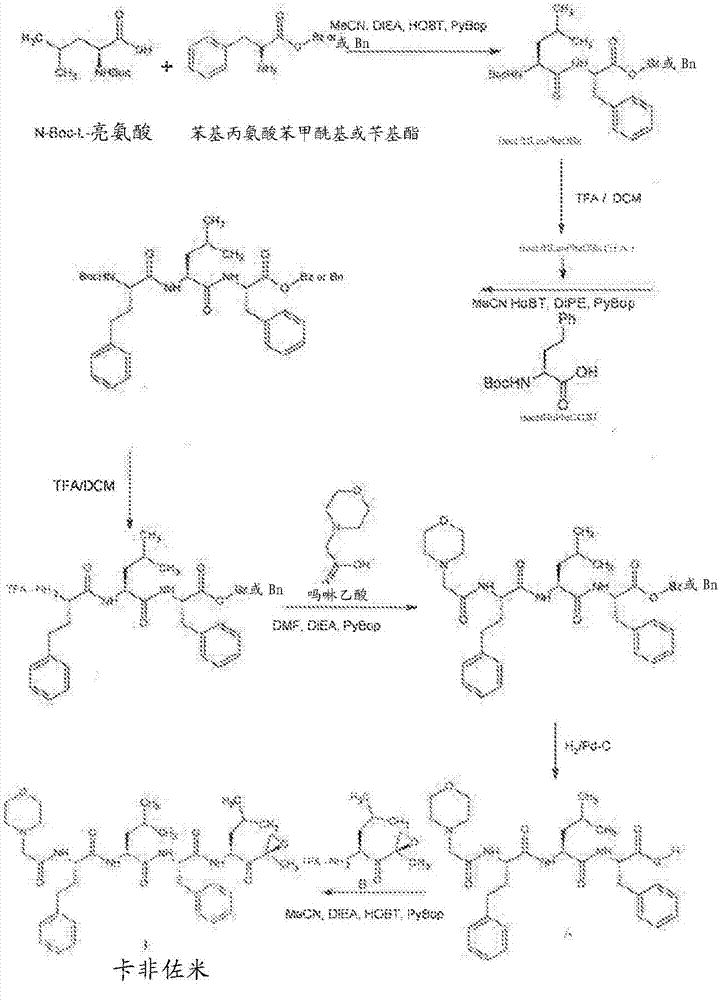
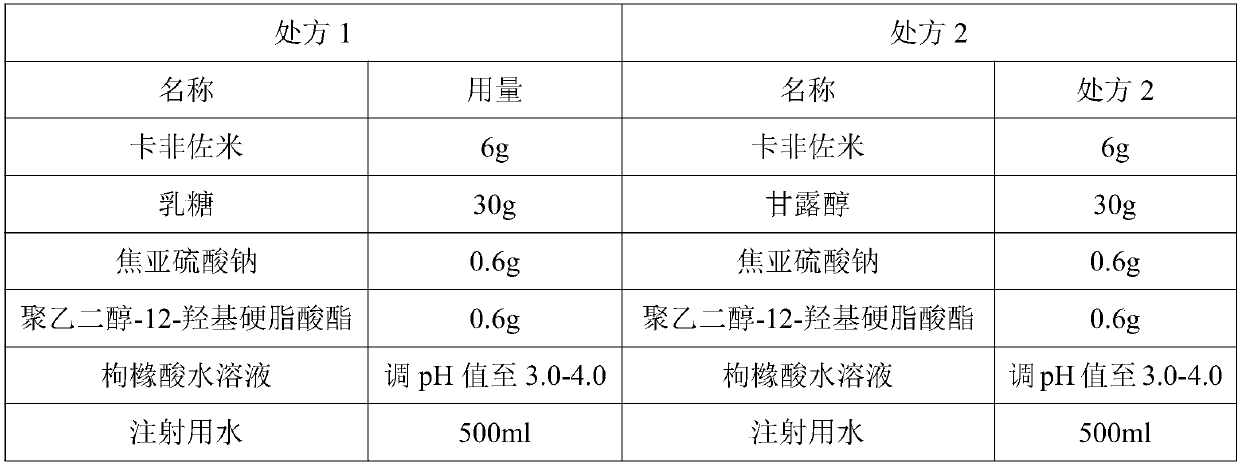
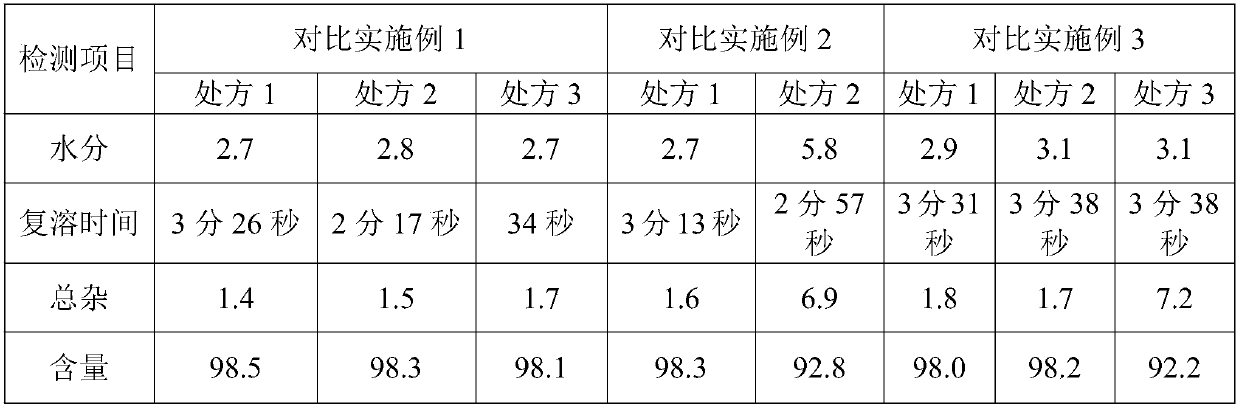
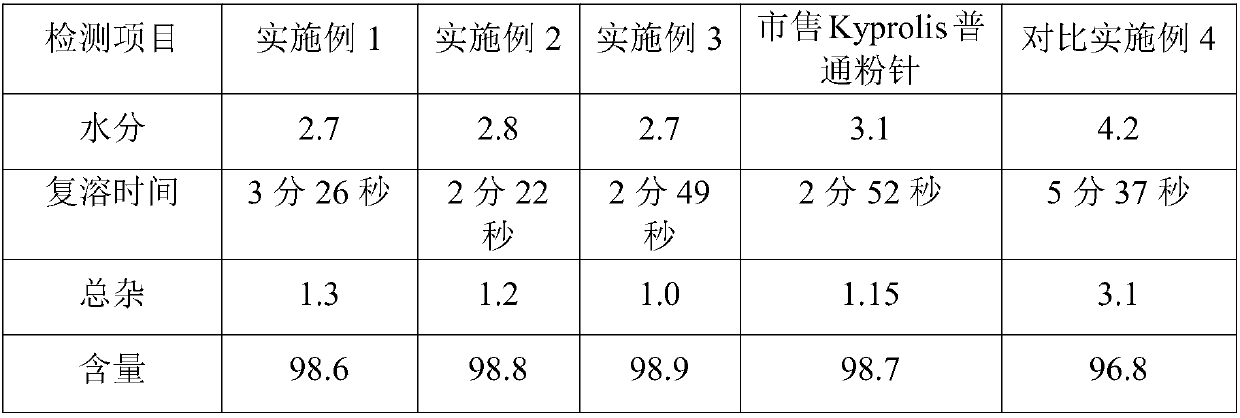
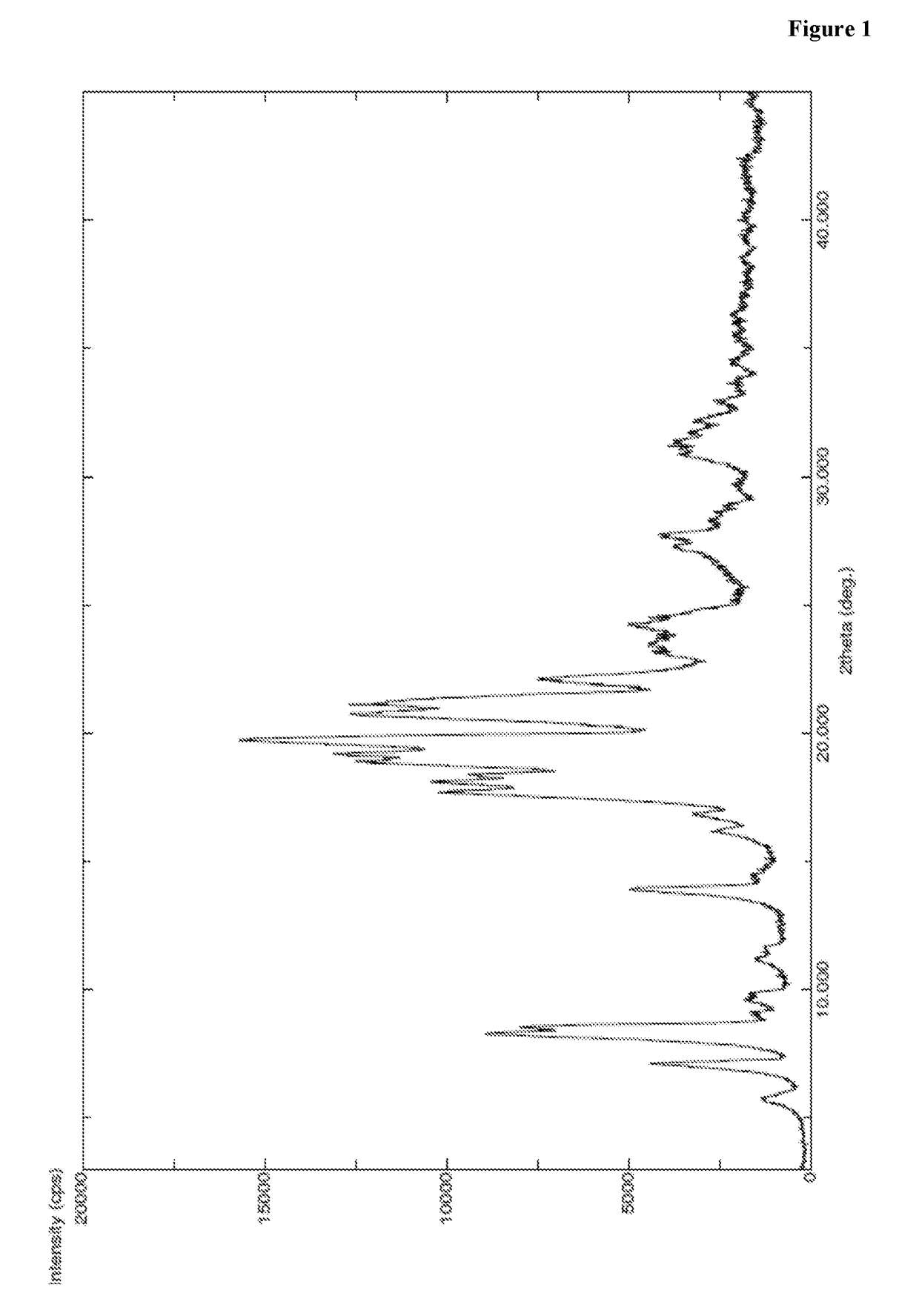
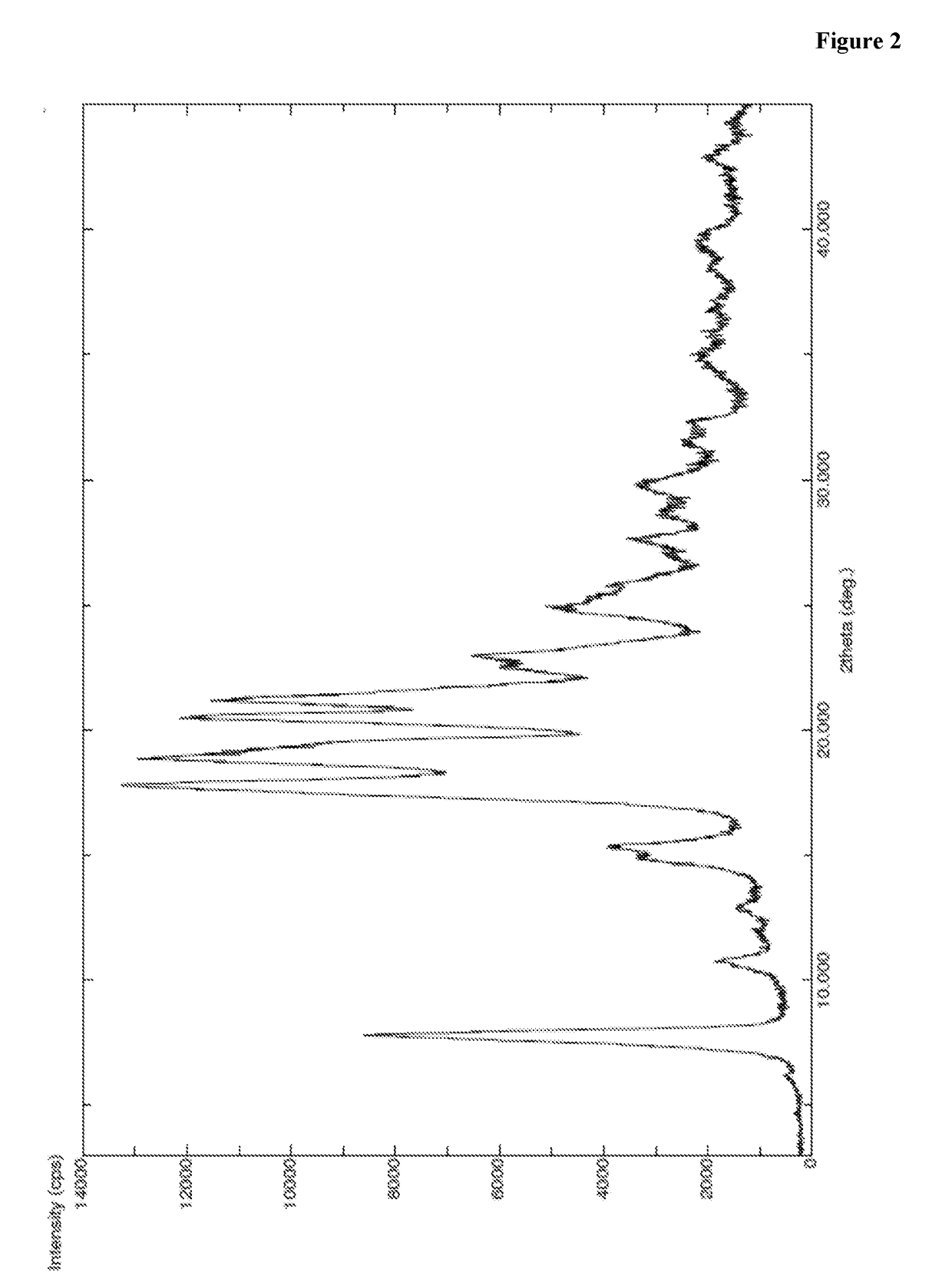
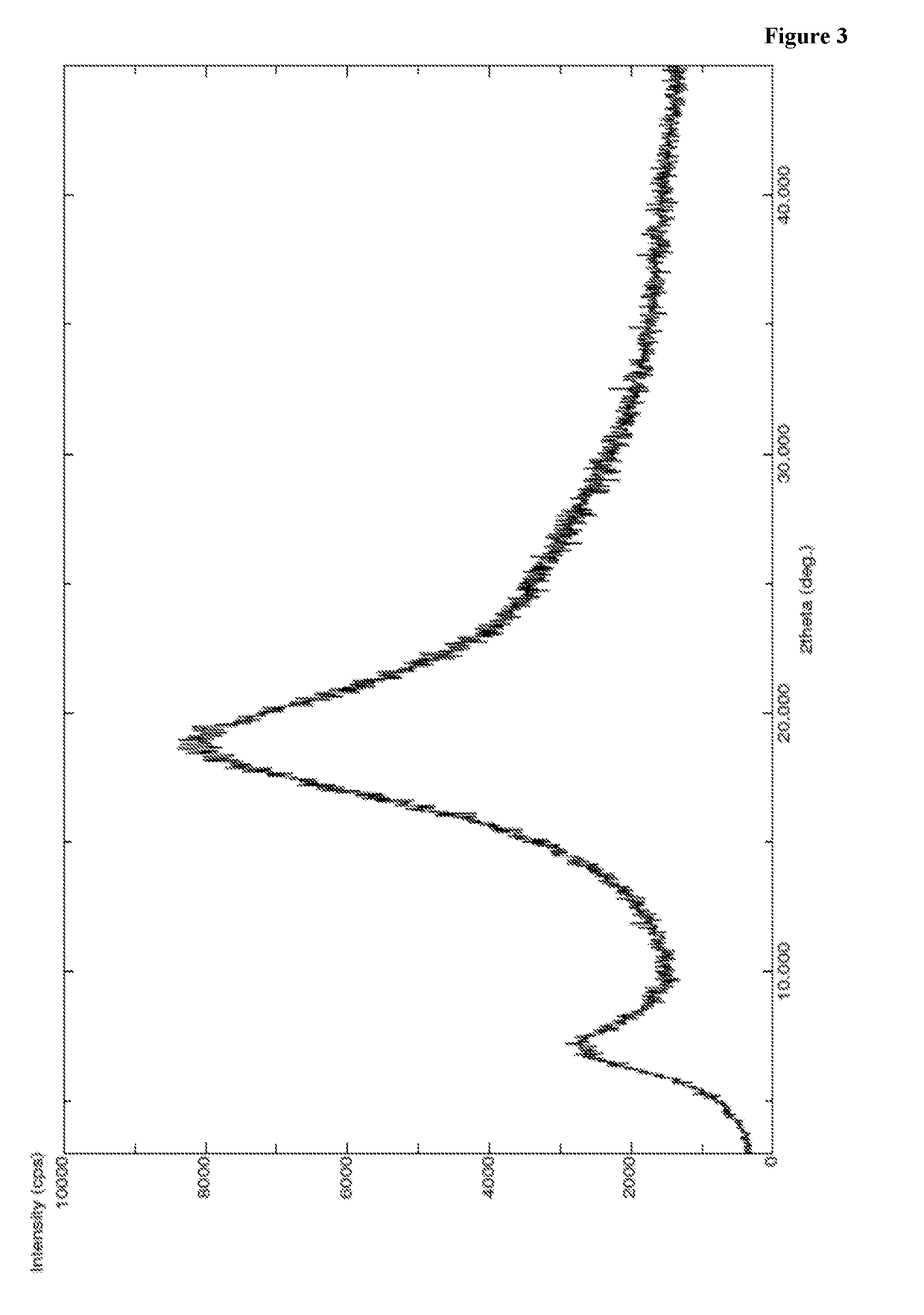
