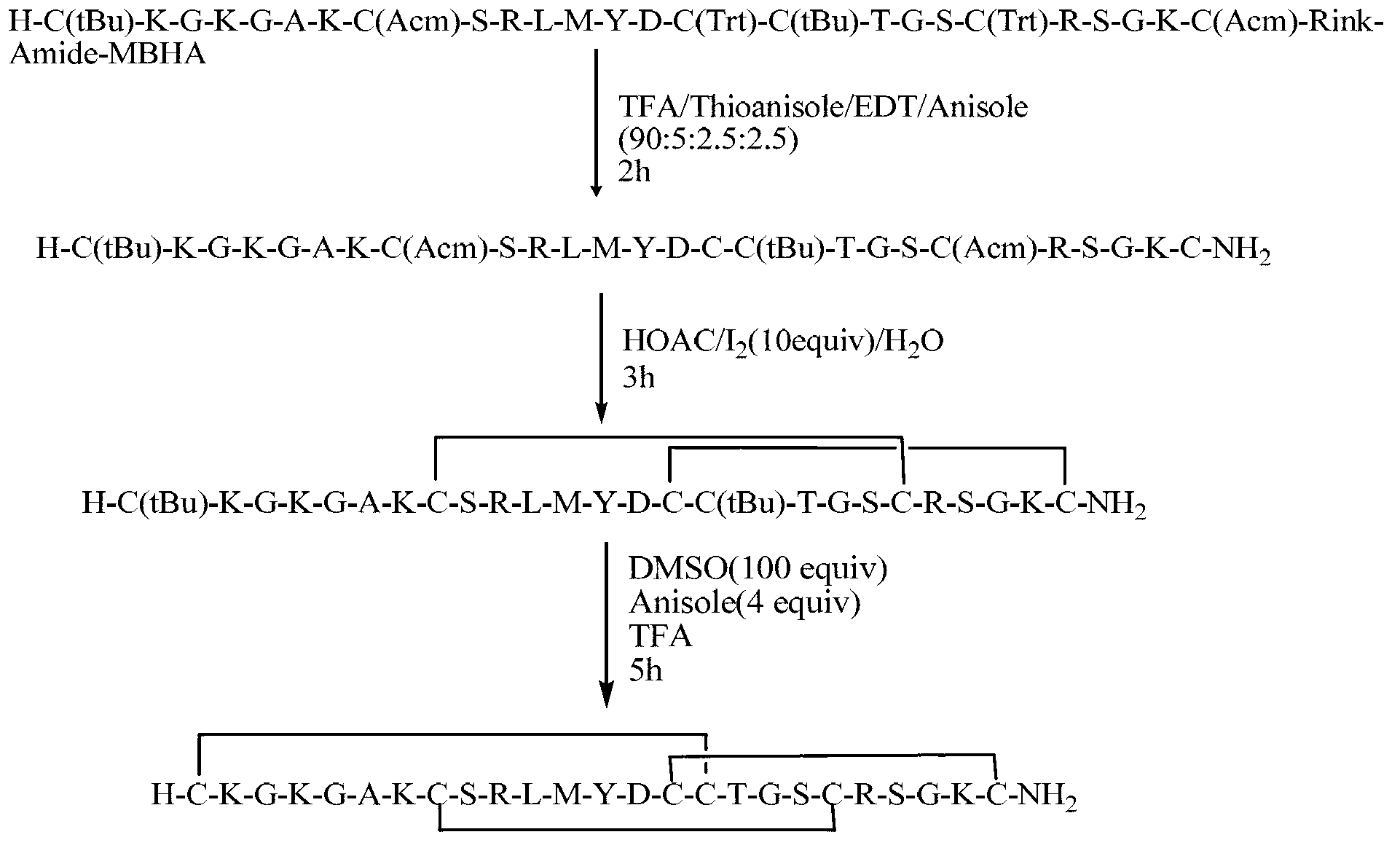
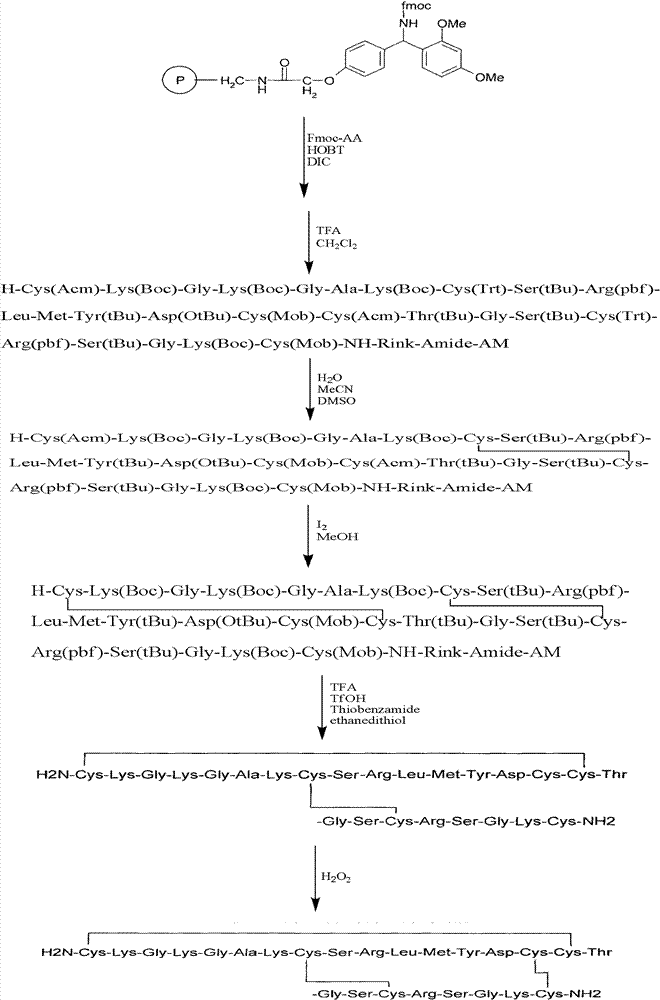
The invention relates to a solid phase synthesis method for ziconotide, which comprises the following steps: an Fmoc-Rink Amide-MBHA resin is taken as a solid phase carrier for program reaction; condensation reactions are orderly performed to link 25 amino acids with protecting groups to obtain a linear peptide complete-protection resin of the 25 amino acids,wherein in three groups of Cyses which form a disulfide bond, the Cyses in the same group are simultaneously linked with one of a Trt, Acm or Mob protecting group, and different groups of the Cyses are linked with different protecting groups; the protecting groups of the three groups of the Cyses are orderly removed; a cyclization reaction is performed to form a disulfide ring bond; a side chain protecting group is removed; and the resin is cut to obtain the ziconotide containing three disulfide rings. The method has the main advantages of few side products, high directive efficiency, simplicity, convenience, high yield of products, and favorability for the purification of the products.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Method for reducing pain
ActiveUS7268109B2Nervous disorderPeptide/protein ingredientsPharmaceutical formulationAnalgesic agents
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
A kind of solid-phase synthesis method of ziconotide
ActiveCN102268082AImprove accuracyHigh yieldPeptide preparation methodsAnimals/human peptidesCombinatorial chemistryProtecting group
The invention discloses a solid-phase synthesis method of ziconotide, and the method comprises the following steps: with Fmoc(9-fluorenylmethyloxycarbonyl)-amino resin as a solid-phase carrier, successively carrying out condensation reaction for connecting 25 protected amino acids to obtain linear fully-protected peptide resin, wherein three groups of Cys (cysteine) with disulfide bond formed areseparately connected with Trt (triphenylmethyl), Acm (acetamidomethyl), or tBu (t-butyl) protecting group; cutting resin, and simultaneously removing all amino acid protecting groups except for Acm and tBu to obtain a linear peptide containing Acm and tBu; oxidizing the linear peptide to form a first pair of disulfide bonds, and simultaneously removing Acm and forming a second pair of disulfide bonds to obtain bicyclic peptide resin; and removing tBu of the bicyclic peptide resin, and simultaneously carrying out cyclization to form a third pair of disulfide bonds and to obtain ziconotide. In the method disclosed by the invention, Trt, Acm and tBu are selected to protect the three groups of Cys, thereby improving the formation accuracy of the disulfide bonds; and after resin is cut off, three pairs of disulfide bonds are sequentially formed through two-step reaction, thereby simplifying steps and improving productivity.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Method for preparing ziconotide
InactiveCN103304655AAvoid it happening againReduce the difficulty of purificationPeptide preparation methodsAnimals/human peptidesSynthesis methodsCombinatorial chemistry
The invention belongs to the technical field of polypeptide drugs, and particularly relates to a method for preparing ziconotide, and the method is used for solving the technical problems of difficult separation and purification and low total product yield and purity in the existing preparation methods. The method comprises the following steps of: preparation of ziconotide linear peptide resin based on a solid phase polytide method, acidolysis for obtaining a ziconotide linear peptide crude product, oxidation for obtaining a ziconotide crude product and purification for obtaining a ziconotide purified product, wherein the solid phase polytide method comprises the following steps of: preparing ziconotide linear peptide resin by sequentially connecting corresponding protected amino acid or protected amino acid fragment in the following sequence starting from amino resin through a solid phase coupling synthesis method: R-Cys(Trt)-X(Boc)-X(Boc)-Ala-Lys(Boc)-Cys(Trt)-Ser(tBu)-Arg(Pbf)-Leu-Met-Tyr(tBu)-Asp(OtBu)-Cys(Trt)-Cys(Trt)-Y(tBu)-Ser(tBu)-Cys(Trt)-Arg(Pbf)-Z(tBu)-Lys(Boc)-Cys(Trt)-amino resin, wherein R is Fmoc, Boc or H, X is Lys-Gly, Y is Thr-Gly, and Z is Ser-Gly. The invention provides a novel method for shortening the production period and improving the product purity and the product yield.
