Purification method of Ziconotide
A technology of ziconotide and purification method, which is applied in the field of purification of polypeptide drugs, can solve problems such as difficult separation, low product purity, and reduced yield of ziconotide, and achieve good polymer impurities, high yield, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1: the solid-phase synthesis of ziconotide
[0082] Weigh 25g of Rink Amide AM resin with a degree of substitution of 0.40mmol / g, add it to the solid-phase reaction column, wash it once with DCM, and swell the resin with DCM for 30 minutes, then wash it with DMF:pyridine The mixed solution with a volume ratio of 4:1 was deprotected by Fmoc, and then washed 6 times with DMF, and 17.57g Fmoc-Cys(Trt)-OH (30mmol) and 4.05g HOBt (30mmol) were added in a volume ratio of 1:1 DCM and DMF mixed solution, add 4.6ml DIC (30mmol) under ice-water bath to activate, then add to the above-mentioned reaction column equipped with resin, react at room temperature for 2 hours, use ninhydrin method to detect and judge the reaction end point, if the resin has no If the color is transparent, it indicates that the reaction is complete; if the resin develops color, it indicates that the reaction is incomplete and needs to be reacted for another 1 hour. This criterion is applicable...
Embodiment 2
[0083] Embodiment 2: the splitting of ziconotide
[0084] Take by weighing 105.02g ziconotide linear peptide resin, join in the three-neck round bottom flask of 2000mL, be EDT, PhSMe, H in volume ratio 2.5:5:5:87.5 2 O and TFA were prepared with 1050ml of lysate, and the lysate was added to the above resin, and reacted at 20~30°C for 2 hours. The reaction solution was added to glacial ether for precipitation for 1 hour, centrifuged, washed with anhydrous ether for 6 times, and vacuum-dried to obtain 27.12 g of ziconotide linear peptide.
Embodiment 3
[0085] Embodiment 3: Cyclization of Ziconotide
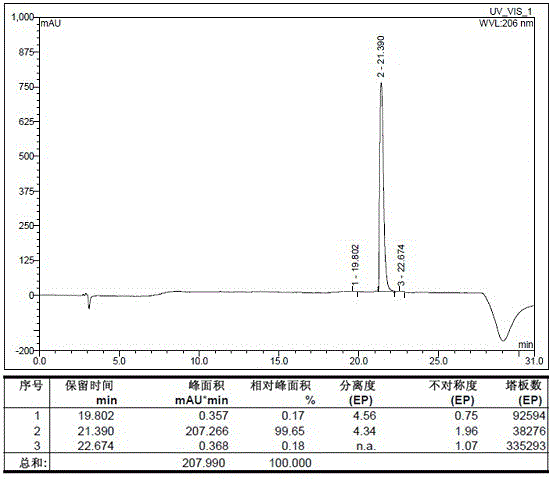
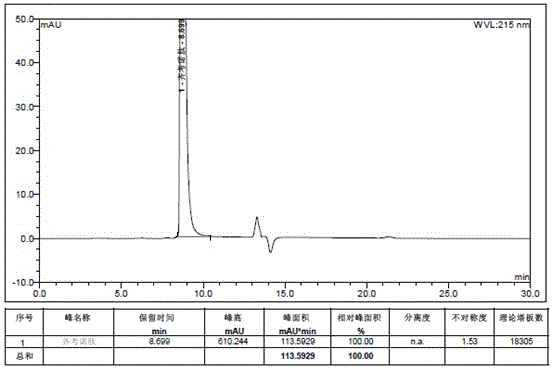
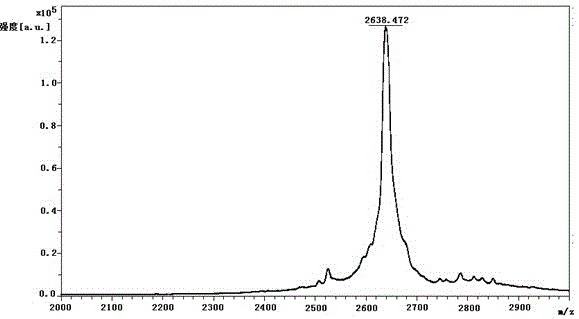
[0086] Example 2 middle Add 27.12g of ziconotide linear peptide, 53.92g of ammonium acetate, 2.61g of disodium ethylenediaminetetraacetic acid and 0.847g of cysteine into 7L of aqueous solution and stir to dissolve, use ammonia water to adjust the pH to 8, and stir at 5-10°C After reacting for 72 hours, it was used for later use. The purity of the crude product of ziconotide was 67.5% (detected by HPLC), the content of polymer impurities was 2.73% (detected by gel chromatography), and the synthesis yield was 62.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com