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
92 results about "Carfilzomib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
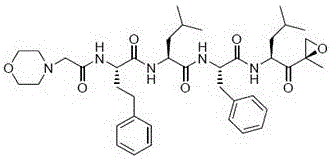
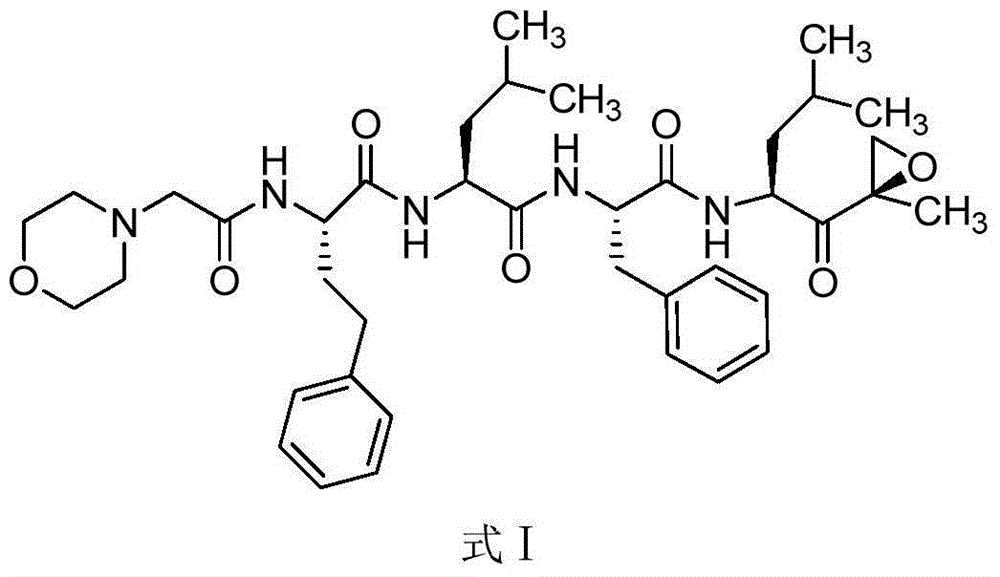
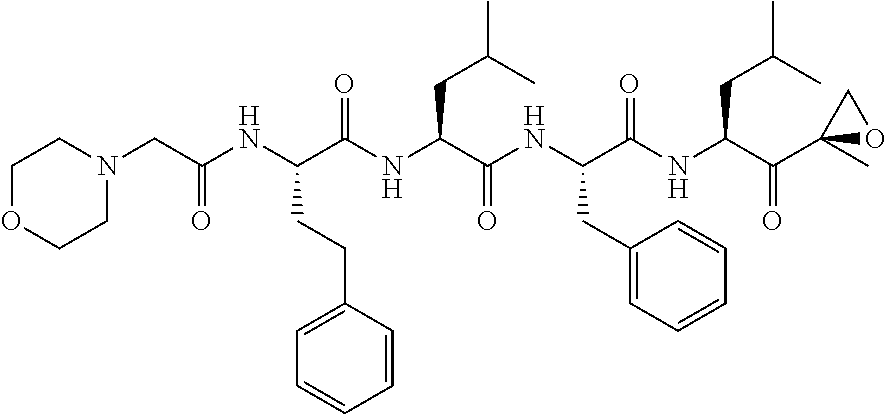
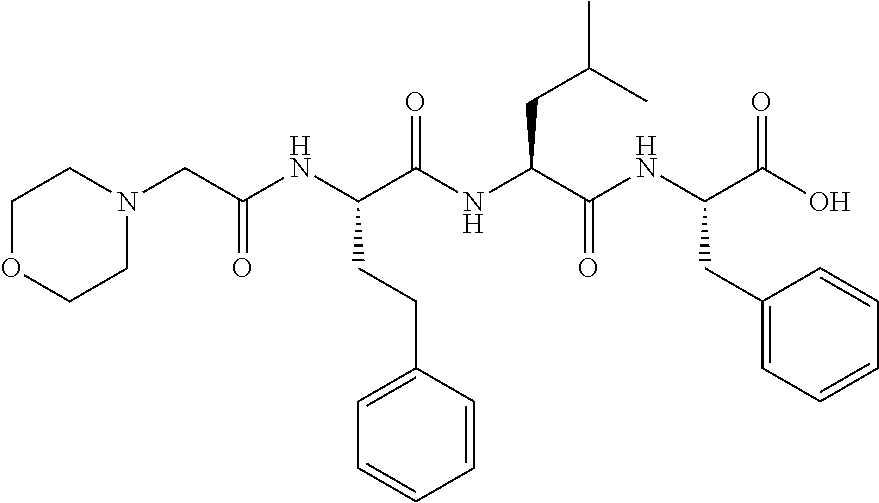
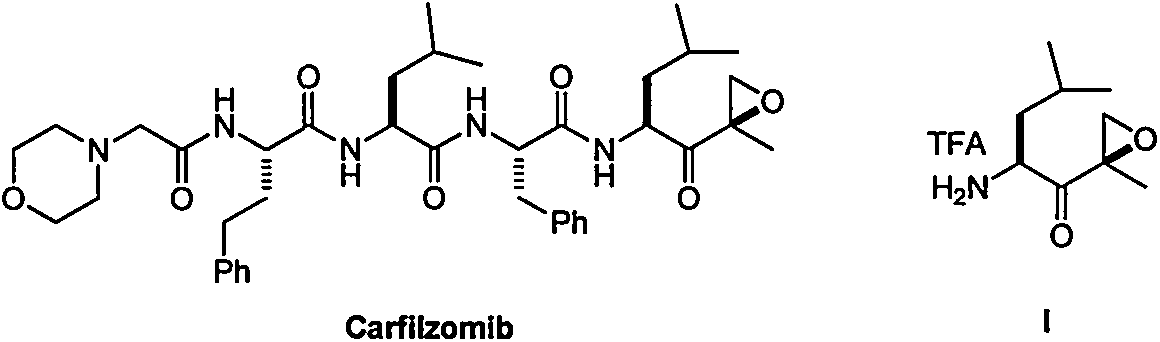