Owner:CHENGDU SHENGNUO BIOPHARM
Method for preparing ziconotide
InactiveCN101709082ASynthetic reactions are inefficientStrong folding effectPeptide preparation methodsAnimals/human peptidesSide chainFreeze-drying
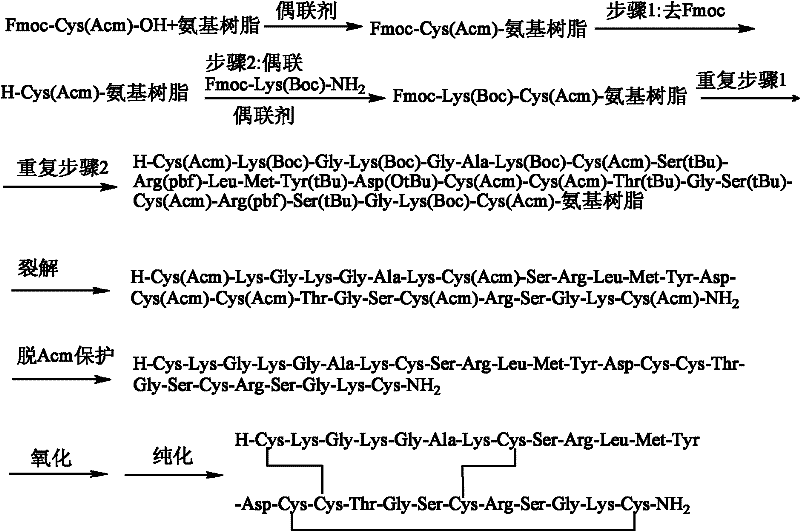
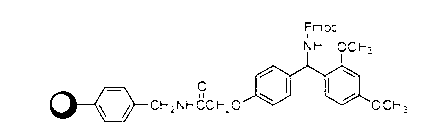
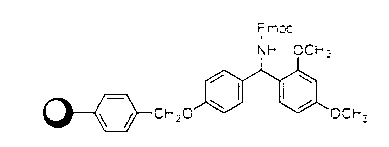
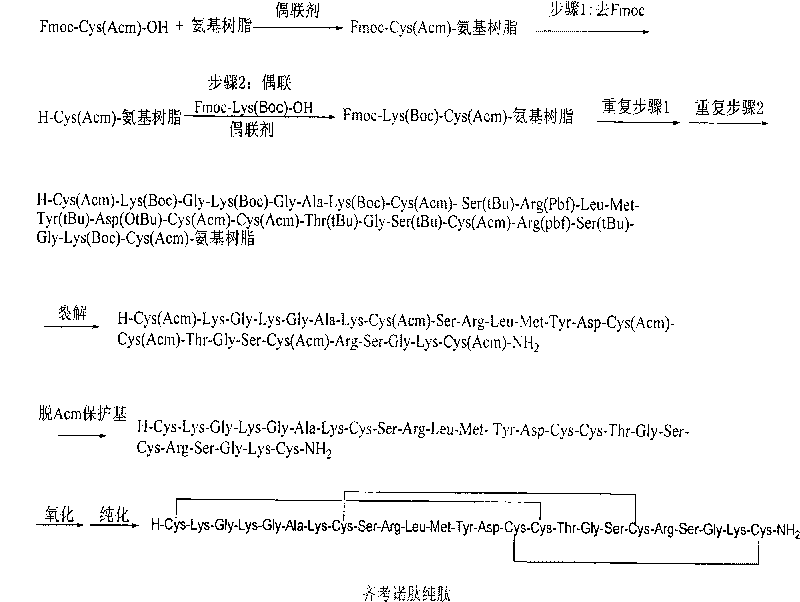
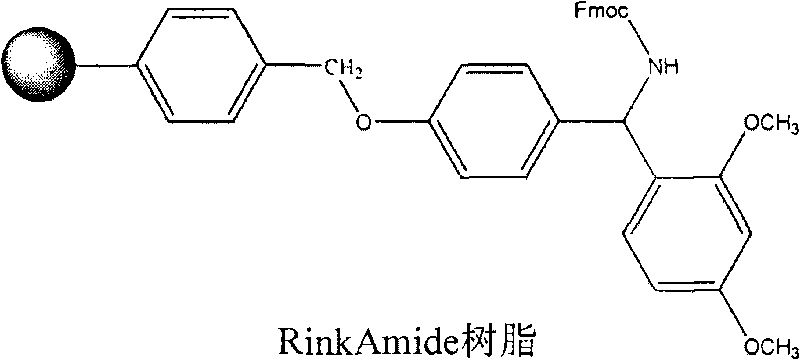
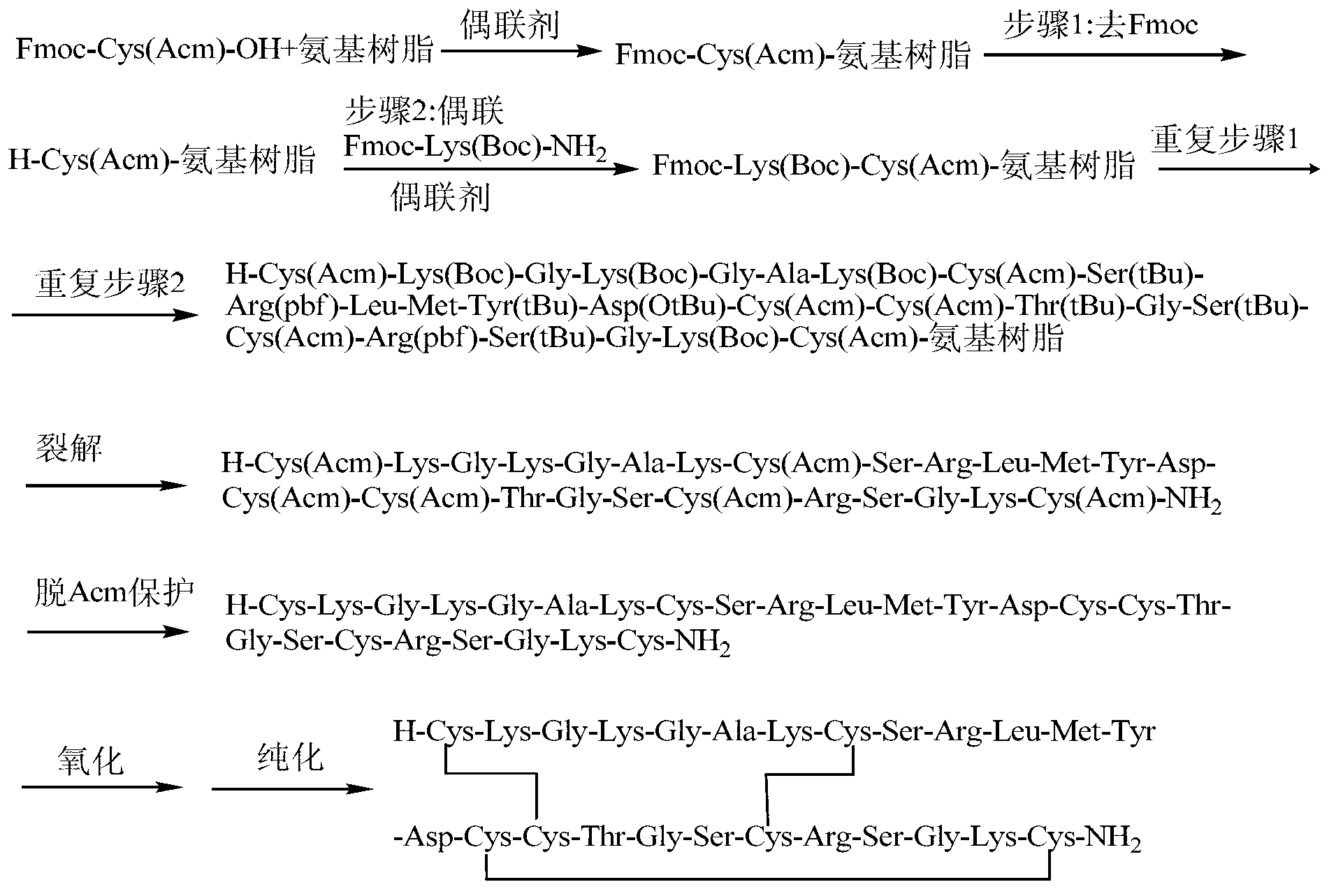
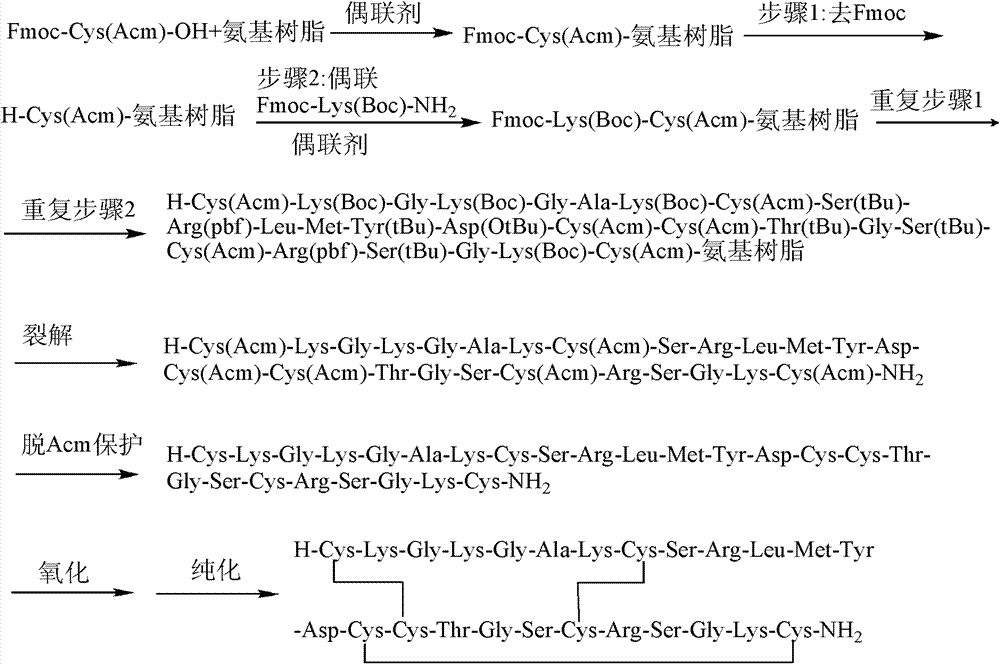
The invention discloses a method for preparing ziconotide. The technical scheme of the invention comprises the following steps: (1) obtaining an Fmoc-Cys(Acm)-amino resin from Fmoc-Cys(Acm)-OH and an amino resin; (2) obtaining a linear-ziconotide-amino resin of which a Cys side chain comprises Acm by performing the solid phase synthesis on the Fmoc-Cys(Acm)-amino resin and an amino acid adopting Fmoc group protection; (3) obtaining a linear crude peptide of which the Cys side chain comprises the Acm by performing cracking on the linear-ziconotide-amino resin of which the Cys side chain comprises the Acm, and obtaining linear ziconotide by removing the Acm, purifying and freeze-drying; (4) and obtaining the ziconotide by performing cyclization, purifying and freeze-drying on the linear ziconotide. The method for preparing ziconotide has the characteristics of simple reaction operation, easy subsequent treatment, low raw material investment, low cost, high yield and the like, and has considerable economic and practical value, and also has wide application prospect in the field of design synthesis of polypeptide drugs.
Owner:HYBIO PHARMA
Solid-phase synthesis method of Ziconotide
