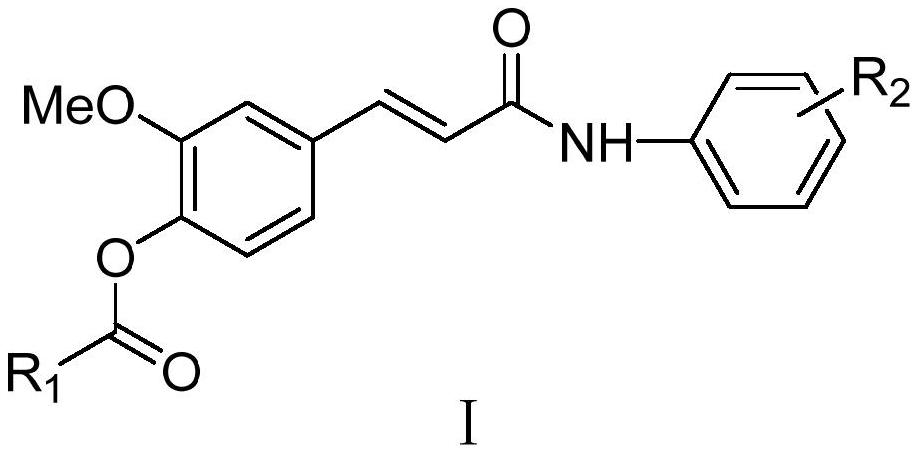
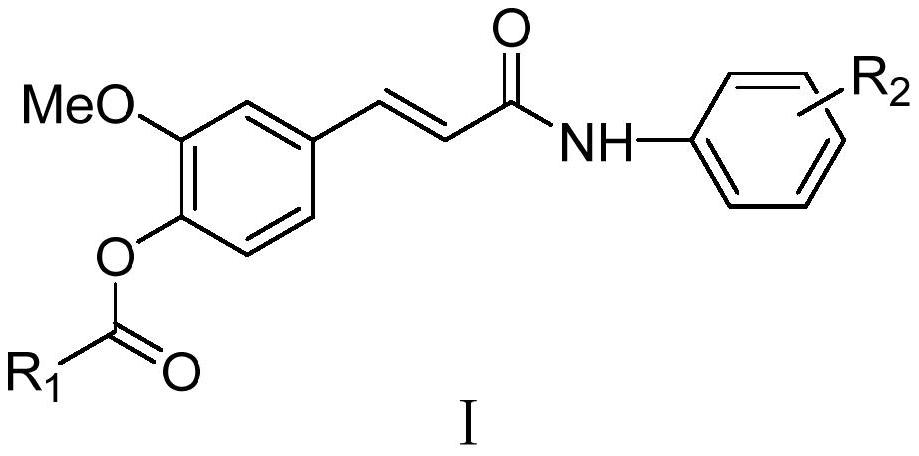
Ferulic acid derivative as well as preparation method and application thereof
A derivative, the technology of ferulic acid, applied in the field of pharmaceutical chemical synthesis, can solve the problems of low bioavailability, poor blood-brain barrier permeability, and clinical application limitations, and achieve high total yield, easy repeatability, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
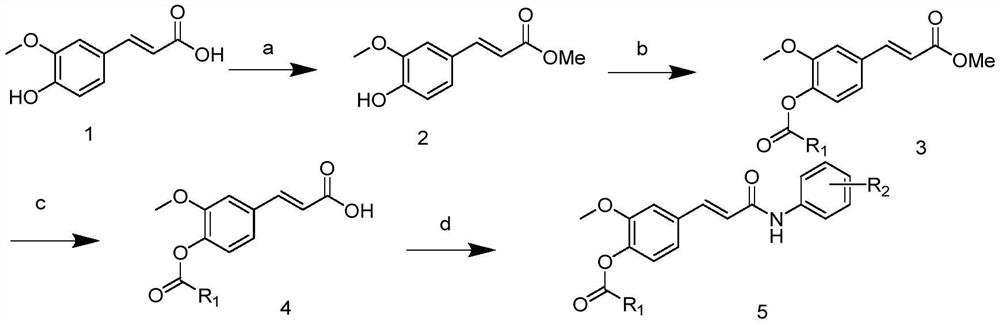
[0017] The preparation method of a kind of ferulic acid derivative of the present invention is specifically implemented according to the following steps:
[0018] Step 1, using ferulic acid as a raw material, under the condition of concentrated sulfuric acid and methanol reflux reaction to prepare methyl ferulate;
[0019] The mass ratio of ferulic acid to methanol was 1:8; the reflux reaction temperature was 70°C, and the reflux reaction time was 3h;
[0020] Step 2, mix methyl ferulate with different substituted carbamoyl chlorides and add acetone K 2 CO 3 , carry out the reflux reaction, the reaction temperature is 60 ℃, and the reaction time is 7h; the ferulic acid carbamate substituted derivative is generated;
[0021] Different substituted carbamoyl chlorides are N-methyl-N-ethylformyl chloride, N,N-dimethylformyl chloride or N,N-diethylformyl chloride;
[0022] The mass ratio of methyl ferulate to carbamoyl chloride is 1:1.1;
[0023] In step 3, the ferulic acid car...
Embodiment 1
[0035] Synthesis of Example 1 Intermediate
[0036] a, (E)-3-(4-hydroxy-3-ethoxyphenyl) methyl acrylate (2)
[0037]