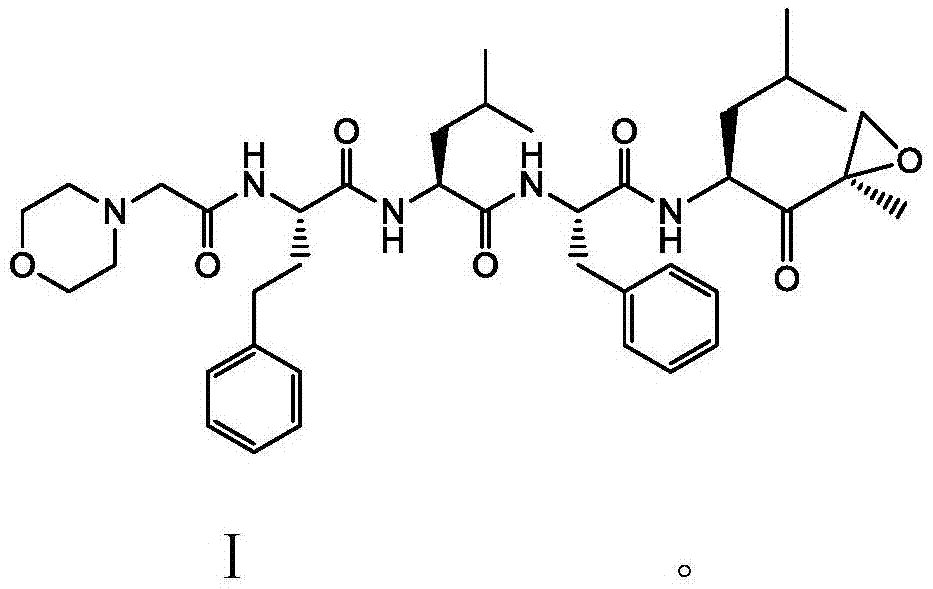
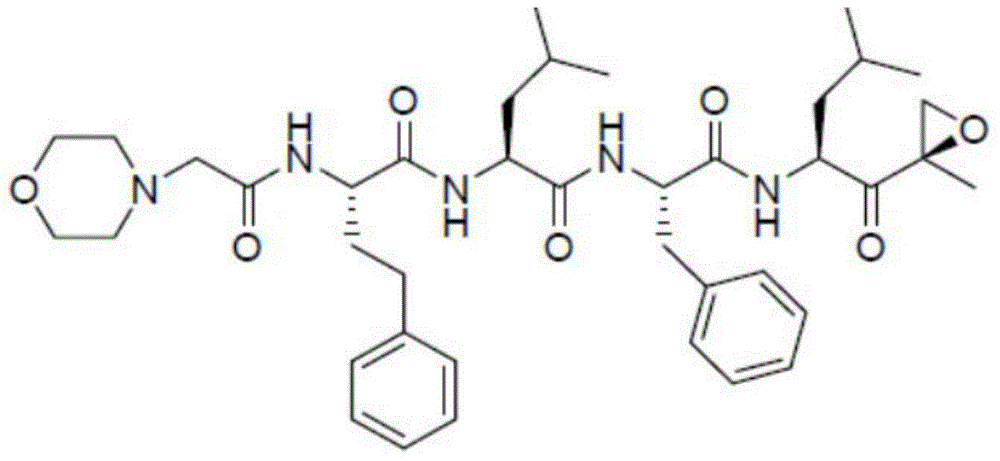
Carfilzomib (marketed under the trade name Kyprolis, developed by Onyx Pharmaceuticals) is an anti-cancer drug acting as a selective proteasome inhibitor. Chemically, it is a tetrapeptide epoxyketone and an analog of epoxomicin.
