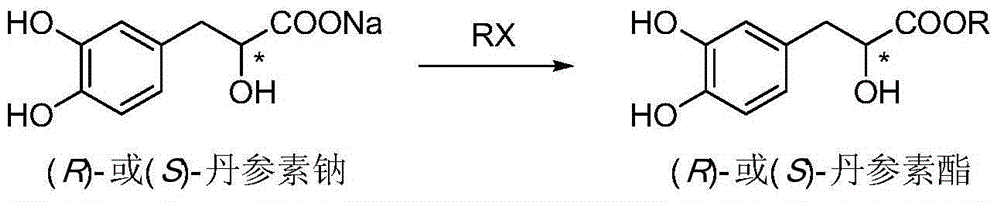
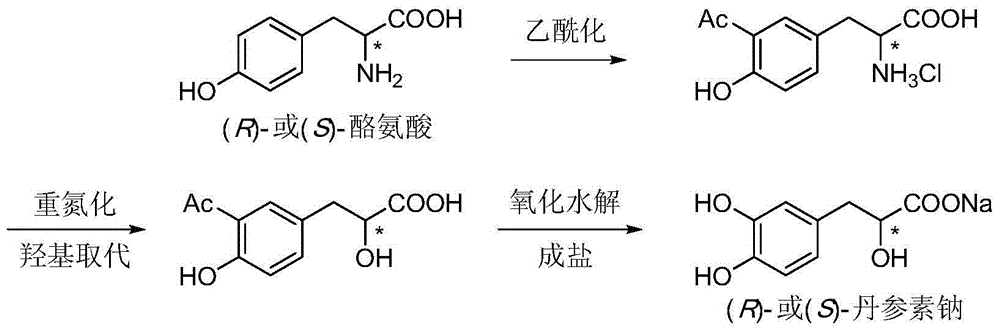
Asymmetric synthesis method for tanshinol ester derivative
A technology of danshensu ester and synthesis method, which is applied in the field of asymmetric synthesis of danshensu ester derivatives and achieves the effects of high yield, high enantioselectivity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, the asymmetric rhodium-catalyzed oxidation of (Z)-2-acetamido-3-(4-hydroxyphenyl) acrylic acid
[0026] Add (Z)-2-acetamido-3-(4-hydroxyphenyl)acrylic acid to a 10mL reaction test tube, catalyst [Rh((R,R)-QuinoxP*)(cod)]SbF6 (the amount is dehydroamino acid 1 / 10000 of the molar equivalent), vacuumize and change the hydrogen for 3 times, then add degassed MeOH (2mL) under the protection of hydrogen, and finally adjust the hydrogen pressure to 20atm and stir vigorously for 48 hours. After the reaction was completed, MeOH was distilled off under reduced pressure to obtain (R)-2-acetylamino-3-(4-hydroxyphenyl)propionic acid.
[0027] 1 HNMR (CD 3 OD, 400MHz): δ7.25(d, J=8.6Hz, 2H), 7.02(d, J=8.6Hz, 2H), 4.65(dd, J=9.2Hz, 5.2Hz, 1H), 3.20(dd, J=13.8Hz, 5.2Hz, 1H), 2.96(dd, J=13.8Hz, 9.2Hz, 1H), 2.25(s, 3H), 1.91(s, 3H).
[0028] HPLC (with (CH 3 ) 3 SiCHN 2 Determination after methyl esterification): DaicelCHIRALCELOD-H, 1.0mL / min, 2-propanol / hexane=10 / ...
Embodiment 2
[0029] Embodiment 2, the asymmetric rhodium catalytic hydrogenation of (Z)-2-acetamido-3-(4-acetoxyphenyl) methyl acrylate
[0030]Add (Z)-2-acetamido-3-(4-acetoxyphenyl)methyl acrylate to a 10mL reaction test tube, catalyst [Rh((S, S)-QuinoxP*)(cod)]SbF6 (amount of 1 / 10000 of the molar equivalent of dehydroamino acid), vacuumize the hydrogen for 3 times, then add degassed MeOH (2mL) under the protection of hydrogen, and finally adjust the hydrogen pressure to 5atm and stir vigorously for 20 hours. After the reaction was completed, MeOH was distilled off under reduced pressure to obtain (S)-methyl 2-acetamido-3-(4-acetoxyphenyl)propionate.
[0031] 1 HNMR (CD 3 OD, 400MHz): δ7.23(d, J=8.6Hz, 2H), 7.02(d, J=8.6Hz, 2H), 4.87(s, 3H), 4.66(dd, J=8.8Hz, 5.8Hz, 1H), 3.14(dd, J=14.0Hz, 5.8Hz, 1H), 2.97(dd, J=14.0Hz, 8.8Hz, 1H), 2.56(s, 3H), 1.91(s, 3H).
[0032] HPLC: Daicel CHIRALCELOD-H, 1.0 mL / min, 2-propanol / hexane=10 / 90, 210 nm. Rt(major)=15.3min, Rt(minor)=19.9min.
Embodiment 3
[0033] The synthesis of embodiment 3, (R)-tyrosine
[0034] In a 500ml single-necked bottle, add (R)-2-acetylamino-3-(4-hydroxyphenyl)propionic acid and hydrochloric acid, stir to dissolve, and heat to reflux. The reaction was monitored by a TLC plate, and after 3 hours the raw material spot disappeared, and the heating was stopped. Add ammonia water to the hydrolyzed solution to adjust the pH value to 3.5, add 1% activated carbon, stir and boil for 10 minutes, stir and heat in a water bath at 90°C for 30 minutes, filter while hot, wash the activated carbon layer with distilled water 3 times, and combine the filtrate and lotion. Place it below 10°C for 24 hours, and the crystalline (R)-tyrosine (ee>99%) will precipitate out.
[0035] 1 HNMRofhydrochloridesaltof5(D 2 (0, 400MHz): δ7.13(d, J=8.4Hz, 2H), 6.83(d, J=8.4Hz, 2H), 4.23(dd, J=7.6Hz, 5.6Hz, 1H), 3.21(dd, J=14.4Hz, 5.6Hz, 1H), 3.09 (dd, J=14.4Hz, 7.6Hz, 1H).
[0036] HPLC: DaicelCHIRALCELCR(+), 0.8mL / min, pH=2.0HC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com