Synthesis method of goserelin
A technology for goserelin and solid-phase synthesis, applied in the field of solid-phase synthesis of goserelin polypeptides, can solve the problems of purification influence, poor stability, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
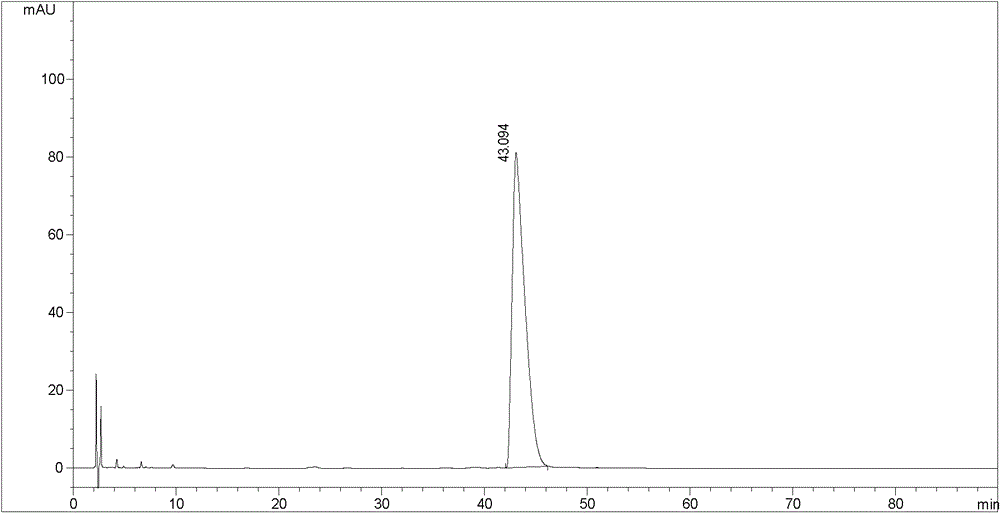
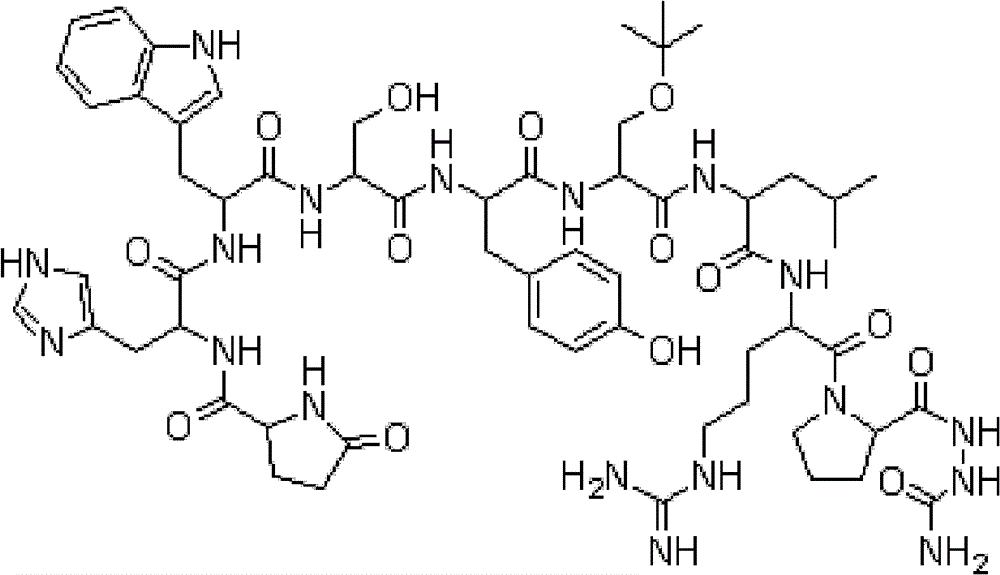
Image
Examples
Embodiment 1
[0109] Example 1 Preparation of Fmoc-NH-NH-CO-NH-Resin
[0110] The abbreviation and the full name of the raw material adopted in the embodiment and the foregoing process are as follows:
[0111] Arg arginine Azgly NH-NH-COOH (azaglycine) D-Ser D-serine Glp pyroglutamic acid His histidine Leu leucine Pro proline Ser serine Trp tryptophan Tyr tyrosine DIC di-isopropylcarbodi-imide DMF N,N-dimethylformamide t-Bu tert-butyl DSC diduccinimido carbonate Su succinimido
[0112] Pip piperidine HOB N-Hydroxybenzotrizole
[0113] In the example:
[0114] Said peptide-connecting reagent is as follows: DIC:DMF=1:10, volume ratio;
[0115] Said decapping reagent is as follows: PIP: DMF=1: 4, volume ratio;
[0116] Said acidifying reagent is: HOBt:DMF=1:20, the same below;
[0117] 1) Take 1g of Rink Amide MBHA (0.5mmol / g, 0.5mmol) resin and put it in...
Embodiment 2
[0123] Example 2 Preparation of Fmoc-Pro-NH-NH-CO-NH-resin
[0124] 1) Add 10ml of decapping reagent, react at 25°C for 15 minutes, drain, wash with DMF five times, and drain.
[0125] 2) Add Fmoc-Pro, add 3ml peptide reagent, and react at 25°C for 60 minutes.
[0126] 3) Drain, wash three times with DMF, and drain.
Embodiment 3
[0127] Example 3 Preparation of Fmoc-Arg HCl-NH-NH-CO-NH-resin
[0128] 1) Add 10ml of decapping reagent, react at 25°C for 15 minutes, drain, wash with DMF five times, and drain.
[0129] 2) Add Fmoc-Arg·HCl, add 3ml peptide reagent, and react at 25°C for 90 minutes.
[0130] 3) Drain, wash three times with DMF, and drain.
[0131] 4) Add 10ml of decapping reagent, react at 25°C for 15 minutes, drain, wash with DMF five times, and drain.
[0132] 5) Add 5ml of acidifying reagent and react for 5 minutes.
[0133] 6) Add Fmoc-Arg·HCl, add 3ml of peptide reagent, and react at 25°C for 90 minutes.
[0134] 7) Drain, wash three times with DMF, and drain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com