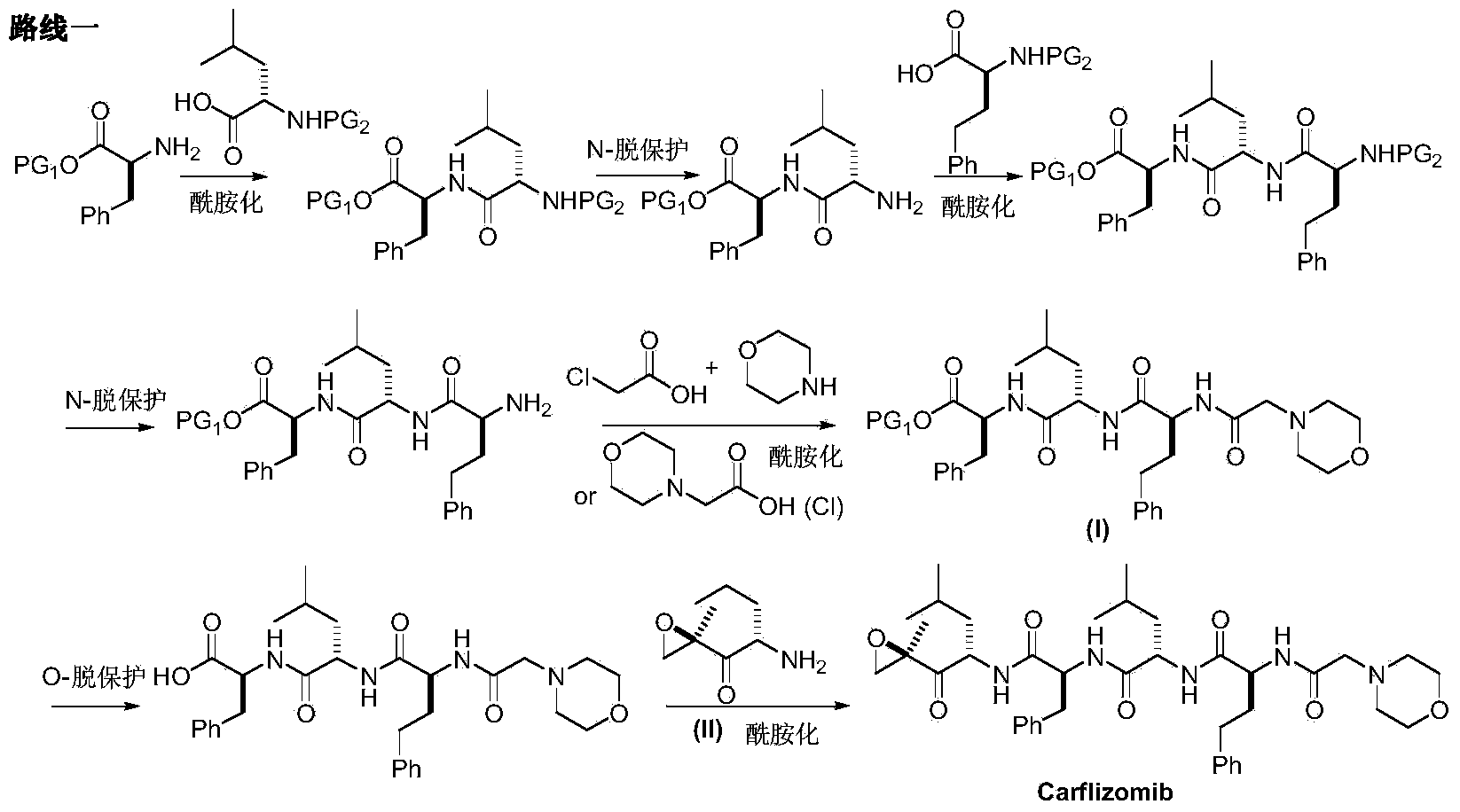
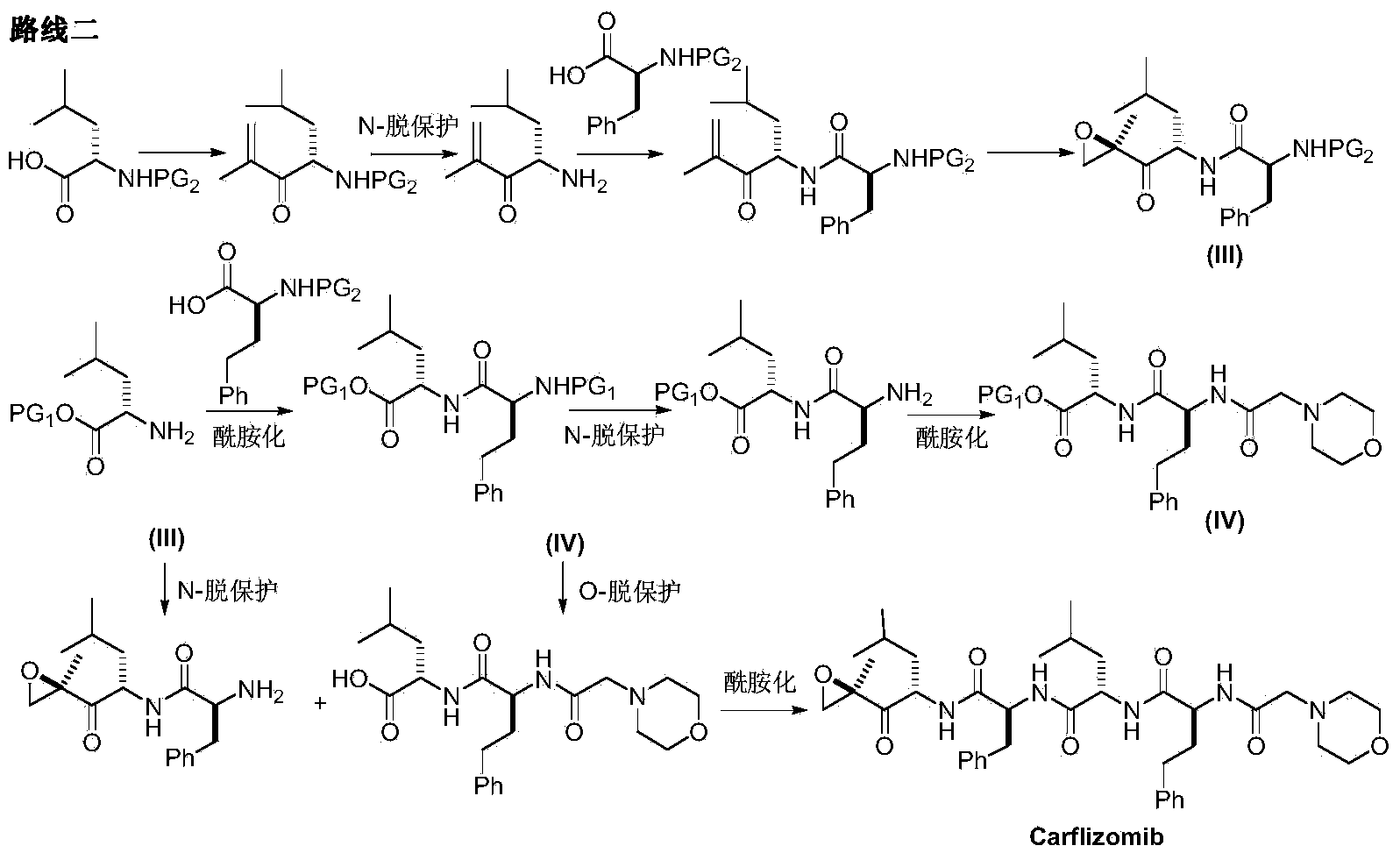
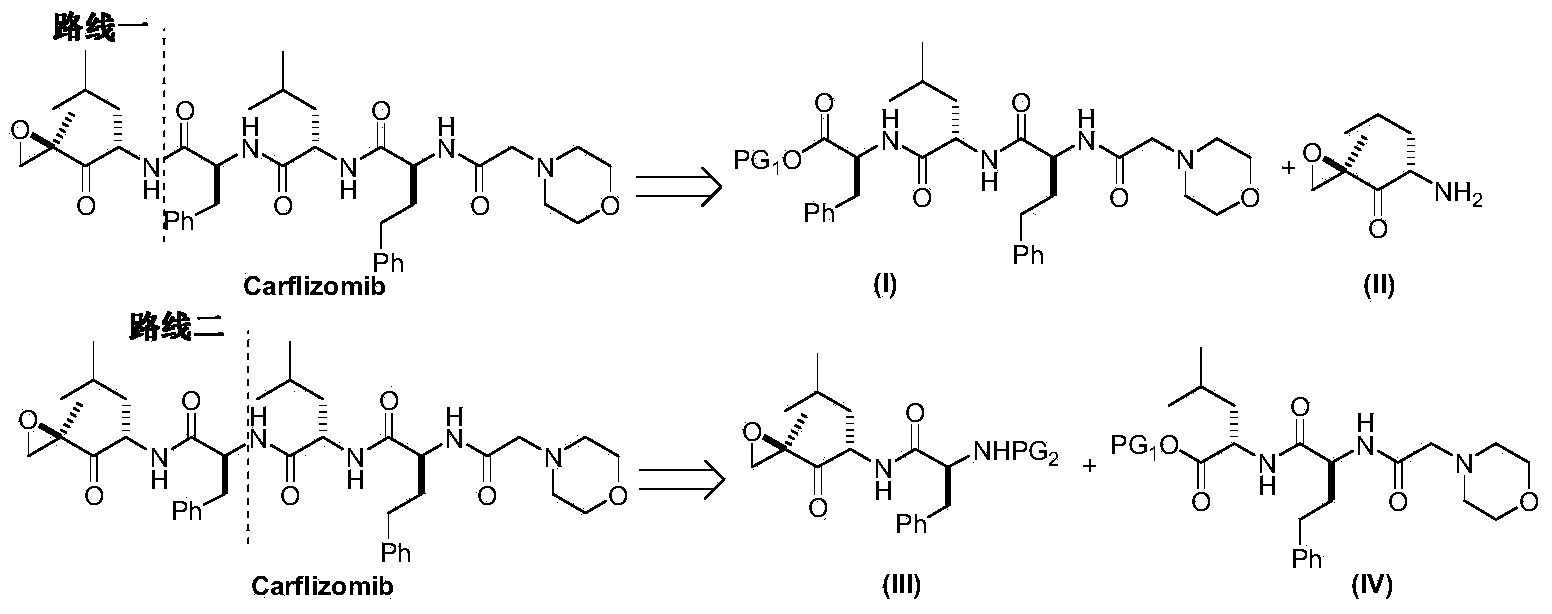
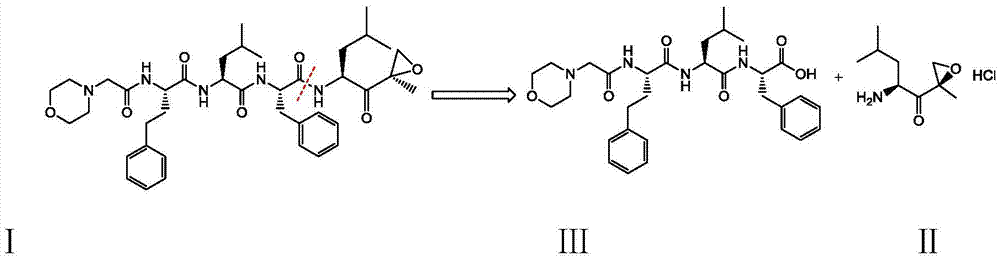
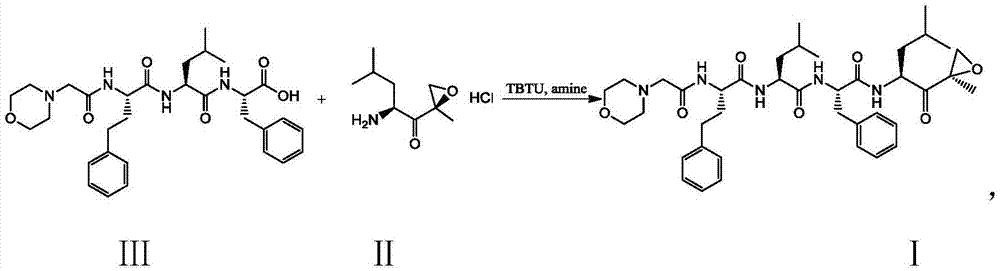
Preparation method for carfilzomib
ActiveCN104086624AShort reaction timeFeeding is simplePeptidesTemperature controlProcess engineering
The invention discloses a preparation method for carfilzomib. The method utilizes HATU as a condensing agent to perform condensation and comprises a plurality of steps. The method has the characteristics of short reaction time, simple feeding, no need for nitrogen protection, appropriate control the feeding temperature, no need for strict temperature control, and easier washing and removal of the by-product of HATU, greatly shortens the preparation time, and improves the work efficiency, thus being suitable for industrial production.