InactiveCN103242441APrecise positioningIncrease the speed of cyclizationPeptide preparation methodsAnimals/human peptidesFreeze-dryingSide chain
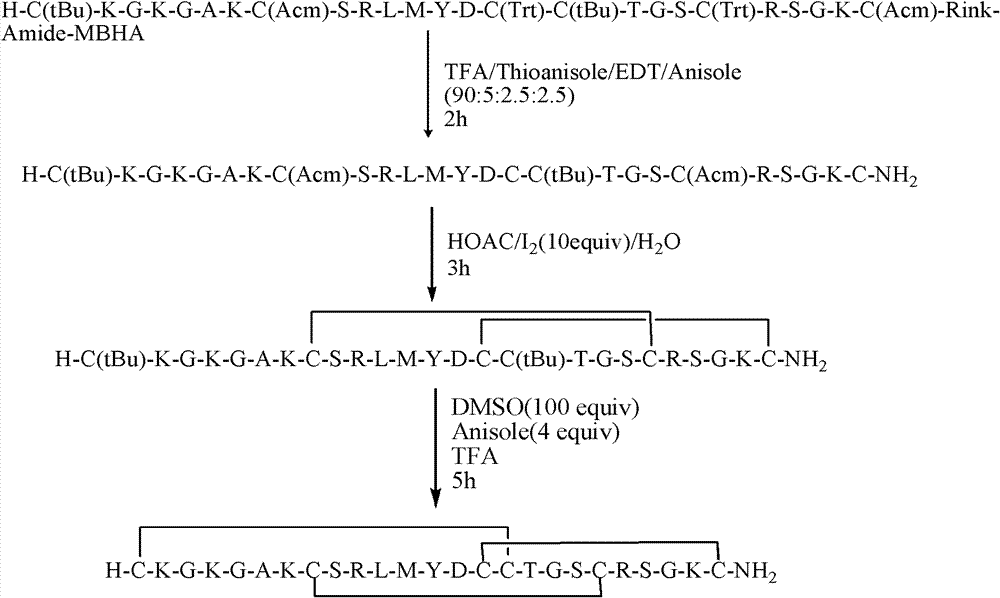
The invention discloses a solid-phase synthesis method of Ziconotide. The method comprises the following steps of: performing condensation reaction by taking Fmoc-amino resin as a solid-phase carrier and connecting 25 side chain protected amino acids to obtain linear fully protected resin, wherein three groups of Cys which form disulfide bonds are connected with Trt, Acm or tBu protecting groups respectively; linearly cutting the resin and removing the side chain protected groups of other amino acids except for Cys(Acm) and Cys(tBu); oxidizing linear peptide to form the first pair of disulfide bonds to obtain single disulfide cyclopeptide; removing the Acm protected group of the Cys(Acm) from the single disulfide cyclopeptide and cyclizing to form the second pair of disulfide bonds to obtain double disulfide cyclopeptide; removing the tBu protected group of the Cys(tBu) from the double disulfide cyclopeptide and cyclizing to form the third pair of disulfide bonds to obtain triple disulfide cyclopeptide; and purifying and freeze-drying the triple disulfide cyclopeptide to obtain the Ziconotide.
Owner:Jiangsu Shimeikang Pharmaceutical Co Ltd
Methods of using ziconotide to treat overactive bladder
The invention relates to methods of using Cav2.2 subunit calcium channel modulators to treat painful and non-painful lower urinary tract disorders and the related genitourinary tract disorders vulvodynia and vulvar vestibulitis in normal and spinal cord injured patients.
