Preparation method of medicine RIP1183 for resisting multi-drug resistance staphylococcia
A technology for RIP1183 and Staphylococcus infection, applied in the biological field, can solve the problems of unfavorable industrial production, low product purity, and poor quality controllability, and achieve the effects of shortening the production cycle, high product purity, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
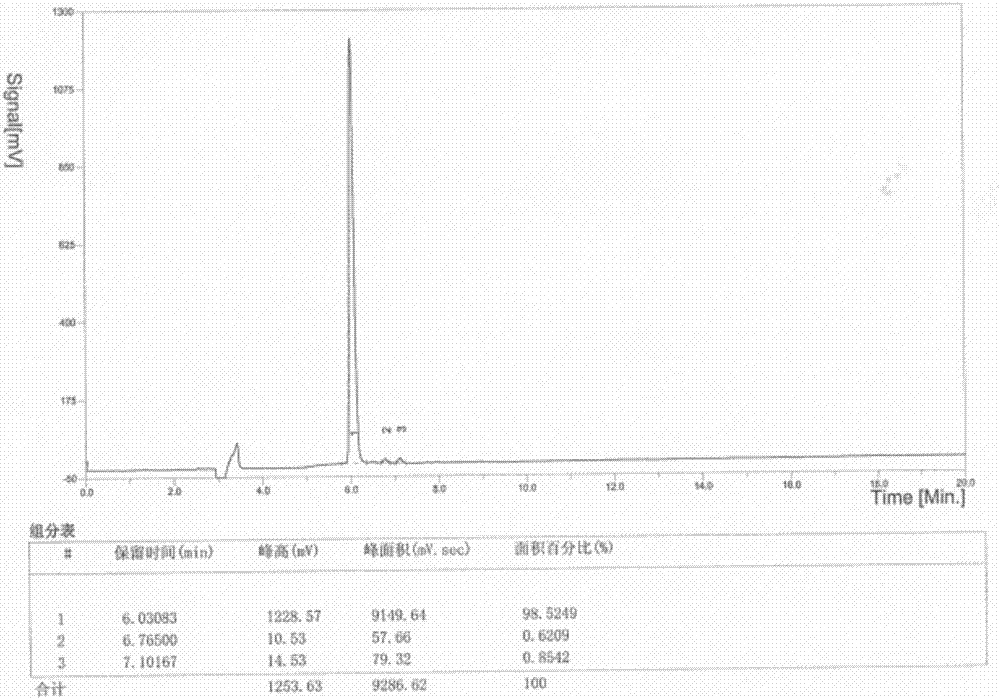
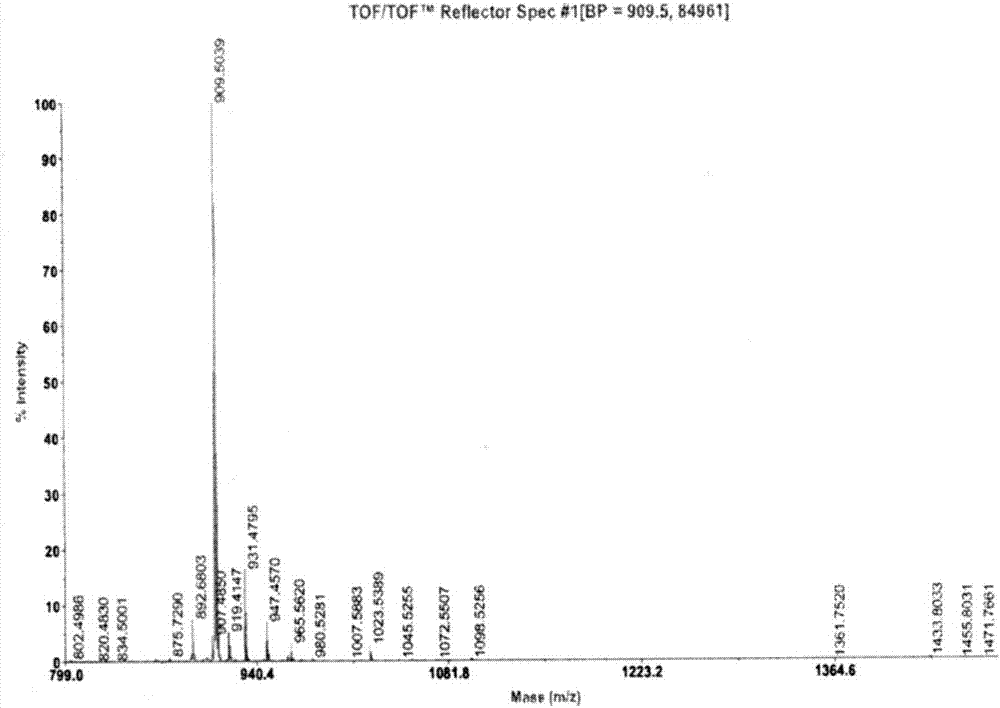
[0053] Two, the preparation method of RIP1183
[0054] The method for synthesizing RIP1183 peptide of the solid-phase polypeptide of the present invention comprises the following steps:
[0055] Using any one of Rink Amide MBHA Resin, MBHA Resin, and Rink Amide-AM Resin as the starting material, according to the solid-phase synthesis method, according to the amino acid sequence of RIP1183, connect the corresponding amino acids with Fmoc protection sequentially, during which Remove the Fmoc protecting group in turn, and use one of DIEA / HOBt or DIEA or TBTU / HOAt / HOBt or HBTU / HOBt or HBTU / HOAt or HATU / HOBt or HATU / HOAt or HCTU / HOBt as a condensing agent for peptide grafting After the reaction, the corresponding RIP1183 resin was obtained, and the side chain protection group was removed and the final peptide was cut synchronously to obtain the crude product of RIP1183, which was subjected to C 18 or C 8 The chromatographic column was used for separation and purification to obtai...