Owner:河南海汇药物研究有限公司
Preparation method of intermediate compounds of carfilzomib and intermediate compounds
InactiveCN104230857AReduce dosageLow costCarbamic acid derivatives preparationOrganic compound preparationHydrogenCarfilzomib
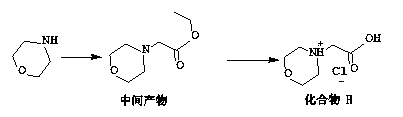
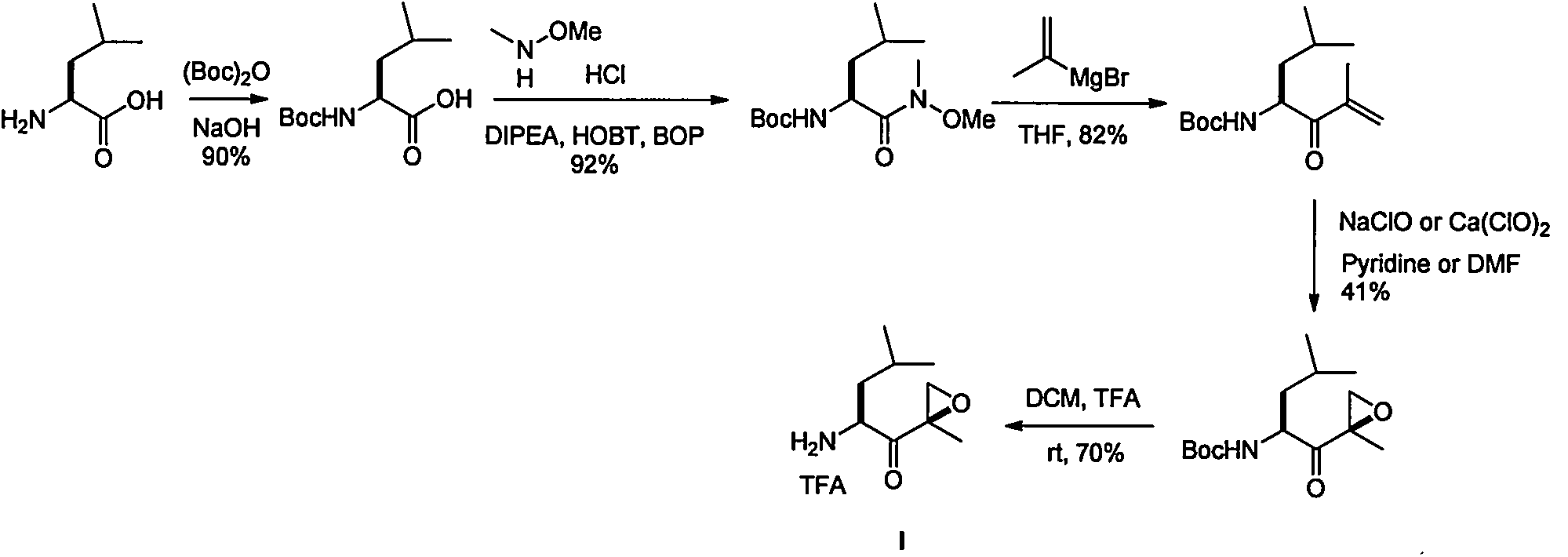
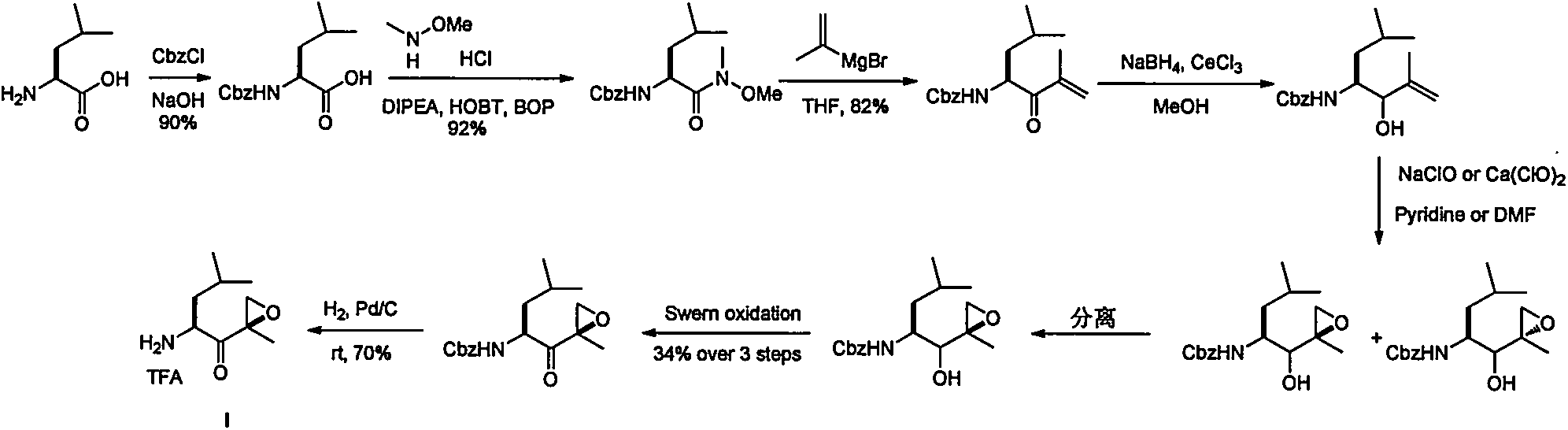
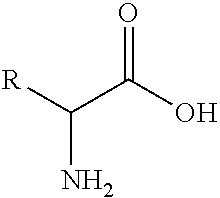
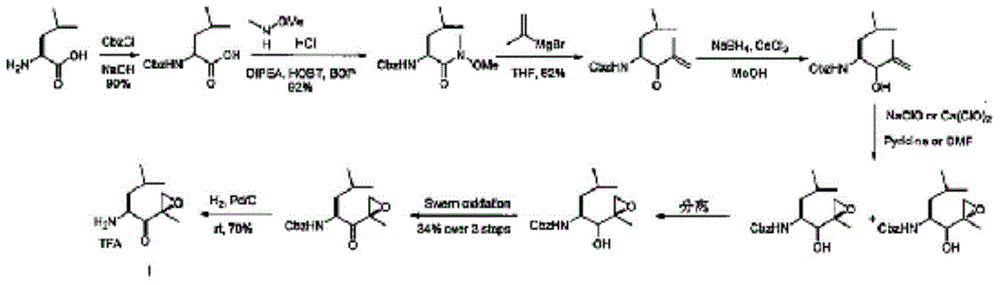
The invention discloses a preparation method of an intermediate compound I of carfilzomib. The method is characterized by comprising the following steps: by using L-leucine as an initial raw material, protecting two active hydrogens on the amino group, carrying out Weinreb amidation, reacting with magnesium 2-propenylbromide, and carrying out epoxidation and amino protection removal to obtain the compound disclosed as Formula I. The invention also discloses intermediate compounds II, III, VI and V of carfilzomib. The preparation method is simple and efficient, has the advantages of low cost and high selectivity, and is beneficial to industrial production.
Owner:SHANGHAI HAOYUAN MEDCHEMEXPRESS CO LTD +1
Stable compositions of peptide epoxy ketones
InactiveUS20140073583A1Overcome limitationsTetrapeptide ingredientsPharmaceutical non-active ingredientsEpoxyKetone
The invention relates to pharmaceutical compositions that provide improved solubility and stability for peptide epoxy ketones. More specifically, the invention relates to pharmaceutical compositions comprising the peptide epoxy ketone proteasome inhibitor carfilzomib.