Owner:EDUSA PHARMA
Application of polypeptide in preparation of intravenous, abdominal or nasal drug-administration drugs
ActiveCN108992661AGood analgesic effectLittle side effectsNervous disorderPeptide/protein ingredientsNasal cavitySide effect
The invention relates to an application of a polypeptide in preparation of intravenous, abdominal or nasal drug-administration drugs. A C end of Ziconotide is connected with an N end of a cell membrane penetrating peptide through three glycines, and the polypeptide that can pass through a blood-brain barrier is obtained. The polypeptide is suitable for an intravenous, abdominal or nasal drug-administration way, has the advantages of convenient operation and low clinical risk, has long in-vivo drug effect time, good analgesic effect and little side effects of peptide through intravenous, abdominal or nasal application, and is suitable for large-scale clinical application. The polypeptide has simple preparation, has controllable quality in preparation technology and preparation process, andis suitable for large-scale industrialized production.
Owner:SHENZHEN RUIJIAN BIOSCI TECH LTD
Methods of administering drugs in an implantable multi-chamber pump
InactiveUS20140296830A1Relieve painReducing severe and chronic painMedical devicesPressure infusionNeuropathic painZiconotide
One embodiment of the present invention is a method for reducing pain using a multi chamber pump to separately administer multiple drugs. For example, one chamber may contain an omega conopeptide, such as ziconotide, and the other chamber or chambers may contain one or more other drugs, which may include of morphine, hydromorphone, fentanyl, sufentanil, bupivacaine, baclofen, clonidine, and buprenorphine, or their pharmaceutically acceptable salts thereof. Other applications of the present invention include methods for treating severe chronic pain due to cancer, failed back syndrome, CRPS, neuropathic pain, mixed neuropathic and nociceptive pain.
Owner:JAZZ PHARMA
Method for preparing ziconotide by solid-liquid combination
InactiveCN109021087AHigh purityHigh yieldPeptide preparation methodsAnimals/human peptidesZiconotideOxidizing agent
The invention discloses a method for preparing ziconotide by solid-liquid combination and mainly solves the technical problems that an existing synthesizing method has a long synthesizing period, highproduction cost, a low product yield and no favorability for later industrialized enlargement. The method disclosed by the invention comprises the following steps: (1) preparing modified polypeptidefragment A by solid-liquid combination; (2) preparing polypeptide fragment B by a solid phase method; (3) linking the modified polypeptide fragment A to the polypeptide fragment B by a natural linkingmethod under the liquid phase condition to prepare and obtain a ziconotide linear peptide crude product solution; (4) adding an oxidizing agent under the same system oxidizing to obtain a ziconotidecrude product water solution and purifying and dry freezing through a high performance liquid chromatography to obtain a ziconotide fine product.
Owner:滨海吉尔多肽有限公司 +1
GLP-1 analogue and ziconotide composite slow-release microsphere preparation
The invention relates to a GLP-1 analogue and ziconotide composite slow-release microsphere preparation and a preparation method thereof. The slow-release microsphere preparation is prepared from a GLP-1 analogue, ziconotide, a biodegradable high polymer material with biocompatibility, a stabilizing agent and a freeze drying protection agent. The preparation method comprises the following steps of (1) adding water for preparing the GLP-1 analogue and the ziconotide into a medicine solution A; adding an organic solvent for preparing the biodegradable high polymer material with biocompatibility into a solution B; (2) mixing the solution A and the solution B; performing ultrasonic processing to form primary emulsion; adding the primary emulsion into a stabilizing agent water solution saturated by an organic mixed solvent; performing homogenizing emulsification to obtain secondary emulsion; (3) performing room-temperature stirring on the secondary emulsion for 1 hour; then, raising the temperature to 40 DEG C to 45 DEG C; maintaining the temperature for one hour; then, lowering the temperature to 10 DEG C; adding the freeze drying protection gent; screening and collecting particles; performing freeze drying; performing radiation sterilization. By using a related technology, the effect of treating diabetes and PDN (painful diabetic neuropathy) complications for a long time can be achieved; the pain of a patient is reduced; the medicine compliance of the patient is improved; the clinic practical significance is realized.
Owner:深圳市健翔生物制药有限公司
Purification method of Ziconotide
ActiveCN105017401AHigh purityEasy to controlPeptide preparation methodsAnimals/human peptidesIsocratic elutionTrifluoroacetic acid
The invention relates to a purification method of Ziconotide. The method is characterized by including the following steps that 1, the pH of a crude Ziconotide solution is regulated to 3-4 through trifluoroacetic acid; 2, the gradient is set according to the volume fraction, a reversed-phase filler column is washed for 10 min through a 50% mobile phase B, and then isocratic elution balancing is performed for 10 min through a 5% mobile phase B; 3, the solution in the step1 is loaded into the reversed-phase filler; 4, the gradient is set according to the volume fraction, the original state mobile phase B of the elution gradient is 5% and is maintained for 5 min, then the proportion of the mobile phase B is increased to 22% within 60 min, and elution fractions are collected; 5, the elution fractions are subjected to salt transfer, concentration and freeze-drying to obtain fine Ziconotide.
Owner:HANGZHOU HEZE PHARMA TECH +1
Application of fusion polypeptide of ziconotide and TAT peptide in drug preparation
ActiveCN109265557ALittle side effectsEasy to makePolypeptide with localisation/targeting motifNervous disorderNasal cavityTat peptide
The invention provides application of fusion polypeptide of ziconotide and TAT peptide in drug preparation. According to the application provided by the invention, the terminal C of ziconotide is connected with the terminal N of cell penetrating peptide to obtain fusion peptide, and the defects that intramuscular injection cannot be realized and the like are overcome. The fusion polypeptide can pass through the blood brain barrier and is suitable for the administration through vein, abdominal cavity or nasal cavity while the operation is convenient and the clinical risk is low; after being applied through the vein, abdominal cavity or nasal cavity, the fusion polypeptide has the advantages of long acting time in vivo, good analgesic effect and few peptide side effects and thus is suitablefor large-scale clinical application. The fusion polypeptide provided by the invention is easy to prepare, the preparation technology and the preparation process are controllable in quality, and the fusion polypeptide is suitable for large-scale industrial production.