Embodiment 1
[0094] Example 1 RIP1183 synthesis process of 1g grade Rink Amide MBHA Resin resin:
[0095] (1) Preparation of Fmoe-Phe-MBHA Resin
[0096] Weigh 1g of Rink Amide MBHA Resin (Gill Biochemical (Shanghai) Co., Ltd.; sample loading: 1.0mmol / g) and pour it into the reaction column, add about 10ml of DMF, soak for 30 minutes (at room temperature) and dry it with a vacuum pump, add the decapping reagent ( 20% piperidine solution in DMF) at 10-50°C for 5-30 minutes, drained with a vacuum pump, re-adding capping reagent at 10-50°C for 10-60 minutes, drained, washed twice with isopropanol, then washed with DMF 2 times, ninhydrin test was positive, drained, added Fmoc-Phe-OH 0.54g, TBTU0.45g, DMF 10ml, HOBt 1ml, DIEA 0.4ml, reacted at room temperature for 2 hours, ninhydrin test was negative, resin Wash twice with isopropanol, twice with DMF, and dry to obtain Fmoc-Phe-MBHA Resin;
[0097] (2) Preparation of Fmoc-Asn(Trt)-Phe-MBHA Resin
[0098] In the Fmoe-Phe-MBHA Resin of step (1...
Embodiment 2
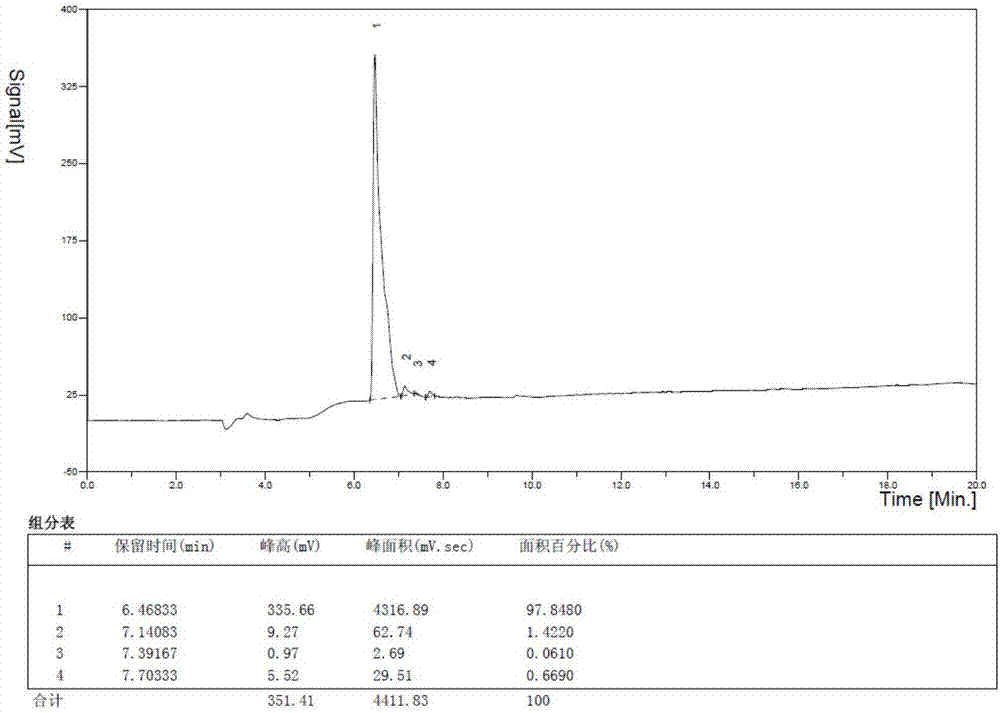
[0114] Example 2 The synthesis process of RIP1183 in pilot scale (taking 20150601 batches as an example)
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com