Liquid-phase synthesis method of snake venom-like tripeptide
A liquid-phase synthesis, snake venom technology, applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of product quality impact, reaction difficulties, process control difficulties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
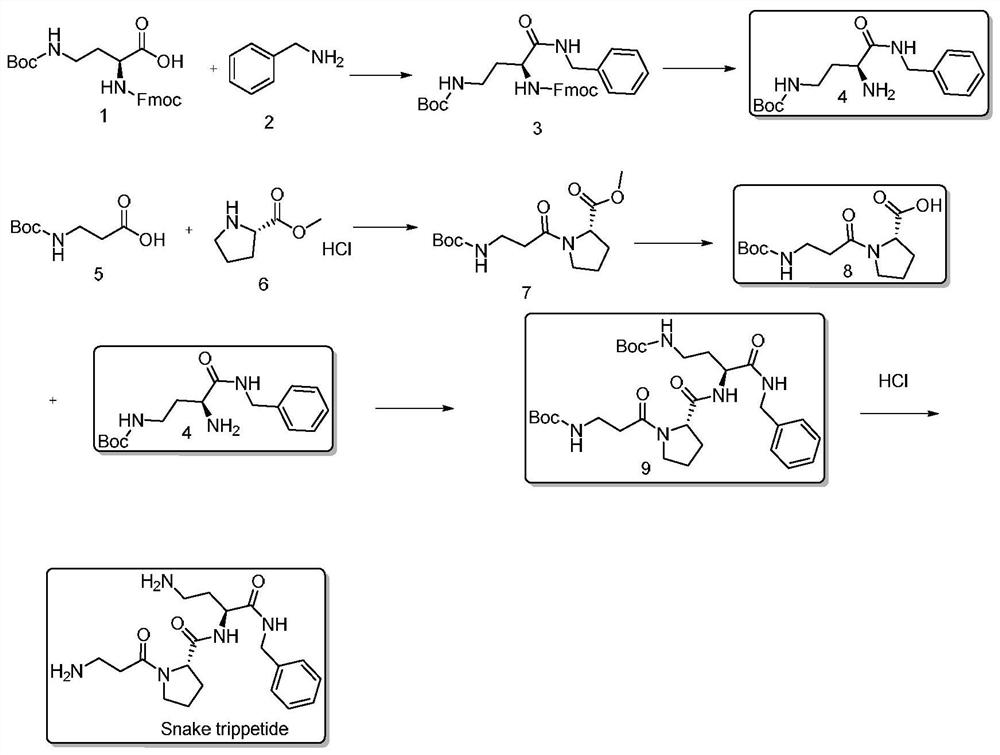
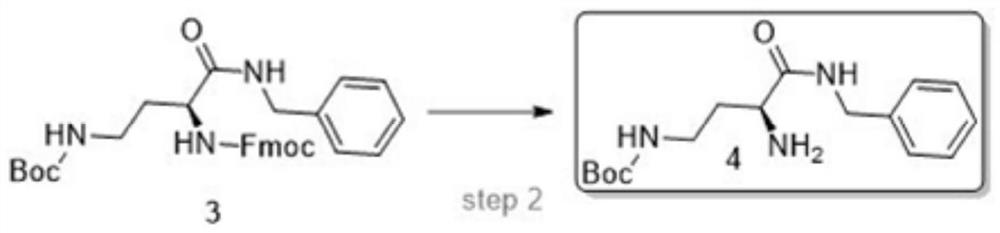
[0037] The embodiment of the liquid phase synthesis method of a kind of snake venom tripeptide of the present invention is described further:
[0038] (1) Synthesis of Fmoc-Dab(Boc)-NHBzl
[0039] Add 18L DMF to the 100L reaction tank, add Fmoc-Dab(Boc)-OH (200.0g, 454mmol) and HoBT (79.9g, 590mmol), BnNH2 (48.6g, 454mmol), NMM (59.7g, 590mmol), and then Add 2L of DMF to wash, stir to dissolve, slowly add EDCI (1132.2g, 5906mmol), and stir at room temperature overnight.
[0040] TLC detected that the reaction of the raw materials was complete, and the reaction solution was added to a large amount of water to precipitate a viscous solid, then the supernatant was discarded, and the solid was washed with water. The solid was dissolved in DCM, and the layers were separated after dissolution, and the aqueous phase was removed. The DCM layer was dried over magnesium sulfate and filtered to obtain a DCM solution. The DCM was removed by rotary evaporation to obtain 200 g of a white...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com