Method for preparing compound Lifitegrast
A compound and catalyst technology, applied in the field of preparation of the compound Lifitegrast, can solve the problems of low purity of Lifitegrast, unsuitable for industrial production, low synthesis efficiency, etc., and achieve the effects of improving the total reaction yield, being suitable for industrial production, and improving quality and safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Preparation of intermediate IV (benzofuran-6-carbonyl chloride).
[0054] Weigh the raw material I (benzofuran-6-carboxylic acid) (1eq) and triphosgene (0.5eq) into the reaction flask, add toluene (10 times the volume) into the flask, and add a catalytic amount of DMF dropwise at room temperature. The temperature of the liquid is raised to 60-70°C for about 4-6 hours, the raw materials are basically reacted (less than 2%), the feed liquid is concentrated to a small volume to obtain a solid, and the toluene is entrained once, which is directly used in the next step reaction (dissolved in dry THF, to be with), crude product yield: 96%.
Embodiment 2
[0055] Example 2: Preparation of intermediate V (2-(benzofuran-6-formyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-carboxylic acid).
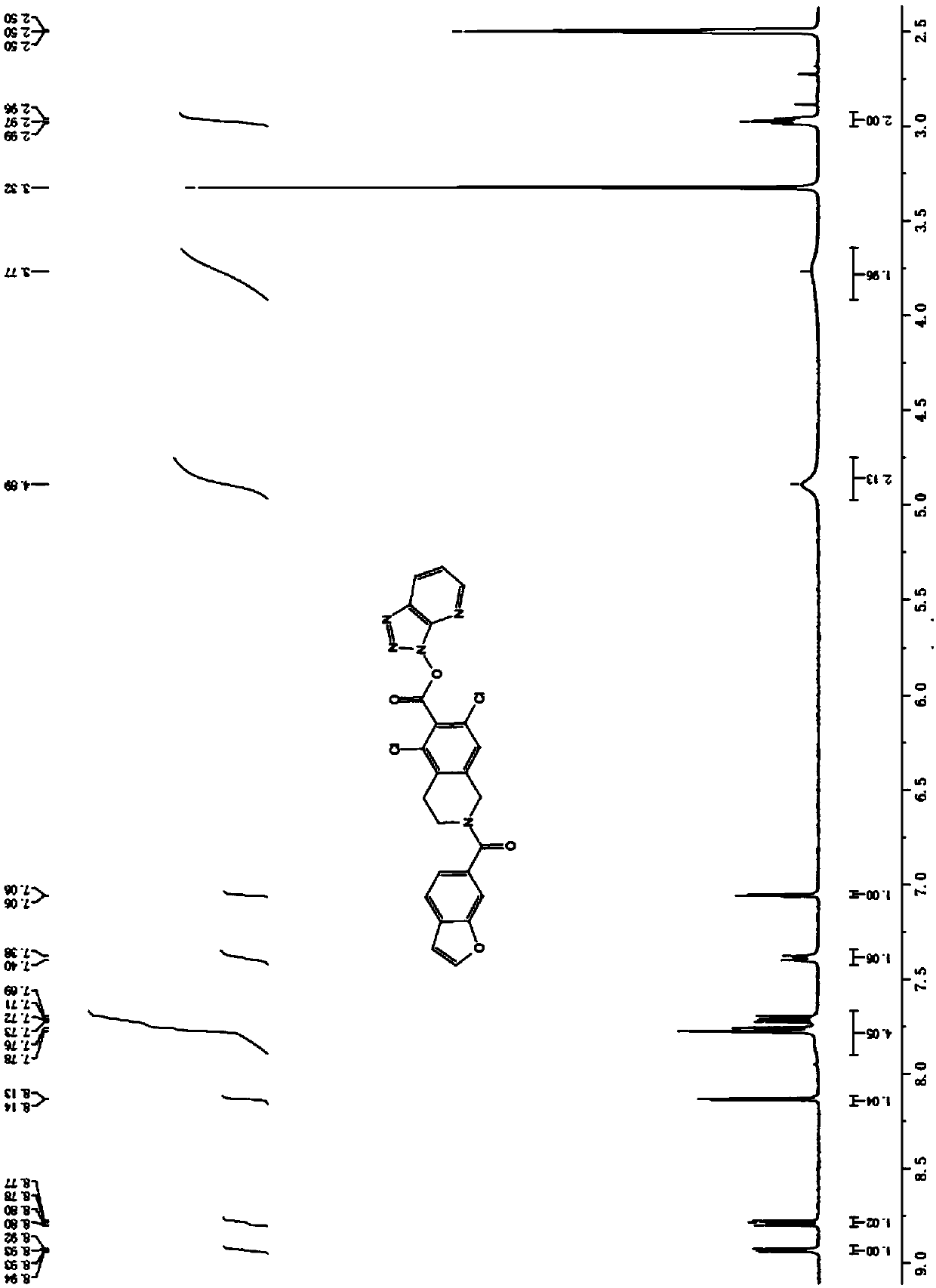
[0056] Weigh the raw material II (1.1eq, relative to the raw material I in the previous step), add DIEA (2.2eq) into the reaction bottle, add anhydrous THF (5 times the volume) into the bottle, stir for 0.5-1h, and control the temperature below 20°C. Add the intermediate IV (THF solution) in the previous step dropwise. After the addition is complete, stir at room temperature for 4 to 6 hours. The intermediate control reaction is basically complete. Quench with an appropriate amount of methanol. Heat and stir for about 0.5 hours. Concentrate the material to a small volume and add isopropanol. Adjust the pH to 3-4 with 1N dilute hydrochloric acid; the solid precipitated out, and the solid was filtered and purified with ethanol to obtain intermediate V with a yield of 83% and a purity of 99%.
