Synthetic route of compound and application thereof in field of preparation of anti-diabetic drugs
A synthesis route and compound technology, applied in the field of pharmaceutical synthesis, can solve the problems of low synthesis yield and complicated purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This example is a specific example of synthesizing SN158.
[0041] 1.1 Step I—preparation of intermediate product 1 (5-bromo-2,4-hydroxybenzaldehyde)
[0042] 2,4-Dihydroxybenzaldehyde (10 g, 72.4 mmol) was dissolved in acetic acid, and the reaction was placed under an ice bath. Under stirring condition, liquid bromine (3.78ml, 72.4mmol) was slowly added dropwise, and the reaction temperature was slowly raised to room temperature. After reacting for 3 hours, the reaction solution was poured into 100 mL of cold water, filtered, washed with 100 mL of cold water, dried, and recrystallized with 1:1 acetonitrile / toluene to obtain 13.09 g of intermediate product 1 crystals (yield: 83%).
[0043] Structural characterization of intermediate product 1:
[0044] Melting point: 165-168°C.
[0045] 1.2 Step II—Preparation of intermediate product 2 (5-bromo-2-hydroxyl-4-(methoxymethyl)benzaldehyde)
[0046] Intermediate 1 (10.9 g, 50 mmol) and potassium carbonate (20.8 g, 150.3 ...
Embodiment 2
[0099] This example is a specific example for verifying that the prepared SN158 improves blood sugar and glucose tolerance.
[0100] Six-week-old male C57BL / 6JHamSlc-ob / ob was used as the animal model of spontaneous insulin resistance diabetes, and the mice were raised under the conditions of 23±2°C, 55±5% humidity and standard light cycle (12 hours light / dark) .
[0101] The experiment was divided into 4 groups, with 7 mice in each group. Four groups of mice were fed with 30mg / kg of pioglitazone, 30mg / kg of SN158, 100mg / kg of SN158 and normal saline every day for 3 consecutive weeks. Blood samples were collected 1 week, 2 weeks, and 3 weeks after administration, and blood glucose levels were measured. After gavage for 3 weeks, fast overnight, take 2g / kg of D-glucose orally, collect blood from the tail vein at 0 minutes, 30 minutes, 60 minutes, 120 minutes, and 240 minutes respectively, use a blood glucose meter to measure blood glucose, and calculate the area under the bloo...
Embodiment 3
[0104] This embodiment is a specific embodiment for measuring the adverse effects of the prepared SN158.
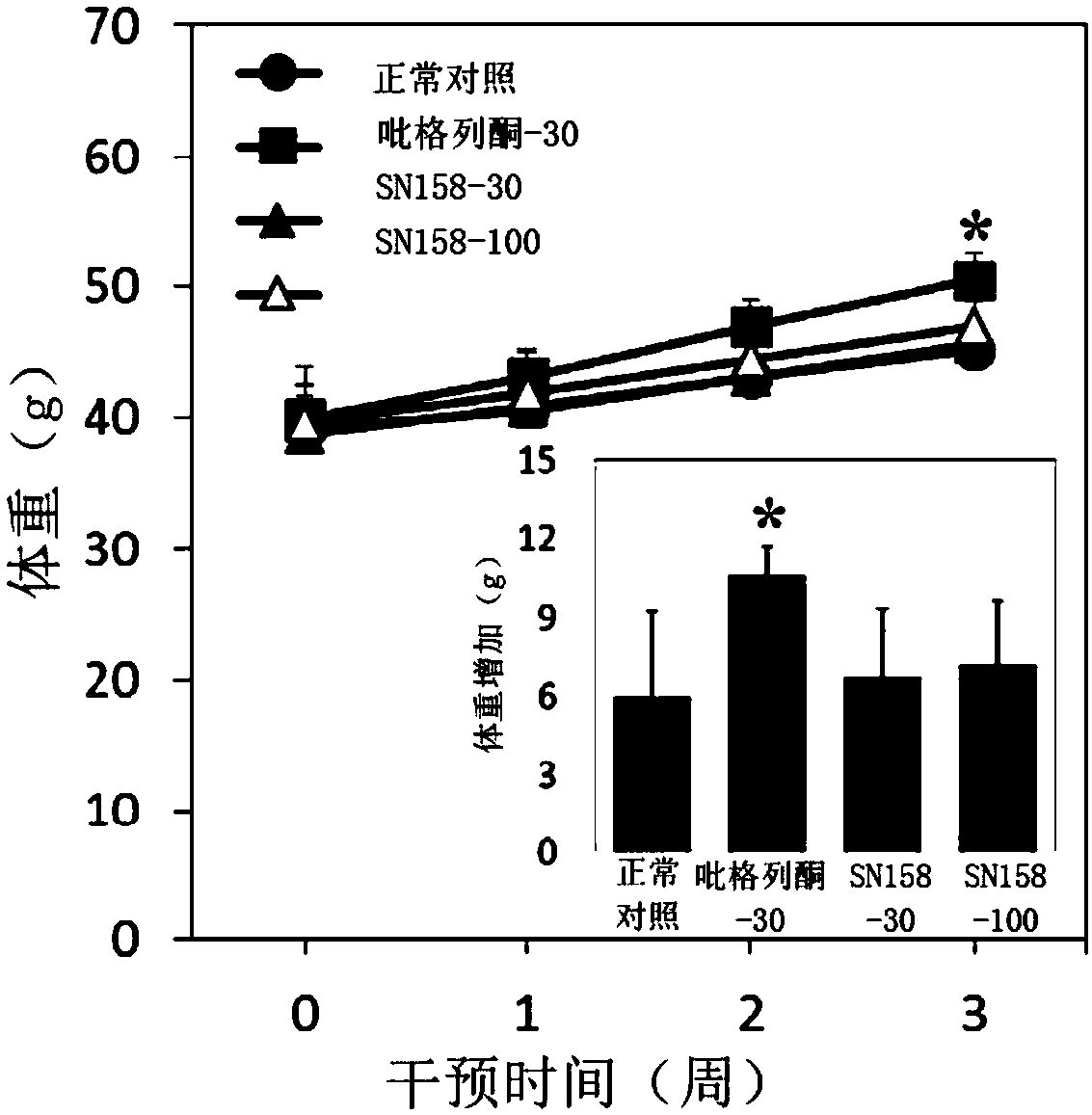
[0105] After the blood glucose and glucose tolerance test in Example 2, the mice were fasted for 12 hours, and the body weight of the mice was measured. Dissect and separate the mouse liver, weigh the weight, and calculate the percentage of liver weight relative to body weight. For the results, please refer to image 3 with Figure 4 .
[0106] from image 3 with Figure 4 It can be concluded that after administration, unlike pioglitazone, SN158 does not cause adverse reactions such as fractional weight gain and hepatomegaly.
[0107] In summary, the present invention provides a synthetic route of SN158, and the present invention also provides an application of the above synthetic route in the field of preparation of antidiabetic drugs. In the technical scheme provided by the present invention, after the raw material 2,4-dihydroxybenzaldehyde is brominated, the para-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com