Owner:SHENZHEN RUIJIAN BIOSCI TECH LTD
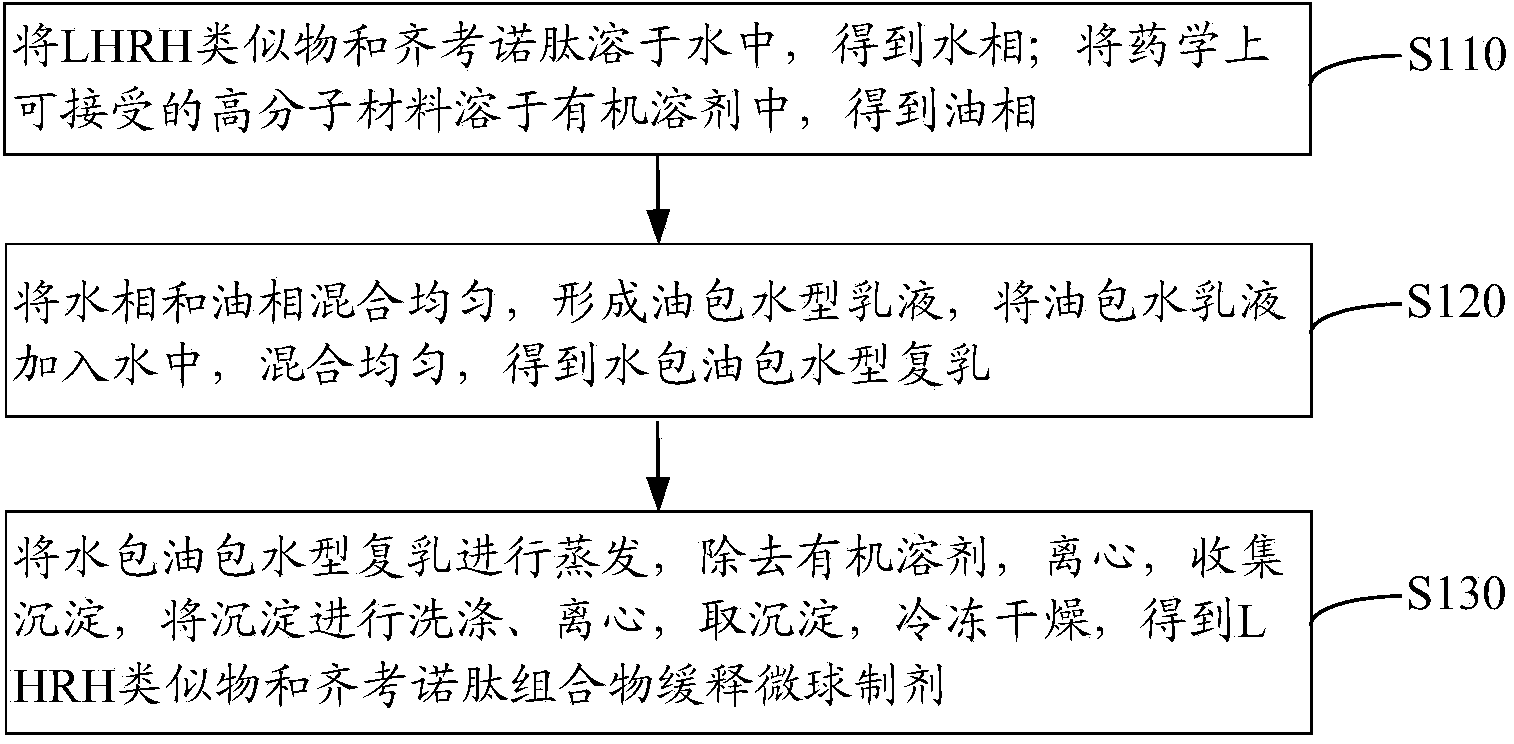
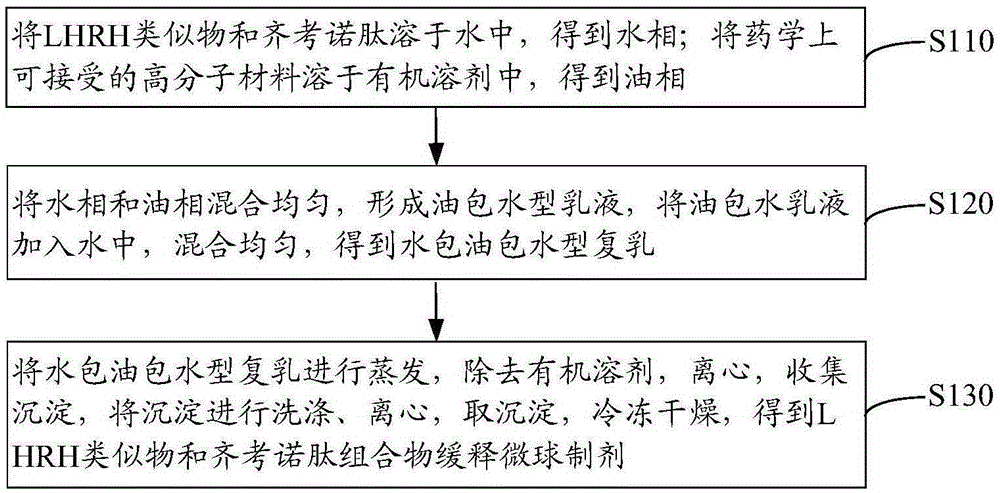
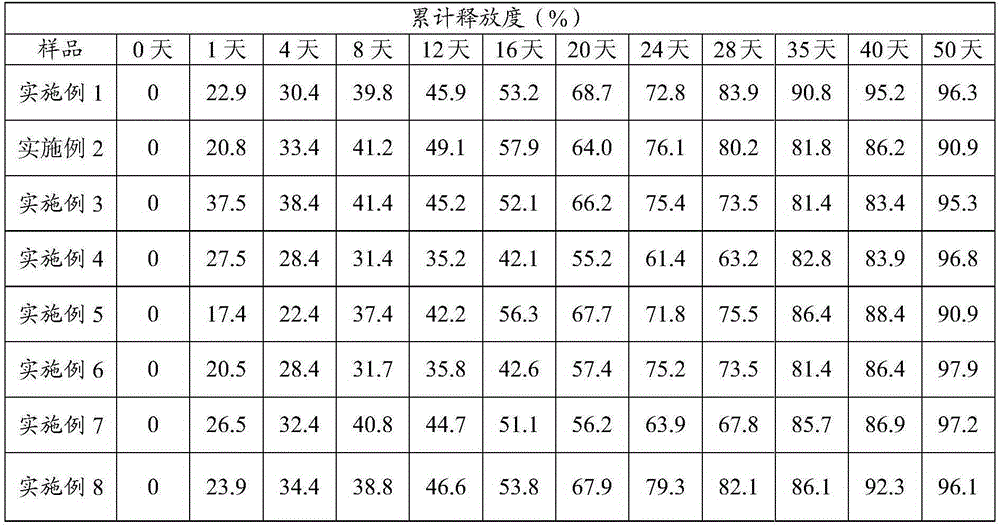
LHRH (luteinizing hormone releasing hormone) analogue and ziconotide composition sustained-release microsphere preparation and preparation method thereof
ActiveCN103908659AReduce releaseSolve the problem of inconvenient clinical administrationPeptide/protein ingredientsAntipyreticMicrosphereCurative effect
The invention relates to an LHRH (luteinizing hormone releasing hormone) analogue and ziconotide composition sustained-release microsphere preparation and a preparation method thereof. The LHRH analogue and ziconotide composition sustained-release microsphere preparation comprises sustained-release microspheres, wherein the sustained-release microspheres comprise an LHRH analogue, ziconotide and a pharmaceutically acceptable high polymer material. The LHRH analogue and ziconotide composition sustained-release microsphere preparation can effectively bring synergistic effect of the LHRH analogue and the ziconotide into play; experiments prove that the LHRH analogue and ziconotide composition sustained-release microsphere preparation has a sustained-release effect, has a better curative effect in treating cancers, is capable of achieving an analgesic effect at the same time, is administrated by regular injection, and can be used for solving a problem that the ziconotide is inconvenient for clinical administration.
Owner:南京星银药业集团有限公司 +1
Application of polypeptide capable of passing through hemato encephalic barrier in preparing drug
ActiveCN109106942AGood analgesic effectLittle side effectsNervous disorderPeptide/protein ingredientsNasal cavitySide effect
The invention relates to application of a polypeptide capable of passing through a hemato encephalic barrier in preparing a drug. An end C of ziconotide is connected with an end N of a cell penetrating peptide by virtue of one glycine to obtain the polypeptide capable of passing through the hemato encephalic barrier. The polypeptide provided by the invention is suitable for the vein, abdominal cavity or nasal cavity drug administration way, convenient in operation, low in clinical risk, capable of being applied by virtue of veins, abdominal cavity or nasal cavity, long in drug effect acting time in the body, excellent in pain alleviating effect, small in peptide side effect and suitable for the large-scale clinical application. The polypeptide provided by the invention is simple in preparation, controllable in quality in the preparation technology and preparation process, and suitable for industrialized mass production.
Owner:SHENZHEN RUIJIAN BIOSCI TECH LTD
Nasal gel composite and preparation method thereof
The invention belongs to the field of chemicals, and relates to a Ziconotide-nasal gel composite and a preparation method thereof. The preparation is composed of Ziconotide, a gel matrix and pharmaceutical acceptable other auxiliary materials including an absorption enhancer, an osmotic pressure regulator, a moisturizing agent, an antioxidant, a preservative and a pH regulator. The preparation is mainly used for treating intolerable or invalid serious chronic pains such as generalized pain and the like. The preparation is simple in preparation process, convenient to take, rapid in onset, high in bioavailability, and beneficial to being accepted by patients.
Owner:SHENZHEN JYMED TECH
Polypeptide capable of passing through hemato encephalic barrier
ActiveCN109232745AGood analgesic effectLittle side effectsPolypeptide with localisation/targeting motifPeptide/protein ingredientsNasal cavitySide effect
The invention relates to a polypeptide capable of passing through hemato encephalic barrier. According to the invention, the polypeptide capable of passing through hemato encephalic barrier is acquired by connecting C end of ziconotide with N end of cell penetrating peptide through a glycine. The polypeptide provided by the invention is suitable for vein, enterocoelia or nasal delivery, is convenient for operation and has small clinic risks. Through vein, enterocoelia or nasal delivery, the polypeptide has the characteristics of long acting time in human body, excellent analgesic effect, smallside effect of peptide and suitability for large-scale clinical application. The polypeptide provided by the invention is simply prepared, the preparation technology and the quality during the preparation process are controllable and the polypeptide is suitable for large-scale industrialized production.
