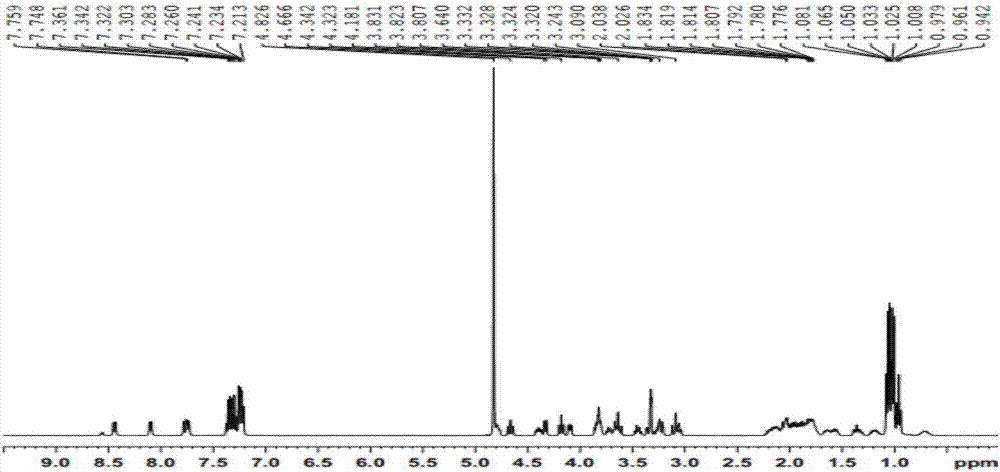
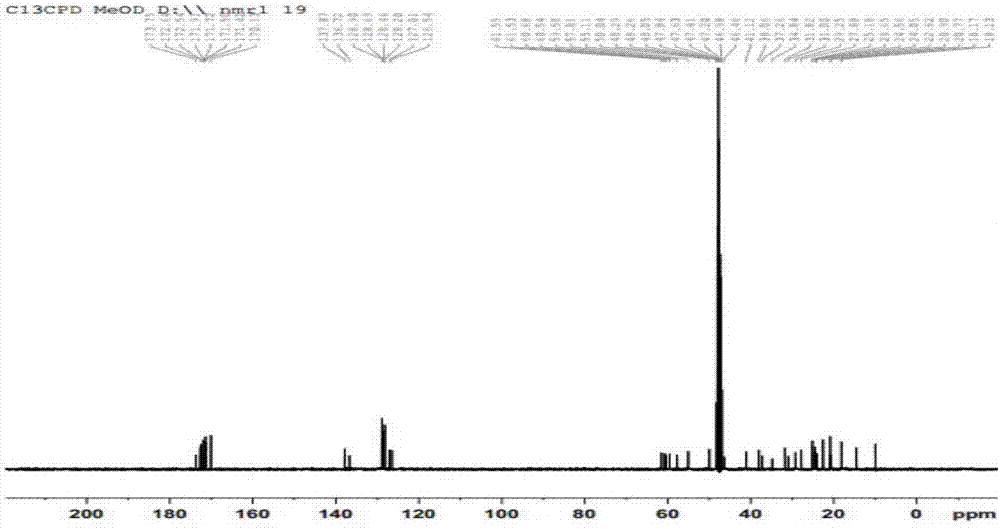
Cyclo(Phe-Pro-lle-Phe-Pro-Pro-Leu-Val)peptide preparation method
A fmoc-phe-oh, cyclization reaction technology, applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve problems such as restricting production and application, high cost, and low content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention provides a method for preparing a cyclic (phenylalanine-proline-isoleucine-phenylalanine-proline-proline-leucine-valine) peptide, comprising:
[0024] 1) The base resin, Fmoc-Phe-OH, and N,N-diisopropylethylamine with the structure shown in formula (I) are contacted in a solvent to prepare a compound with the structure shown in formula (II). Grade resin, then by methanol capping primary resin, then remove the Fmoc of the primary resin by Fmoc eluent elution, finally through piperidine deprotection to obtain the secondary resin of structure shown in formula (III);
[0025] 2) The secondary resin is subjected to multiple continuous modification reactions with various modified amino acids in turn to obtain a tertiary resin with a structure shown in formula (IV); each step of the modification reaction is: the resin and the modified amino acid are mixed in N , in the presence of N-diisopropylethylamine, solvent and HATU, carry out contact reaction, then remove ...
Embodiment 1
[0055]
[0056] 1) Soak 2-chlorotrityl chloride resin (2g, represented by formula I-1) in dichloromethane (1mL) for 30min, add N-fluorenylmethoxycarbonylphenylalanine (1029.4mg, 2.65mmol ), DIPEA (1390uL, 7.95mmol), stirred and reacted at 25°C for 1h, and the resulting N-fluorenylmethoxycarbonylphenylalanine-2-chlorotrityl chloride resin (shown in formula II-1) was added to methanol To seal the head, rinse 3 times with DCM and DMF respectively (15-25mL of DCM and DMF each time). After deprotection with 20% by weight of piperidine (20 mL) at 25° C. for 30 min to remove Fmoc, wash with DCM and DMF 3 times each (15-25 mL for each DCM and DMF) to obtain phenylalanine-2-chlorotris Benzyl chloride resin (shown in formula III-1).
[0057] 2) N-Fremoxyproline (2692.5mg, 7.95mmol), DIPEA (1390uL, 7.95mmol), HATU (1517mg, 3.99mmol), and DCM (20mL) were added to phenylalanine-2- In chlorotrityl chloride resin, stir and react at 25°C for 1h, remove the filtrate, wash 3 times with DCM...
Embodiment 2
[0071] Carry out according to the method for embodiment 1 and obtain cyclic (phenylalanine-proline-isoleucine-phenylalanine-proline-proline-leucine-valine) peptide (total yield 48.8%, weight 1017.56mg), the difference is that the amount of Fmoc-Phe-OH in step 1) is 3mmol, the amount of Fmoc-Pro-OH in step 2) is 9mmol, and the amount of Fmoc-Ile-OH in step 2) The consumption of OH is 9mmol, the consumption of Fmoc-Phe-OH in step 2) is 9mmol, the consumption of the Fmoc-Pro-OH that adds for the first time in step 2) is 9mmol, the Fmoc-Pro-OH that adds for the second time in step 2) The amount of Pro-OH used is 9 mmol, the amount of Fmoc-Leu-OH used in step 2) is 9 mmol, and the amount used of Fmoc-Val-OH in step 2) is 9 mmol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com