A kind of preparation method of crude drug olbitasvir
A technology for obitasvir and bulk drug, which is applied in the field of preparation of bulk drug obitasvir, can solve the problems of high cost, complicated process and the like, and achieves the effects of high yield and easy separation and purification of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] With A4 as raw material, according to the valine peptide coupling reaction conditions mentioned in CN102333772 A, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride as valine peptide coupling reagent , dichloromethane is used as solvent, and the concrete technological process is:
[0044] Add 50L of dichloromethane, 25L of nitrogen methylmorpholine, 10kg of MOC-L-valine, 11kg of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride into a 11kg In the reactor of A4, stir for 3 hours, keep warm at 15-25° C., monitor by TLC, and TLC shows that the reaction is complete.
[0045] Post-processing: start stirring, add ethanol into the reaction kettle, put in the crude product and activated carbon, heat up to 50-60°C, stir for 30 minutes, dissolve, filter, and the filtrate enters the crystallization kettle, cools down to 20-25°C, and crystallizes for 4 hours. Centrifuge, dry under reduced pressure at 40-45° C. for 4-6 hours, and weigh to obtain 11 kg of white solid...
Embodiment 2
[0048] The difference between embodiment 2 and embodiment 1 is that nitrogen methylmorpholine is added in the reaction system, and the volume ratio of dichloromethane to nitrogen methylmorpholine is 1:1.
Embodiment 3
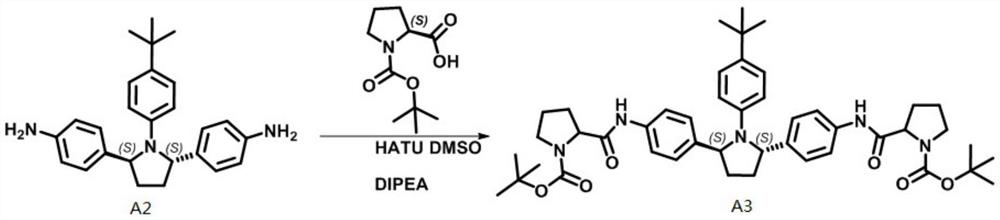
[0050] Example 3 Using A3 as a raw material, obitasvir was prepared through a two-step reaction of amino deprotection and valine peptide coupling.
[0051] The process is as follows: in a dry 500L reactor, add 45kg of ethanol, 15kg of raw material intermediates, 20L of hydrochloric acid ethanol solution, control the temperature at 10-15°C, and stir for 1 hour to obtain 10.5kg of A4 intermediates with a yield of 93 %.
[0052] In the above process, the solvent for the amino group deprotection reaction is ethanol, which can be replaced by other reaction solvents or combinations of solvents in the summary of the invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com