Owner:INNOPHARMA
Preparation method of carfilzomib intermediate
The invention discloses a preparation method of a carfilzomib intermediate (alpha S)-[[2-(4-morpholinyl)acetamido]phenylbutyryl]-L-leucyl-L-phenylalanine (I), which comprises the following steps: with 2-(4-morpholinyl)acetic acid (V), 4-phenyl homophenylalanine ester (VI), L-leucine ester (VIII) and L-phenylalanine ester (X) as raw materials, performing an amidation reaction and a hydrolysis reaction to generate (alpha S)-[[2-(4-morpholinyl)acetamido]phenylbutyryl]-L-leucyl-L-phenylalanine (I). Compared with the prior art, the preparation method has the advantages of simple process, easily available raw materials and few side reactions, adapts to industrial production and promotes the economic and technological development of the raw medicine.
Owner:铜陵尚东高新科创有限公司
Carfilzomib intermediate and preparation method thereof, as well as preparation method of carfilzomib
ActiveCN104356197AHigh yieldHigh purityPeptide preparation methodsBulk chemical productionCarfilzomibAmino acid
The invention provides a carfilzomib intermediate with a structure shown as the formula (XIII). The preparation process of the intermediate is simple, only amino acid condensation and deprotection reactions are related, and the reaction condition is mild and controllable. The intermediate is taken as a raw material to prepare carfilzomib, only an epoxidation reaction is needed, the epoxidation reaction condition has higher selectivity, yield and purity of carfilzomib are improved, the yield of the prepared carfilzomib is higher than 35%, the purity is higher than 99%, and an economical and environment-friendly synthetic process is provided for preparation of carfilzomib.
Owner:重庆兴泰濠制药有限公司
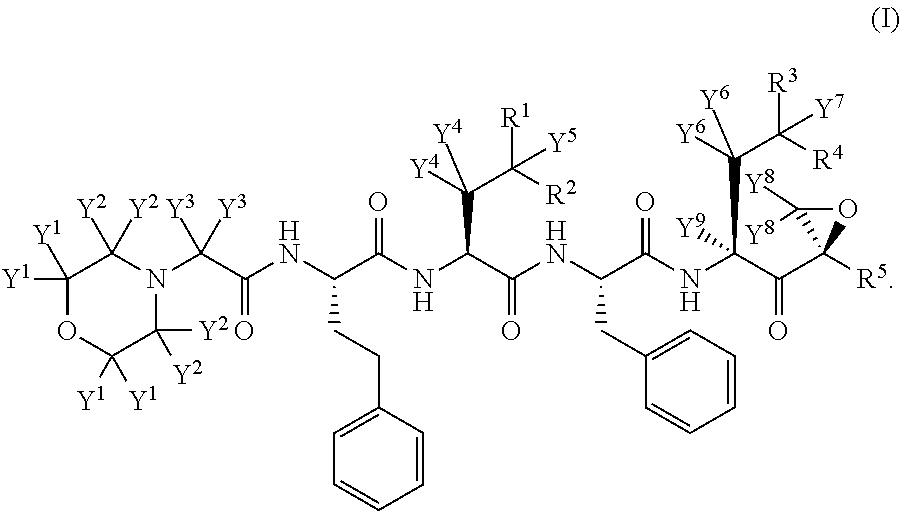
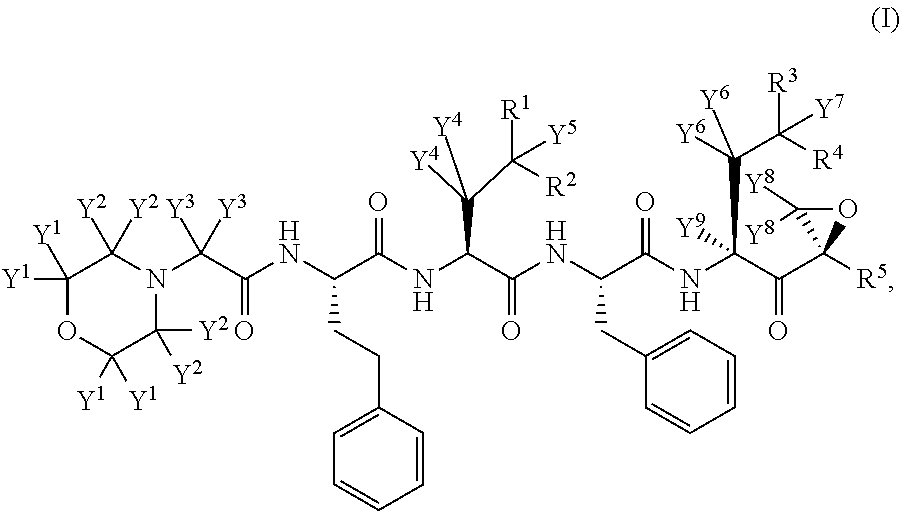
Deuterated carfilzomib
InactiveUS20150166601A1Minimizing toxic side effectIncrease usageTetrapeptide ingredientsAntiviralsDiseaseCarfilzomib
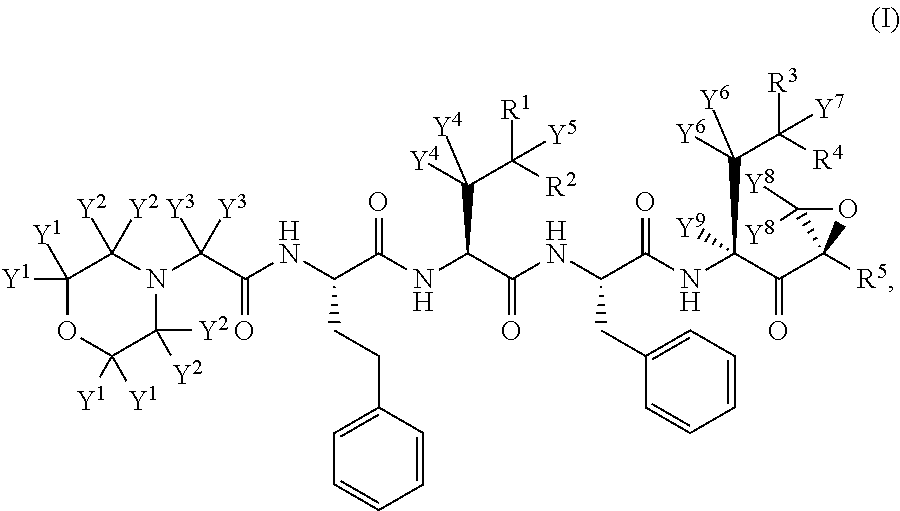
The present invention in one embodiment provides a compound of Formula (I): or a pharmaceutically acceptable salt thereof, wherein the variables shown in Formula (I) are as defined in the specification. The compound of Formula (I) is useful for the treatment of diseases such as a disease selected from the group consisting of multiple myeloma, solid tumors, lymphoma, and leukemia.
Owner:CONCERT PHARMA INC
Preparation method of carfilzomib intermediate and carfilzomib
InactiveCN103936828AFew reaction stepsMild reaction conditionsPeptide preparation methodsDipeptidesAcetic acidCoupling
The invention relates to a preparation method of a carfilzomib intermediate and carfilzomib. The method comprises the following steps: (1) enabling a solid-phase resin carrier to couple with Fmoc-Phe-OH in the presence of an activator to obtain Fmoc-Phe-resin; (2) enabling Fmoc-Phe-resin to sequentially couple with Fmoc-Phe-OH, Fmoc-HoPhe-OH and 4-morpholine acetic acid through a solid-phase synthesis method to obtain tetrapeptide intermediate resin, wherein the coupling is performed in the presence of a coupling agent system, the coupling agent system comprises a condensing agent and a reaction solvent, and the resin firstly removes Fmoc protecting group before coupling; (3) performing pyrolysis on the tetrapeptide intermediate resin by a pyrolysis agent, and removing the resin to obtain the carfilzomib tetrapeptide intermediate. The method provided by the invention has the advantages of high yield, low pollution and simple and controllable reaction, and is suitable for industrial production.
Owner:苏州科耐尔医药科技有限公司
Preparation method of carfilzomib
InactiveCN105440106AEasy to removeEasy to operatePeptide preparation methodsBenzeneTetrafluoroborate
The invention discloses a preparation method of carfilzomib. According to the method, TBTU namely 2-(1H-benzotriazoleL-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate is taken as the condensing agent to carry out condensation. The method has the advantages that the reaction time is short, the nitrogen gas protection is not needed, the operation is convenient and feasible, the byproduct of condensing agent TBTU can be easily removed, the tedious steps such as column chromatography are not needed; a mixed solvent is cured in the post treatment to obtain the solid carfilzomib, the column chromatography is not needed, and the crude product is recrystallized by alcohol so as to obtain the refined product of carfilzomib. The preparation method can be applied to industrial production.
Owner:KUNMING GUIYAN PHARMA
Method for preparing carfilzomib amorphous crystal
InactiveCN104402973AReduce moisture contentImprove stabilityPeptide preparation methodsDevitrificationOrganic solvent
The invention provides a method for preparing a carfilzomib amorphous crystal. The method comprises the following steps: dissolving carfilzomib in an organic solvent to obtain a carfilzomib solution; concentrating the carfilzomib solution to obtain the carfilzomib amorphous crystal. The method has the advantages that as the solvent is organic, no water is needed, and the organic solvent is easy to remove during concentration, no residual can be generated in the product, the moisture content is extremely low, no crystal water is formed, and the obtained carfilzomib amorphous crystal is relatively high in stability. The carfilzomib amorphous crystal is prepared by dissolving carfilzomib and directly concentrating and drying the carfilzomib solution, that is, the processes of crystal growth and devitrification are not avoided, so that the obtained carfilzomib crystal is amorphous. Therefore, the method is simple, high in operability, and suitable for large-scale industrialized production.