Owner:SHENZHEN RUIJIAN BIOSCI TECH LTD
A kind of method for solid-phase synthesis of ziconotide by fragment method
InactiveCN104974237BHigh yieldShort synthesis cyclePeptide preparation methodsAnimals/human peptidesEnvironmental resistanceAfter treatment
The invention belongs to the field of polypeptide synthesis, and relates to a solid-phase synthesis method of ziconotide by a segment process. The method aims to solve the technical problems of long synthesis period and low crude peptide purity in the existing preparation method, and the technical problems of complex operation, low total yield and the like in the disulfide-bond formation process. The technical scheme is as follows: the method comprises the following steps: synthesizing four segment peptides [19-25]A, [12-18]B, [6-11]C and [1-5]D of ziconotide by a solid-phase process; sequentially connecting the all-protected peptide fragments B, C and D to the peptide resin A, and cracking to obtain a ziconotide linear peptide; and finally, carrying out liquid-phase one-step oxidization, which has the advantages of mild conditions, simplified operation, easy after-treatment and environment friendliness, to obtain the ziconotide. The technical scheme can greatly shorten the synthesis period, lower the synthesis and purification difficulty and the production cost, and enhance the purity of the linear peptide and the total yield of the product. The method is beneficial to industrial large-scale production.
Owner:JINAN KANGHE MEDICAL TECH
Fused polypeptide of ziconotide and TAT peptide
ActiveCN109232746ALittle side effectsEasy to makePolypeptide with localisation/targeting motifPeptide/protein ingredientsHuman bodyTat peptide
The invention provides a fused polypeptide of ziconotide and TAT peptide. According to the invention, the fused polypeptide is acquired by connecting C end of ziconotide with N end of cell penetratingpeptide. The fused polypeptide can overcome the defects, such as, unsuitability for intramuscular injection. The fused polypeptide is capable of passing by blood brain barrier, is suitable for vein,enterocoelia or nasal delivery, is convenient for operation and has small clinic risks; through vein, enterocoelia or nasal delivery, the fused polypeptide has the characteristics of long acting timein human body, excellent analgesic effect, small side effect of peptide and suitability for large-scale clinical application; the fused polypeptide provided by the invention is simply prepared; the preparation technology and the quality during the preparation process are controllable; the fused polypeptide is suitable for large-scale industrialized production.
Owner:SHENZHEN RUIJIAN BIOSCI TECH LTD
Efficient ziconotide synthesis and preparation method
InactiveCN106496315AHigh yieldReduce manufacturing costPeptide preparation methodsAnimals/human peptidesSide chainFreeze-drying
The invention discloses an efficient ziconotide synthesis and preparation method. The efficient ziconotide synthesis and preparation method comprises the following synthesis and preparation steps: enabling reaction between amino resin subjected to swelling, de-protection and washing and an activated protected amino acid solution in a constant-temperature oscillator, bonding protected amino acids corresponding to the first to the twenty-fifth amino acids from the resin in sequence to obtain ziconotide precursor peptide-amino resin, removing side-chain protecting groups of cysteine separately, performing disulfide bond cyclization reaction, purifying and freeze-drying to obtain ziconotide pure product polypeptide. The method adopts common, easily available and low-cost raw materials and reagents to perform synthesis and preparation, the yield of the ziconotide pure product reaches 30 to 40 percent through process optimization, and the yield of the ziconotide is greatly increased; and the preparation method is simple to operate, convenient to sample in the reaction process, easy in central control, short in synthesis cycle, low in production cost, high in product yield and favorable for industrial production.
