Efficient ziconotide synthesis and preparation method
A high-efficiency technology of ziconotide, applied in the field of polypeptide drug preparation, can solve the problems of unfavorable large-scale production of ziconotide, long synthesis cycle, and many synthesis steps, and achieve industrial production, short synthesis cycle and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of Ziconotide Precursor Peptide-Amino Resin
[0038] 1. Swelling of amino resin
[0039] Weigh 0.1g of Rink-MBHA-Resin with a degree of substitution of 0.38mmol / g, add it to the polypeptide synthesis reactor from the open end, take the DCM reagent and add it to the reactor, so that the resin is completely immersed in the DCM solvent, fully mixed with the solvent Contact, swelling for 2h.
[0040] 2. Synthesis of ziconotide precursor peptide-amino resin
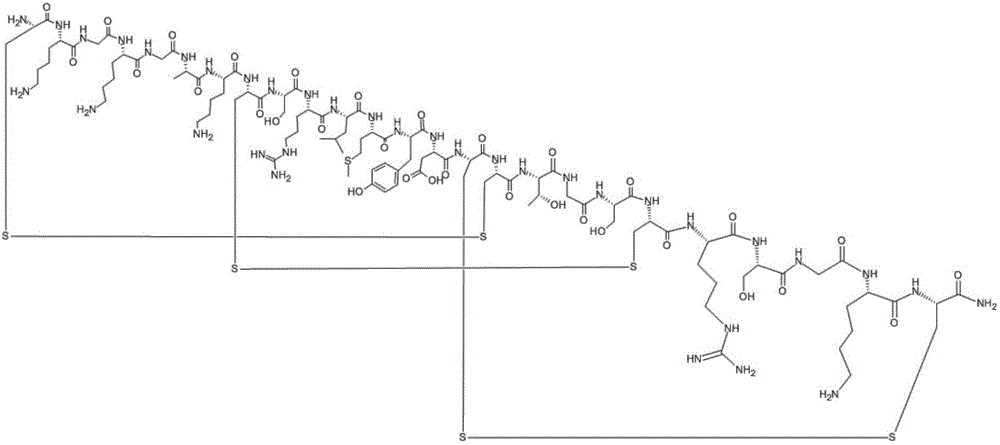
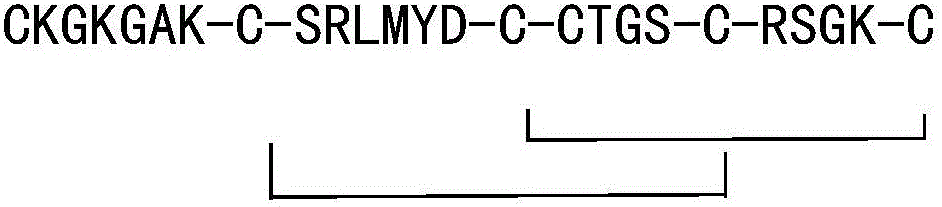
[0041] Ziconotide precursor peptide-amino resin is:
[0042] Fmoc-CKGKGAKCSRLMYDCCTGSCRSGKC-Rink Resin
[0043] The protected amino acids used in this example are listed in Table 1 below for the protected amino acids and molecular weights corresponding to the 1-25th amino acid from the resin:
[0044] Table 1
[0045]
[0046]
[0047]Some commonly used abbreviations in the present invention have the following meanings:
[0048]
[0049] Activation methods for protected amino acids
[0050] Tak...
Embodiment 2
[0055] Synthesis of Ziconotide Precursor Peptide Ⅰ-Amino Resin
[0056] The ziconotide precursor peptide-amino resin was eluted to remove the side chain protecting groups of Cys8 and Cys20, and the elution reaction reagent was a mixed solution of 0.5% TFA+10% dichloroacetic acid+DCM, and the elution reaction was 20min Drain the eluate, then form the first pair of disulfide bonds of ziconotide with the solution cyclization reaction of DCM:DMF=1:1, add triethylamine to adjust the pH to 9, and obtain the ziconotide precursor Peptide I - amino resin.
Embodiment 3
[0058] Synthesis of Ziconotide Precursor Peptide Ⅱ
[0059] The ziconotide precursor peptide I-amino resin was eluted to remove the side chain protecting groups of Cys15 and Cys25, and the elution reagent was 6% TFA + 2.5% H 2 O + 2.5% Tis + 1% EDT + 88% DCM mixed solution, elution reaction for 40 minutes; the reaction solution was centrifugally settled and freeze-dried with anhydrous ether pre-cooled at -20°C, and the obtained sample was reversed-phase high-efficiency Purified by liquid chromatography, freeze-dried to obtain a pure product sample; the sample was dissolved and then dissolved in (NH 4 ) 2 CO 3 Adjust the pH to 8.6 to carry out the second disulfide bond cyclization reaction of ziconotide, and adjust the pH to 5 with 5% acetic acid aqueous solution to obtain the ziconotide precursor peptide II.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com