Preparation method of natural active polypeptide Tubulysin U
An active peptide and natural technology, applied in the direction of peptides, can solve problems such as unsatisfactory synthesis and imperfect synthesis routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] According to the basic idea of the present invention, this embodiment provides a kind of preparation method of natural active polypeptide Tubulysin U, the route of this preparation method is as follows:
[0089]
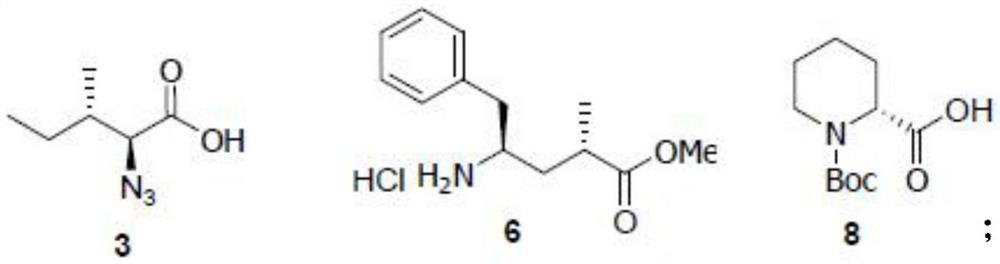
[0090] Wherein, the structures of compound 3, compound 6 and compound 8 used in the above route are as follows:
[0091]
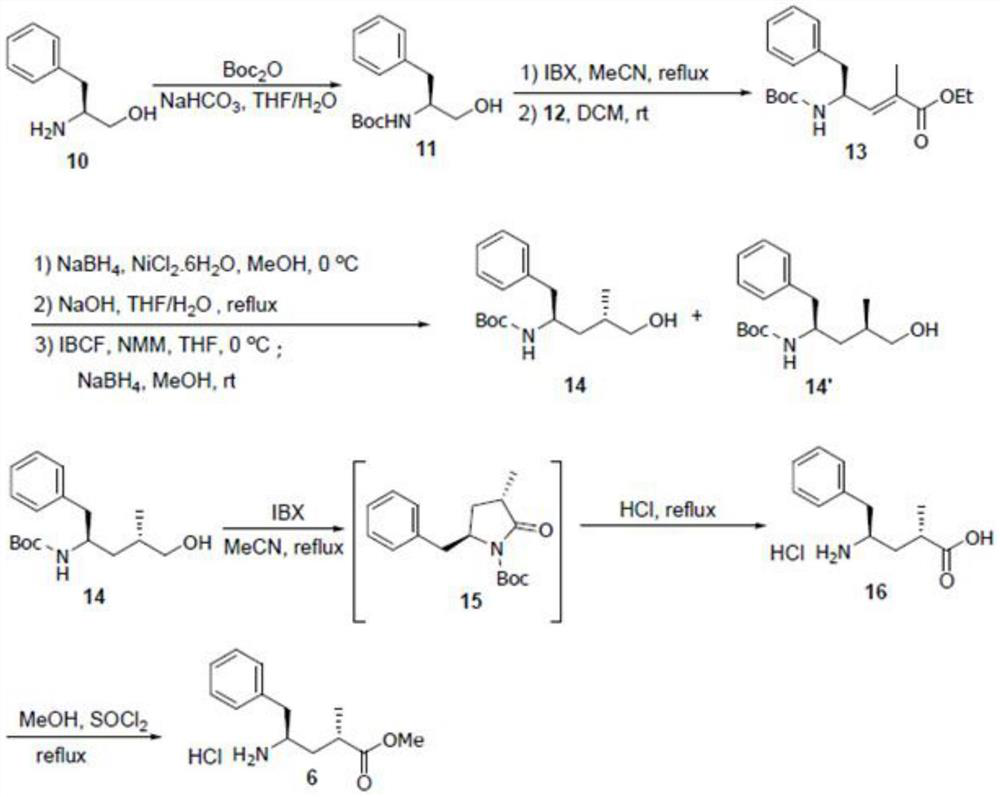
[0092] The compound 6 in the above route is synthesized by the route shown below:
[0093]
[0094] Wherein, the structure of compound 12 used in the above-mentioned route is as follows:
[0095]
[0096] In conjunction with the above-mentioned preparation route, the preparation method of Tubulysin U-compound 1 is specifically set forth below, and the preparation method comprises:
[0097] (1) Synthesis of Compound 4
[0098] Compound 2 (2.0 g, 5.1 mmol) was dissolved in trifluoroacetic acid (50 mL), heated to reflux for 3 h, concentrated under reduced pressure, and dried in vacuo to obtain an intermediate.
[0099] The ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com