Owner:合肥国肽生物科技有限公司
Method for preparing ziconotide
InactiveCN101709082BPurity is easy to controlPrecise positioningPeptide preparation methodsAnimals/human peptidesFreeze-dryingSide chain
The invention discloses a method for preparing ziconotide. The technical scheme of the invention comprises the following steps: (1) obtaining an Fmoc-Cys(Acm)-amino resin from Fmoc-Cys(Acm)-OH and an amino resin; (2) obtaining a linear-ziconotide-amino resin of which a Cys side chain comprises Acm by performing the solid phase synthesis on the Fmoc-Cys(Acm)-amino resin and an amino acid adopting Fmoc group protection; (3) obtaining a linear crude peptide of which the Cys side chain comprises the Acm by performing cracking on the linear-ziconotide-amino resin of which the Cys side chain comprises the Acm, and obtaining linear ziconotide by removing the Acm, purifying and freeze-drying; (4) and obtaining the ziconotide by performing cyclization, purifying and freeze-drying on the linear ziconotide. The method for preparing ziconotide has the characteristics of simple reaction operation, easy subsequent treatment, low raw material investment, low cost, high yield and the like, and has considerable economic and practical value, and also has wide application prospect in the field of design synthesis of polypeptide drugs.
Owner:HYBIO PHARMA
Solid-phase synthesis of ziconotide
InactiveCN101412752BImprove orientation efficiencyHigh yieldPeptide preparation methodsAnimals/human peptidesSide chainCombinatorial chemistry
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Solid-phase synthesis method of ziconotide
ActiveCN102268082BImprove accuracyHigh yieldPeptide preparation methodsAnimals/human peptidesCombinatorial chemistryTwo step
The invention discloses a solid-phase synthesis method of ziconotide, and the method comprises the following steps: with Fmoc(9-fluorenylmethyloxycarbonyl)-amino resin as a solid-phase carrier, successively carrying out condensation reaction for connecting 25 protected amino acids to obtain linear fully-protected peptide resin, wherein three groups of Cys (cysteine) with disulfide bond formed areseparately connected with Trt (triphenylmethyl), Acm (acetamidomethyl), or tBu (t-butyl) protecting group; cutting resin, and simultaneously removing all amino acid protecting groups except for Acm and tBu to obtain a linear peptide containing Acm and tBu; oxidizing the linear peptide to form a first pair of disulfide bonds, and simultaneously removing Acm and forming a second pair of disulfide bonds to obtain bicyclic peptide resin; and removing tBu of the bicyclic peptide resin, and simultaneously carrying out cyclization to form a third pair of disulfide bonds and to obtain ziconotide. In the method disclosed by the invention, Trt, Acm and tBu are selected to protect the three groups of Cys, thereby improving the formation accuracy of the disulfide bonds; and after resin is cut off, three pairs of disulfide bonds are sequentially formed through two-step reaction, thereby simplifying steps and improving productivity.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Pharmaceutical formulation comprising ziconotide
ActiveUS20070269528A1Nervous disorderPeptide/protein ingredientsAntioxidantPharmaceutical formulation
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, an antioxidant, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
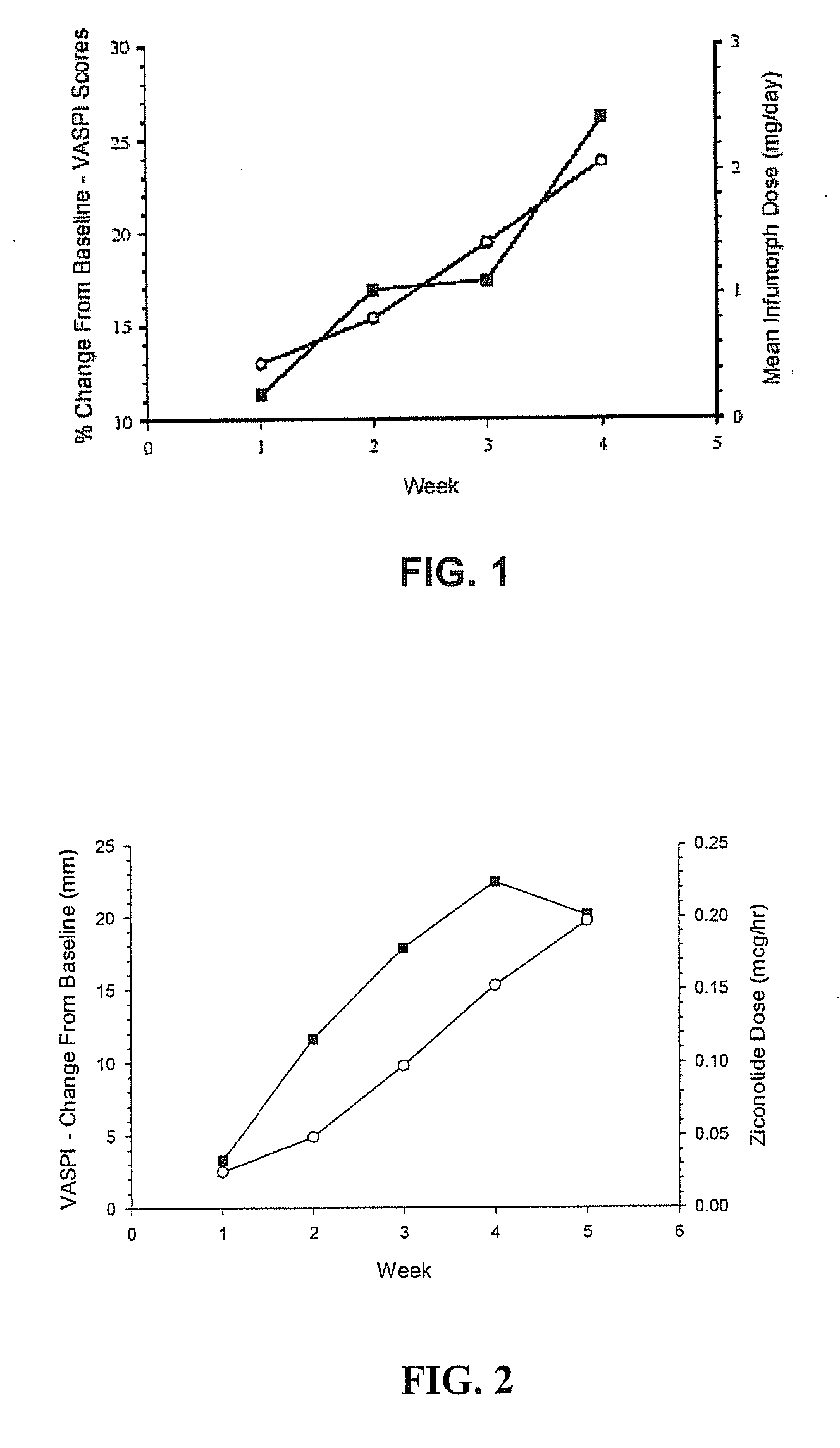