[0038] Add 3 g of ferulic acid (FA) (15.5 mmol) to a 100 mL eggplant-shaped flask, dissolve it in 30 mL of methanol, slowly add 1.2 mL (22.5 mmol) of concentrated sulfuric acid, and heat the system to 70 °C for 3 h. After the reaction was completed, the pH was adjusted to 7 with 10% NaOH after concentration under reduced pressure, extracted with ethyl acetate, washed with water, and the organic phase was washed with Na 2 SO 4 Then, concentrated under reduced pressure to obtain 3.1 g of yellow oil with a yield of 97%; it was identified as (E)-methyl 3-(4-hydroxy-3-ethoxyphenyl)acrylate.
[0039] Among them, the product is characterized as follows:
[0040] (E)-Methyl 3-(4-hydroxy-3-ethoxyphenyl)acrylate: 1 H-NMR (400MHz, CDCl 3 )δ7.64(1H,d,J=16.0Hz,H-7),7.08(1H,d,J=8.2Hz,H-5),7.04(1H,s,H-3),6.93(1H, d,J=8.0Hz,H-6),6.31(1H,d,J=16.0Hz,,H-8),6.07(1H,brs,O...
Embodiment 2
[0062] Example 2 Preparation of (E)-2-methoxy-4-(3-oxo-3-(phenylamino)-1-propenyl)phenyldimethylcarbamate (5a)
[0063]
[0064] First add compound (4a) 0.2mol, HATU (1.2eq), DIEPA (2eq), stir for 15min, add aniline (0.2mol), react at room temperature for 2h, spot plate detection after the reaction is completed, use petroleum ether: acetone (105: 30) Purify by column chromatography; white solid; yield 46.2%; identified as (E)-2-methoxy-4-(3-oxo-3-(phenylamino)-1-propenyl) Phenyldimethylcarbamate.
[0065] mp 178-180℃; 1 H-NMR (400MHz, CDCl 3)δ8.34(1H,s,H-9),7.65(2H,d,J=8.0Hz,H-11,15),7.47(1H,d,J=16Hz,H-7),7.32(2H ,t,H-12,14),7.09(1H,t,H-13),7.04(1H,d,J=8.0Hz,H-4),6.96(1H,d,J=8.0Hz,H- 3),6.89(1H,s,H-6),6.00(1H,d,J=16Hz,H-8),3.66(3H,s,OCH 3 ),3.19(3H,s,-CH 3 ),3.10(3H,s,-CH 3 ); ESI-MS m / z: 341.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com