Sustained-release microsphere preparation of Lhrh analogue and ziconotide composition and preparation method thereof
A slow-release microsphere preparation, technology of ziconotide, applied in the direction of drug combination, anti-inflammatory agent, pharmaceutical formula, etc., can solve the problems of inconvenient administration of ziconotide, and achieve clinical drug administration inconvenience and good curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
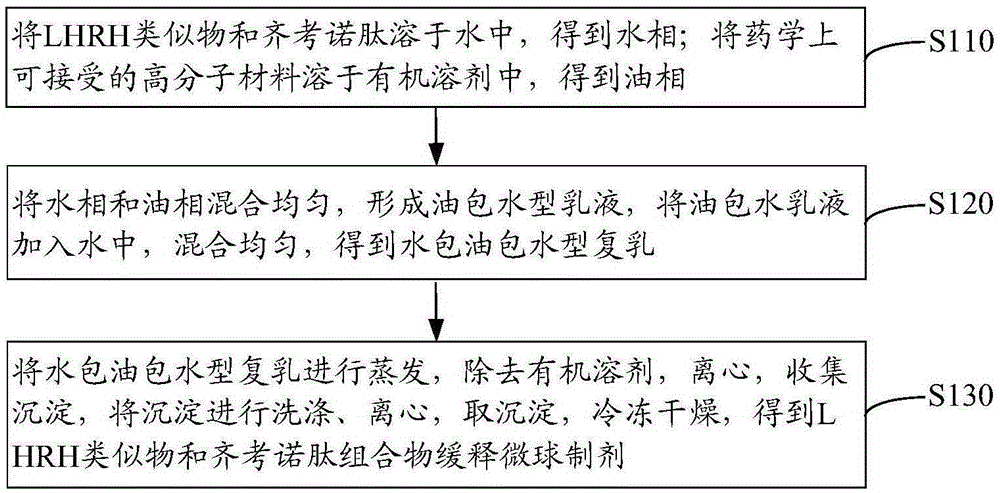
[0048] see figure 1 , the preparation method of the slow-release microspheres of LHRH analogue and ziconotide composition according to one embodiment, comprising the following steps S110 to S130.
[0049] Step S110: dissolving the LHRH analog and ziconotide in water to obtain an aqueous phase; dissolving a pharmaceutically acceptable polymer material in an organic solvent to obtain an oil phase.
[0050] The LHRH analogue and ziconotide were dissolved in water to obtain an aqueous phase. Water is sterile water. The mass ratio of LHRH analogs to ziconotide is preferably 0.1-30:60-90. In the aqueous phase, the mass concentration of LHRH analogs and ziconotide was 60 mg / L.
[0051] In other embodiments, the aqueous phase also includes gelatin and glycerin. In this case, an aqueous solution containing gelatin and glycerin is prepared first, and then LHRH analogues and ziconotide are dissolved in the aqueous solution containing gelatin and glycerin to obtain water box.
[0052...
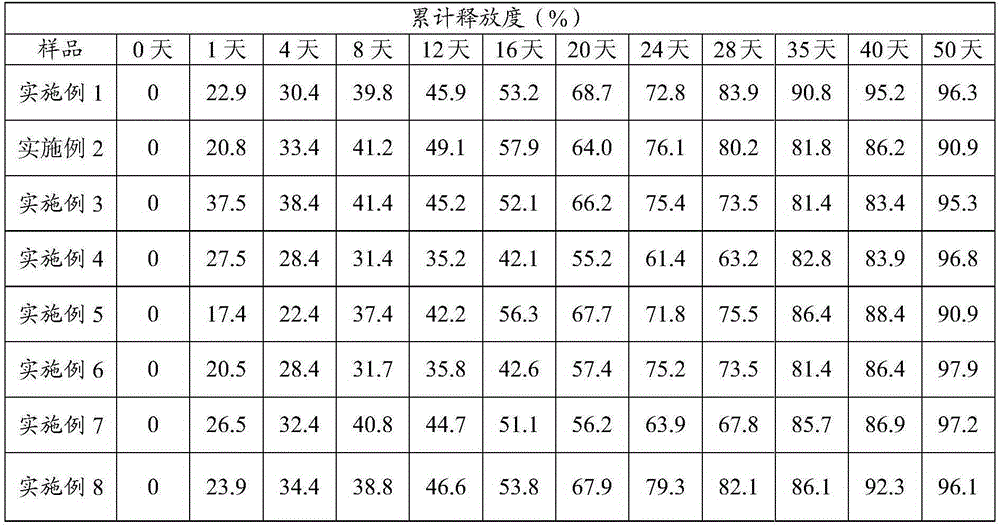
Embodiment 1
[0067] 1. Dissolve gelatin and glycerin of equal quality in sterile water to prepare an aqueous solution containing gelatin and glycerin, wherein the mass concentrations of gelatin and glycerin are both 5 mg / L, and dissolve goserelin acetate and ziconotide in In the aqueous solution that contains gelatin and glycerol, the total mass concentration that obtains goserelin acetate and ziconotide is the water phase of 60mg / L; Add PVP in the water phase, obtain mixture; Polylactide that molecular weight is 5000— Glycolide was dissolved in dichloromethane to obtain an oil phase with a mass concentration of 2400 mg / L; wherein, the mass ratio of goserelin acetate and ziconotide was 1:1, polylactide and polylactide-B The mass ratio of lactide is 1:3;
[0068] 2. Put the above mixture and the oil phase in the mixer, and mix evenly at a speed of 12000r / min to form a water-in-oil emulsion. Add the water-in-oil emulsion to water and mix evenly to obtain a water-in-oil-in-water emulsion. Do...
Embodiment 2
[0072] 1. Dissolve gelatin and glycerin of equal quality in sterile water to prepare an aqueous solution containing gelatin and glycerin, wherein the mass concentrations of gelatin and glycerin are both 5 mg / L, and dissolve goserelin acetate and ziconotide in In the aqueous solution that contains gelatin and glycerin, the total mass concentration that obtains goserelin acetate and ziconotide is the aqueous phase of 60mg / L; Add PVA in the aqueous phase, obtain mixture; Polylactide and the polylactide that molecular weight is 20000 and Polylactide-glycolide with a molecular weight of 20,000 is dissolved in ether to obtain an oil phase with a mass concentration of 540 mg / L; wherein, the mass ratio of goserelin acetate and ziconotide is 1:10, and the polylactide The mass ratio of polylactide to glycolide is 3:2;
[0073] 2. Put the above mixture and the oil phase in the mixer, and mix evenly at a speed of 12000r / min to form a water-in-oil emulsion. Add water to the water-in-oil em...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com