[0057] 1 HNMR (DMSO-d 6 ,400MHz)δ:3.33(m,2H),3....
Embodiment 3
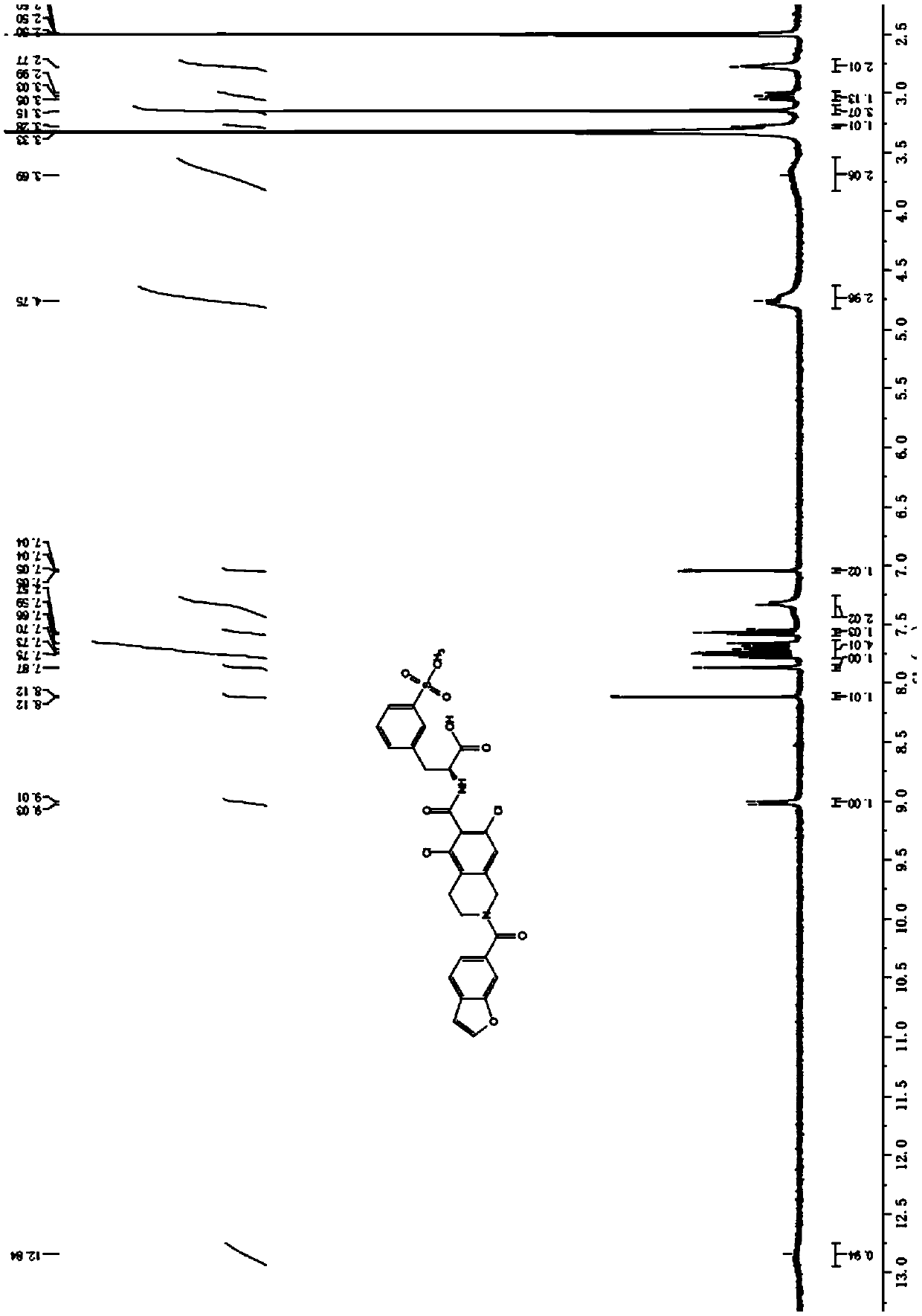
[0058] Example 3: Lifitegrast ((S)-2-[2-(benzofuran-6-formyl)-5,7-dichloro-1,2,3,4-tetrahydroisoquinoline-6-methan Amino]-3-(3-methylsulfonylphenyl)propionic acid) preparation.
[0059] Weigh the intermediate V (1eq) obtained in the previous step, add HATU (1.1eq) into the reaction flask, add THF (10 times the volume) into the flask, stir, control the temperature below 20°C, and add DIEA (3.1eq) dropwise , the feed liquid was kept warm for 1 hour, the system was dissolved first, then heated to 30°C, and the reaction was continued for 15 hours, the raw material V basically reacted completely, and H was added to the feed liquid. 2 O (10 times the volume), stir, add raw material III (1.1eq) in batches, after the addition is complete, react the feed solution below 10°C for about 15 minutes, then slowly raise the temperature to about 30°C, stir and react for 15h, the reaction solution dissolves, and the middle The basic reaction of state VI is complete. Under the ice-water bath, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com