Owner:CHONGQING TAIHAO PHARM CO LTD
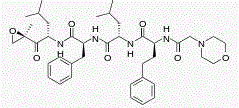
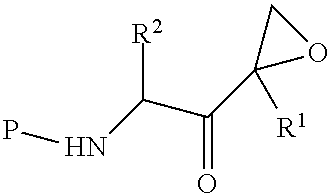
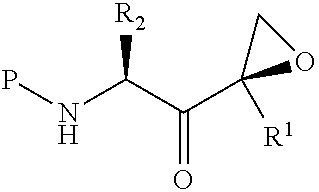
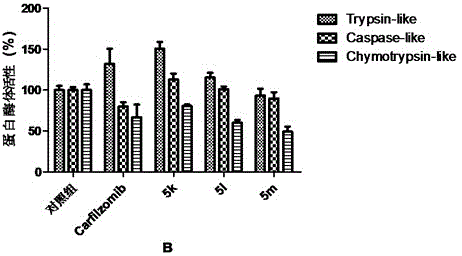
Tripeptide epoxy ketone compound constructed by heterocycle as well as preparation method and application thereof
ActiveCN104945470AReasonable designRaw materials are easy to getNervous disorderDigestive systemEpoxyProteasome Inhibition
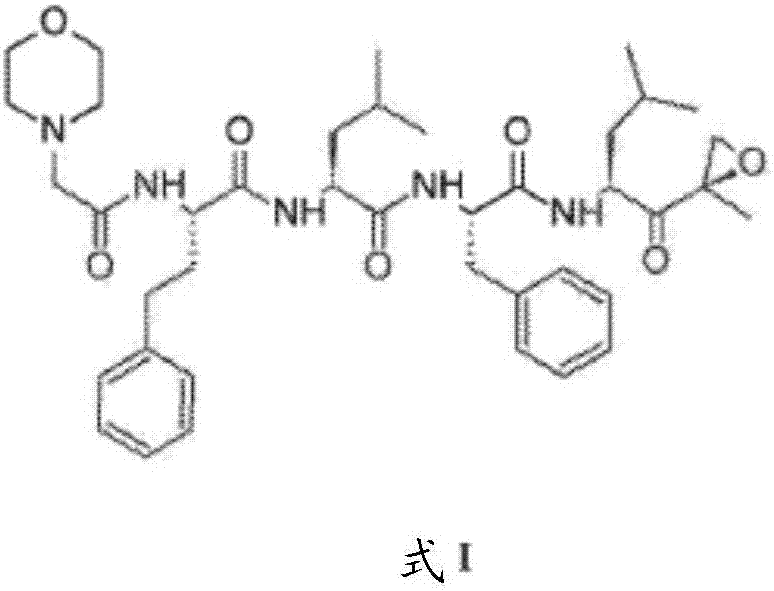
The invention provides a tripeptide epoxy ketone compound constructed by heterocycles. Carfilzomib is taken a pilot compound, a Boc protecting group is removed under condensation and acid conditions, reaction is carried out under alkaline condition to obtain amino acid methyl ester isocyanate, and hydrolysis is carried out under the action of a condensation agent, so that the tripeptide epoxy ketone compound is obtained. The tripeptide epoxy ketone compound is a micromolecule short-peptide proteasome inhibitor; the tripeptide epoxy ketone compound has extremely strong proteasome inhibitory activity and cell prolifation inhibitory activity and has a prospect, and a new way is provided for study of a cancer treatment medicine; raw materials for synthesizing the tripeptide epoxy ketone compound are available, route design is reasonable, reaction conditions are mild, yield of each step is high, operation is easy, and the tripeptide epoxy ketone compound is applicable to industrial production; and the tripeptide epoxy ketone compound has a general structural formula shown in a formula I (described in the specification).
Owner:ZHEJIANG UNIV +1
Preparation method of carfilzomib intermediate and intermediate chemical compounds of carfilzomib
InactiveCN105218488AReduce dosageLow costOrganic compound preparationAmino-carboxyl compound preparationHydrogenChemical compound
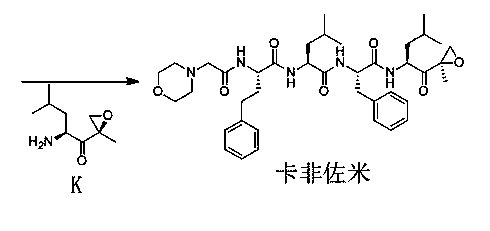
The invention discloses a novel preparation method of a carfilzomib key intermediate. L-leucine is taken as an original raw material, two reactive hydrogen on an amino group are protected, a chemical compound V is prepared and subjected to Weinreb amidation, a chemical compound IV is prepared, IV and 2-allyl magnesium bromide have a reaction, a chemical compound III is prepared and has an epoxidation reaction, a chemical compound II is prepared and subjected to deamination protection, and a chemical compound I is prepared. The invention further discloses intermediate chemical compounds II, III, IV and V of carfilzomib. The preparation method is simple to operate, the total yield is high, the cost is low, the selectivity is high, and the preparation method is suitable for industrial production.
Owner:湖南华腾制药有限公司
Carfilzomib prodrug and preparation method thereof
ActiveCN105924500AGood water solubilityEasy to manufacturePeptide preparation methodsHigh concentrationSolubility
The invention provides a carfilzomib prodrug with a structural formula (I). The invention uses a short-chain PEG molecule as a hydrophilic end, and carfilzomib as a hydrophobic end; and the two parts are connected by L-leucine in the form of imine, so as to prepare a novel carfilzomib prodrug. The carfilzomib prodrug improves the water solubility, is easy for preparation into high-concentration solution, and can be produced into a finished product with enhanced stability by freeze-drying. At the same time, the carfilzomib prodrug is a compound sensitive to acid, maintains stability in blood (alkaline) circulation, and can quickly release carfilzomib drug once arriving in the tumor tissue (acidic), in order to achieve targeted drug delivery, enhance curative effect of chemotherapy and reduce side effects.
Owner:LIANGJIANG MEDICINE CO LTD
Liposome lyophilized composition of carfilzomib drug and preparation method thereof
InactiveCN105497871AChemically stableImprove molecular structurePowder deliveryPeptide/protein ingredientsAntioxidantCholesterol
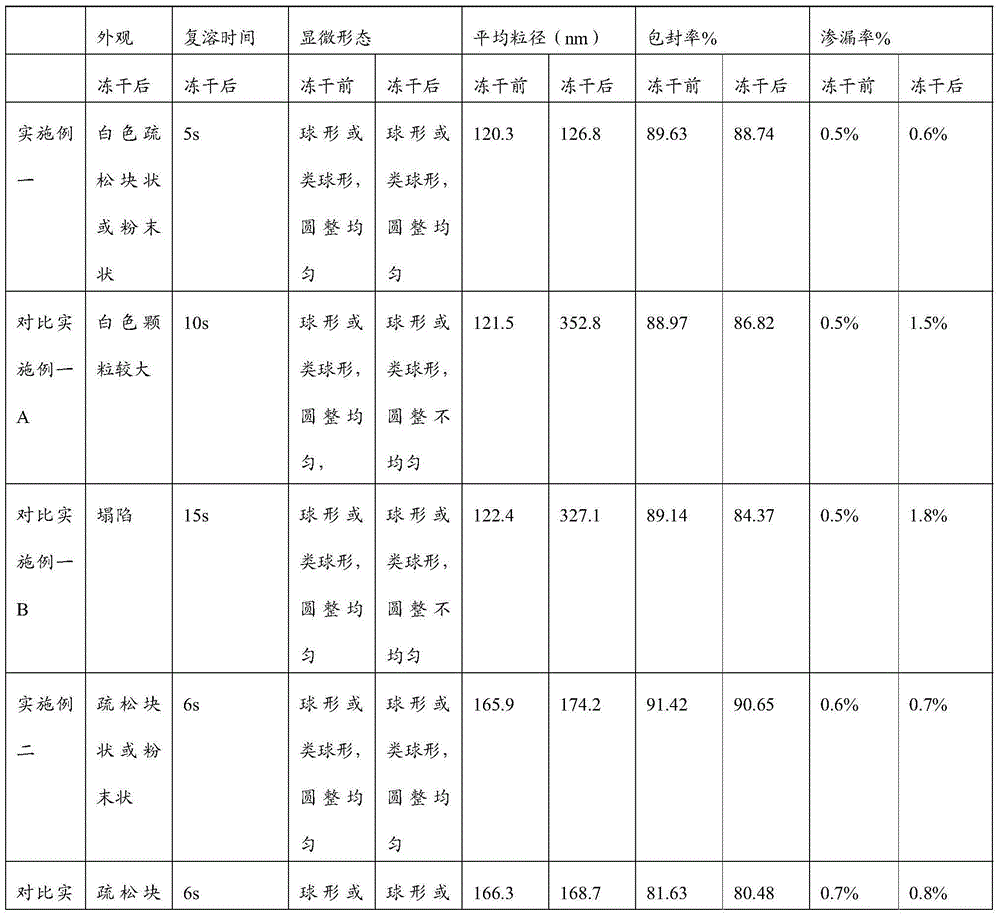
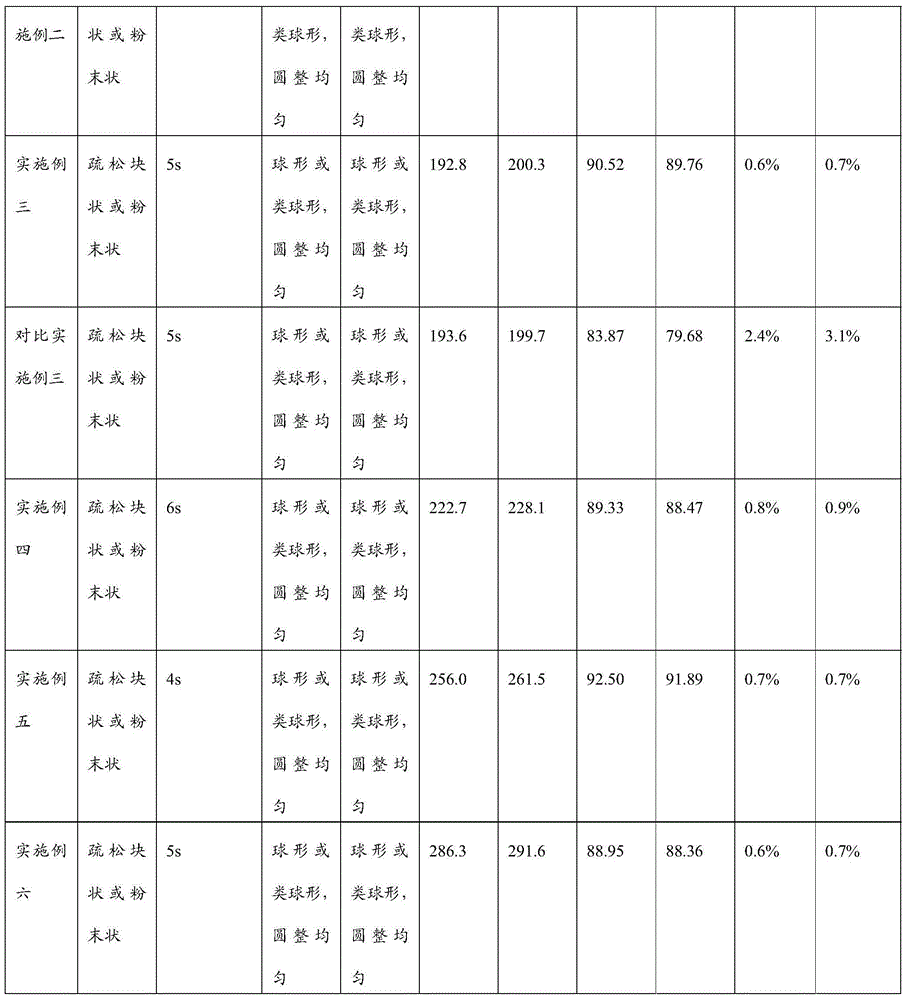
Belonging to the technical field of drug synthesis, the invention in particular relates to a liposome lyophilized composition of carfilzomib drug and a preparation method thereof. The liposome lyophilized composition of carfilzomib drug comprises the following components by weight: 5-20 parts of carfilzomib; 0.1-1 part of an antioxidant; 1-5 pars of a pH value regulator; 40-100 parts of phospholipids; 5-20 parts of cholesterol; and 5-25 parts of a lyophilization protective agent. The preparation method provided by the invention comprises the steps of: 1) preparation of a multilamelar liposome suspension; 2) preparation of a unilamelar liposome; and 3) preparation of carfilzomib liposome lyophilized powder. The carfilzomib liposome lyophilized composition obtained by the method provided by the invention has stable pH value and particle size variation before and after lyophilization, maintains stable encapsulation rate, and has good water re-dissolution after lyophilization, water redissolved particles have uniform size, the particles are spherical or sphere-like, and the appearance is round and even.