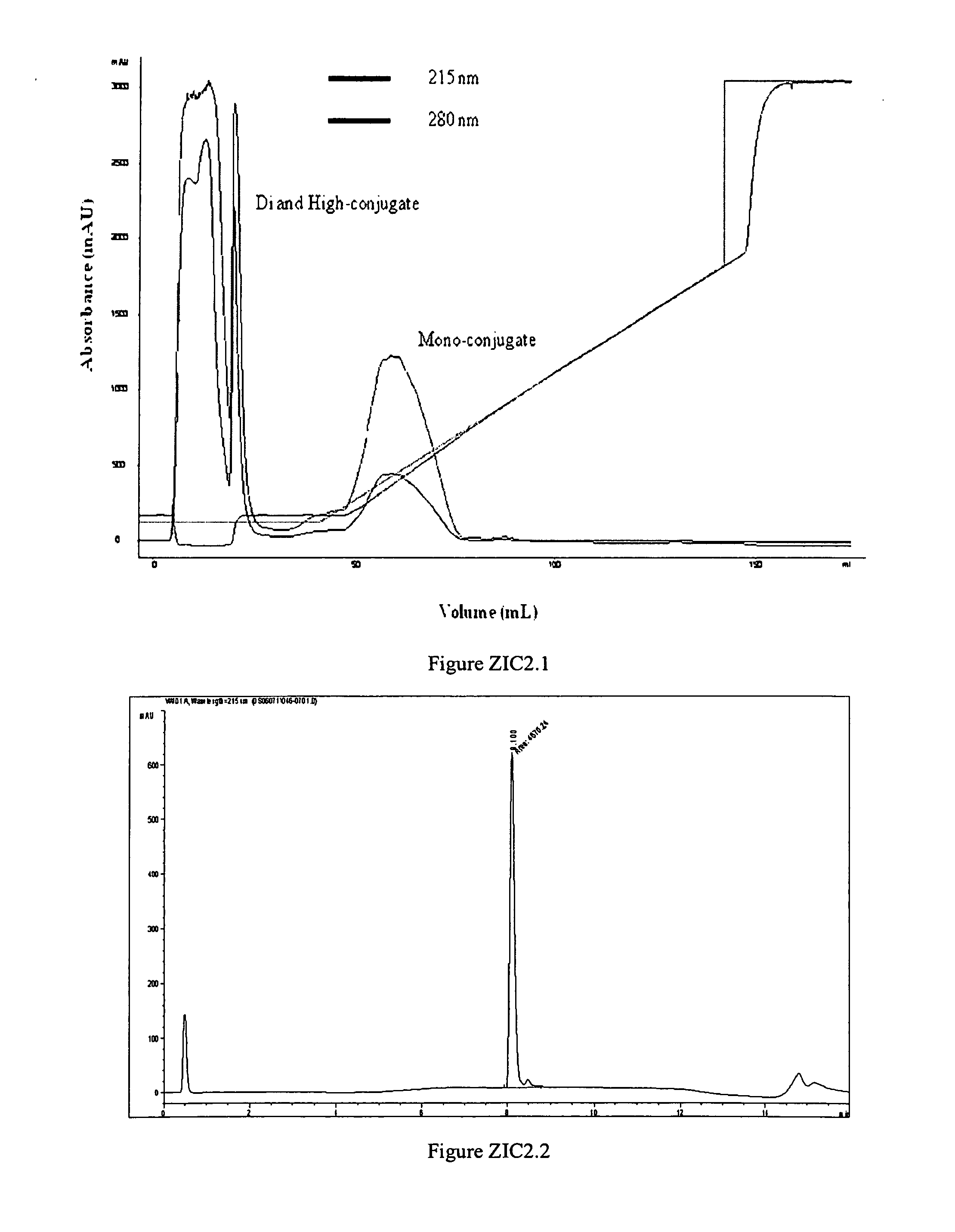
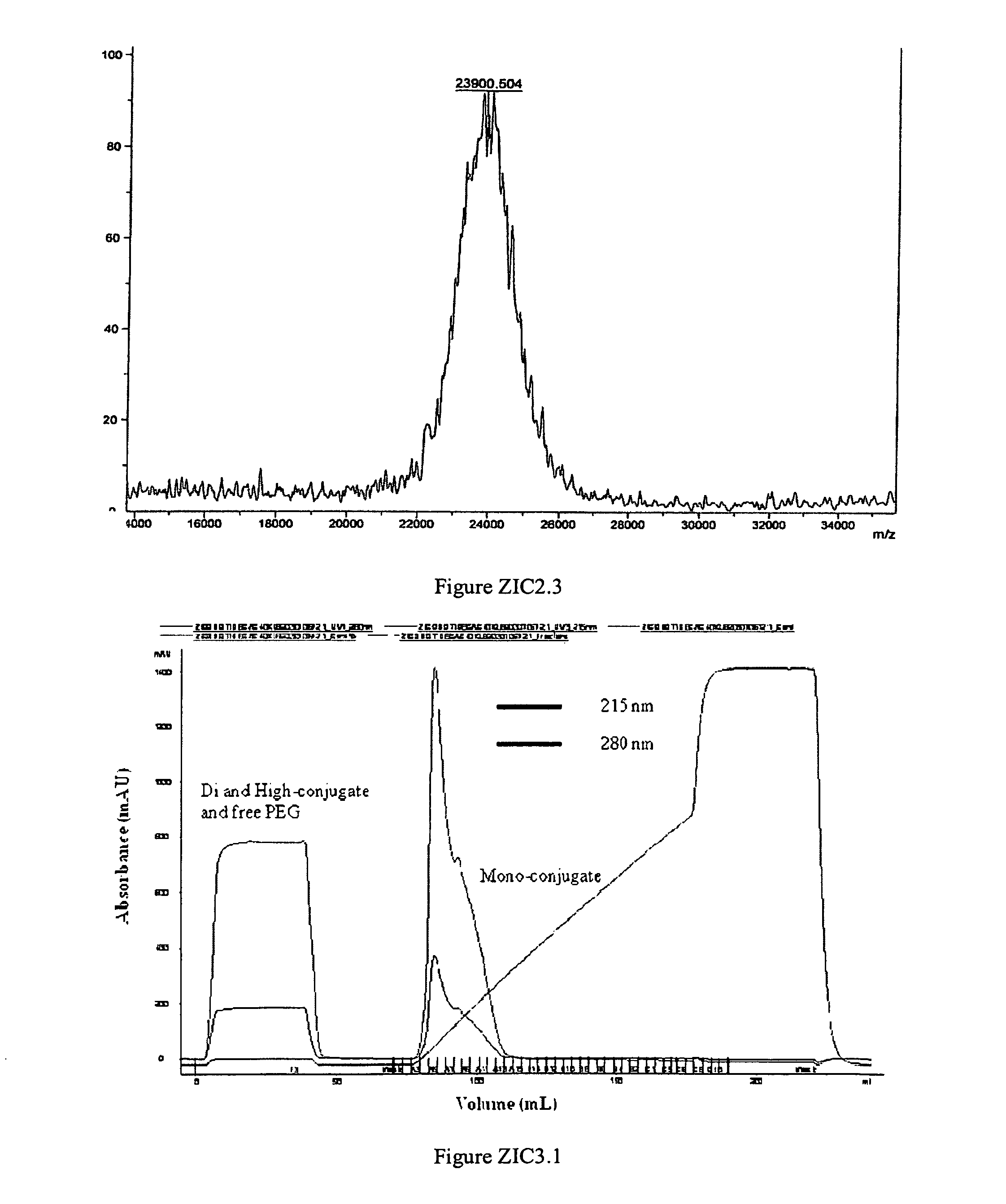
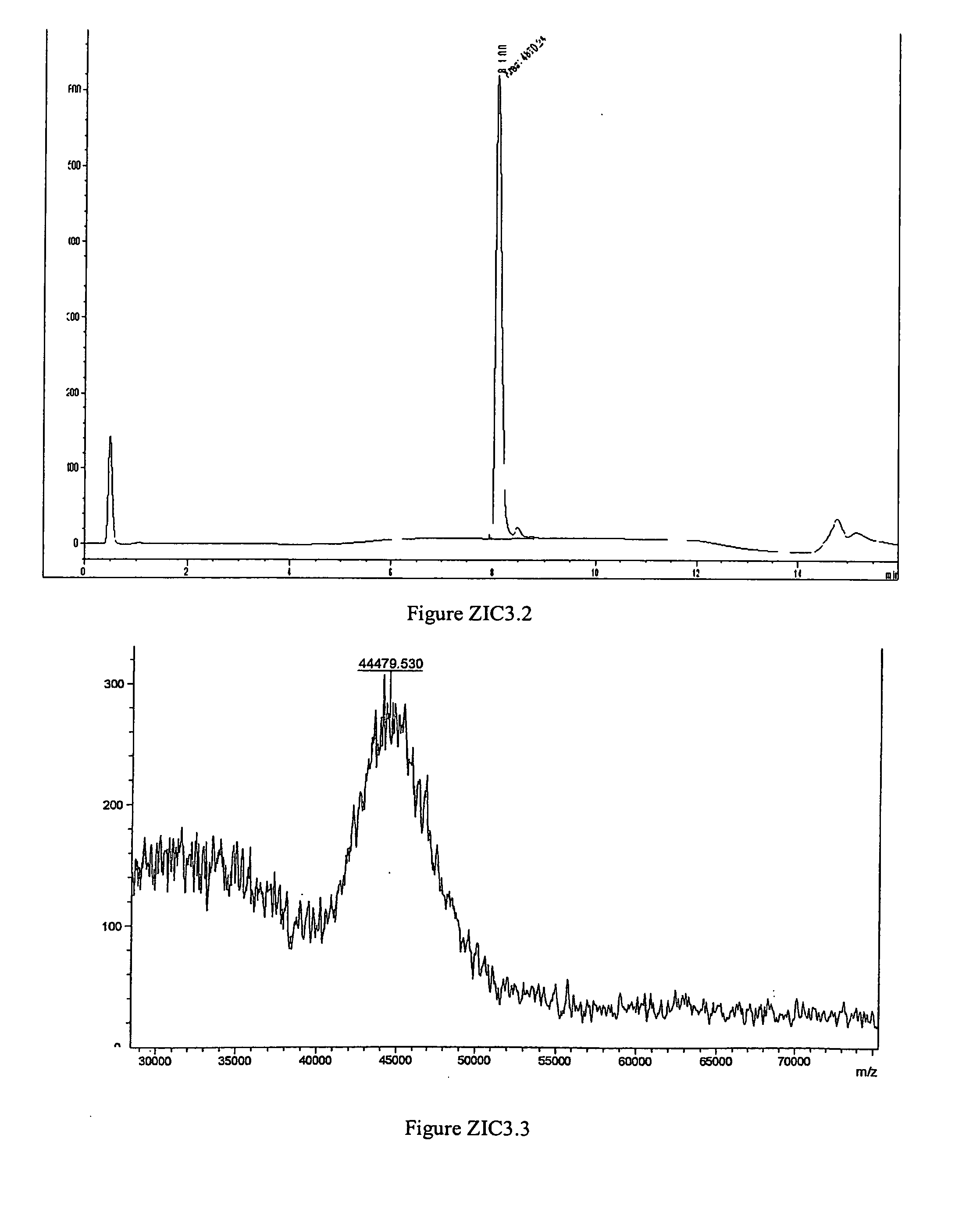
Polymer conjugates of ziconotide peptides
The invention provides peptides that are chemically modified by covalent attachment of a water soluble oligomer. A conjugate of the invention, when administered by any of a number of administration routes, exhibits characteristics that are different from the characteristics of the peptide not attached to the water soluble oligomer.
Owner:NEKTAR THERAPEUTICS INC
Ziconotide injection and preparation method thereof
PendingCN113842361AFix less stable situationsImprove product qualityNervous disorderPeptide/protein ingredientsZiconotide InjectionAnoxomer
The invention relates to a ziconotide injection and a preparation method thereof. The injection comprises the following components: 0.015-0.035 mg / ml of an active component ziconotide, 0.025-0.1 mg / ml of an antioxidant, 7.5-10 mg / ml of an osmotic pressure regulator and a pH adjusting agent; and the pH value of the injection ranges from 4.5 to 5.0. The prepared injection is stable in quality, and the preparation method is simple.
Owner:杭州澳亚生物技术股份有限公司
Polypeptide capable of passing through blood-brain barrier
PendingUS20220112250A1Good analgesic effectLonger effect timePolypeptide with localisation/targeting motifPeptide/protein ingredientsGlycinePharmacologic action
The present invention provides a polypeptide capable of crossing the blood-brain barrier. In the present invention, C-terminal of the ziconotide is linked to N-terminal of a cell membrane penetrating peptide via one glycine to obtain a polypeptide capable of crossing the blood-brain barrier The polypeptide of the present invention is suitable for intravenous, intraperitoneal or nasal administration with convenient operation and low clinical risk. It has a long pharmacological effect in vivo, excellent analgesic effect, and slight peptide side effect after intravenous, intraperitoneal or nasal administration, and is suitable for large-scale clinical applications. The polypeptide of the invention has the advantages of simple preparation, controllable preparation process and quality during the preparation, and is suitable for large-scale industrial production.
Owner:SHENZHEN RUIJIAN BIOSCI TECH LTD
A fusion polypeptide of ziconotide and tat peptide
ActiveCN109232746BLittle side effectsEasy to makePolypeptide with localisation/targeting motifPeptide/protein ingredientsTat peptideSide effect
The invention provides a fusion polypeptide of ziconotide and TAT peptide. The fusion peptide obtained by linking the C terminal of the ziconotide with the N terminal of the cell membrane penetrating peptide overcomes the disadvantages of intramuscular injection and the like. The fusion polypeptide can pass through the blood-brain barrier and is suitable for intravenous, intraperitoneal or nasal administration. It is easy to operate and has low clinical risk. It is administered through intravenous, abdominal or nasal cavity. It has a long drug effect in the body and excellent analgesic effect. The peptide has few side effects and is suitable for large-scale clinical application. The fusion polypeptide of the invention is simple to prepare, the preparation process and the quality of the preparation process are controllable, and is suitable for large-scale industrial production.
Owner:SHENZHEN RUIJIAN BIOSCI TECH LTD
Sustained-release microsphere preparation of Lhrh analogue and ziconotide composition and preparation method thereof
ActiveCN103908659BReduce releaseSolve the problem of inconvenient clinical administrationPeptide/protein ingredientsAntipyreticMicrosphereCurative effect
Owner:南京星银药业集团有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com