Owner:HYBIO PHARMA
Method for preparing carfilzomib intermediate compound
ActiveCN105294501AMild conditionsSignificant technological progressCarbamic acid derivatives preparationOrganic compound preparationTert-Butyloxycarbonyl protecting groupCarfilzomib
The invention provides a method for preparing a carfilzomib intermediate compound. The method comprises the following steps: taking L-leucine V as a raw material, and enabling amidation between the raw material and di-tert-butyl dicarbonate ester to produce a compound IV; enabling Weinreb amidation between the compound IV and N,O-dimethylhydroxylamine hydrochloride under the action of N,N'-carbonyldiimidazole to produce a compound III; enabling grignard reaction between the compound III and an ethyl magnesium halide solution to produce a compound II; enabling aldol reaction between the compound II and formaldehyde or paraformaldehyde to produce a carfilzomib intermediate compound I ((S)-4-(tert-butoxycarbonylamino)-2,6-dimethyl-1-hepten-3-one). The method avoids use of an expensive reagent 2-bromopropylene, adopts ethylmagnesium chloride, and conforms to the design principles of highly accessible raw materials and simple operation. The synthetic route is mild in condition, the synthetic yield in each step is excellent or favorable, and the method is suitable for industrial production.
Owner:SHANGHAI INST OF TECH
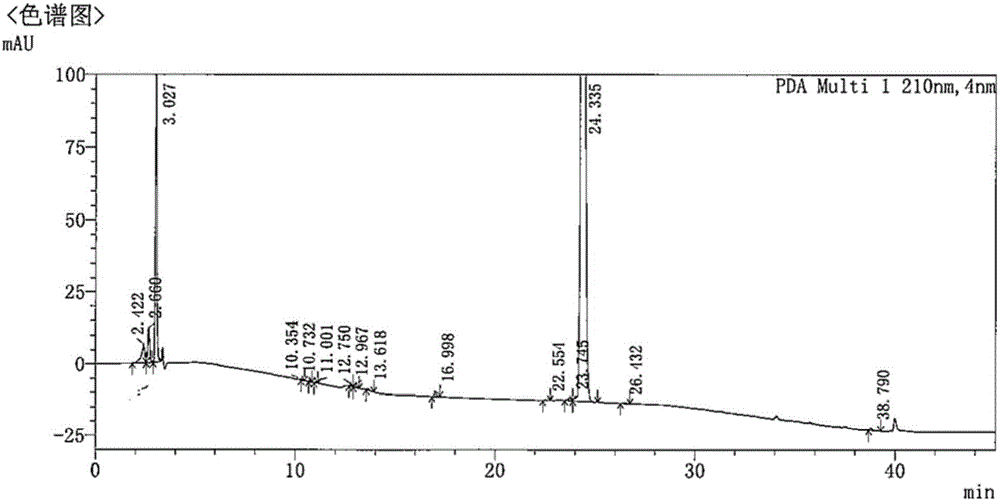
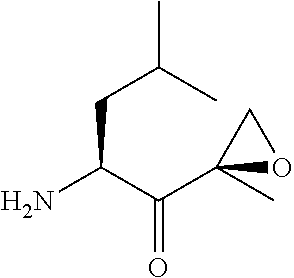
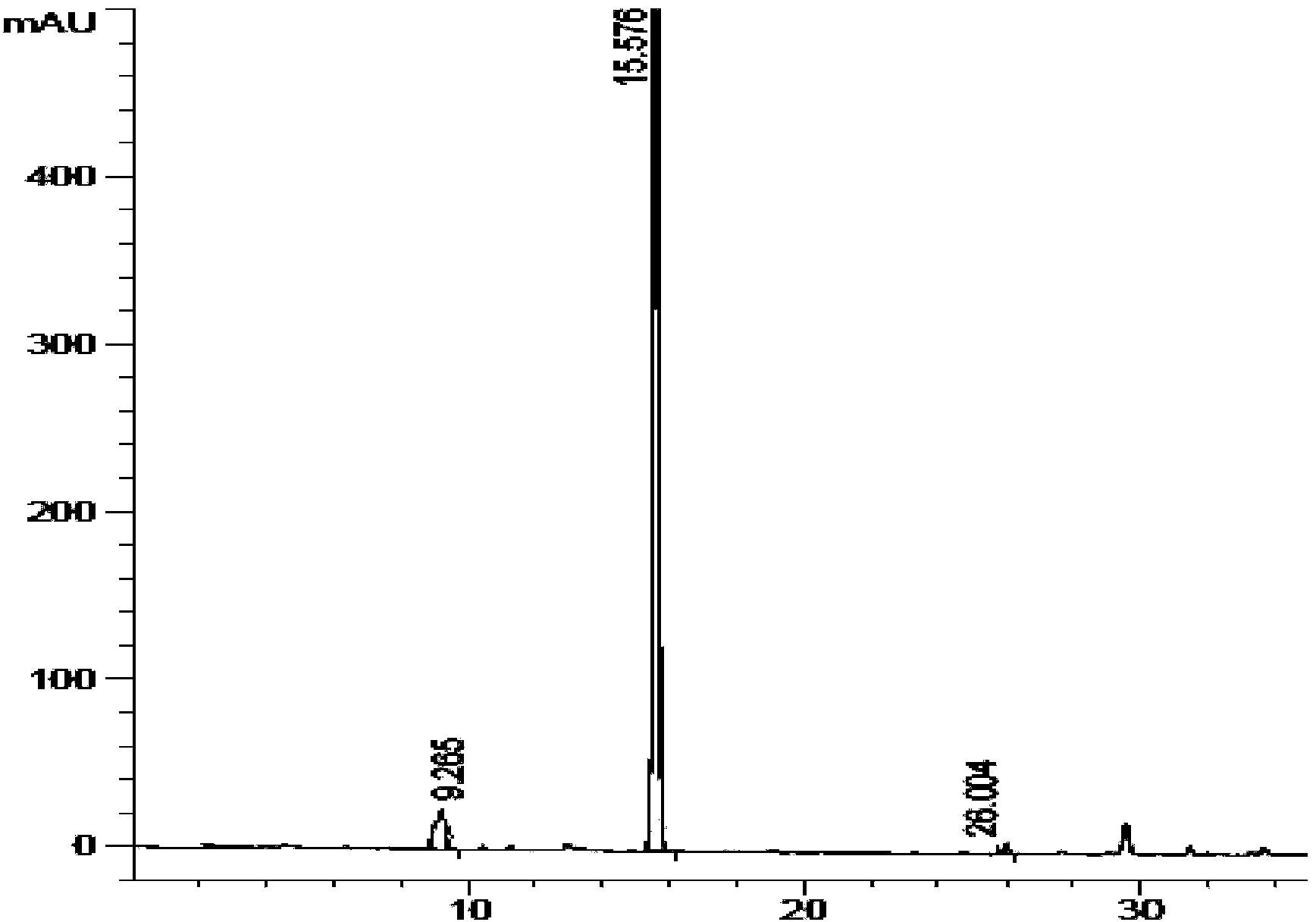
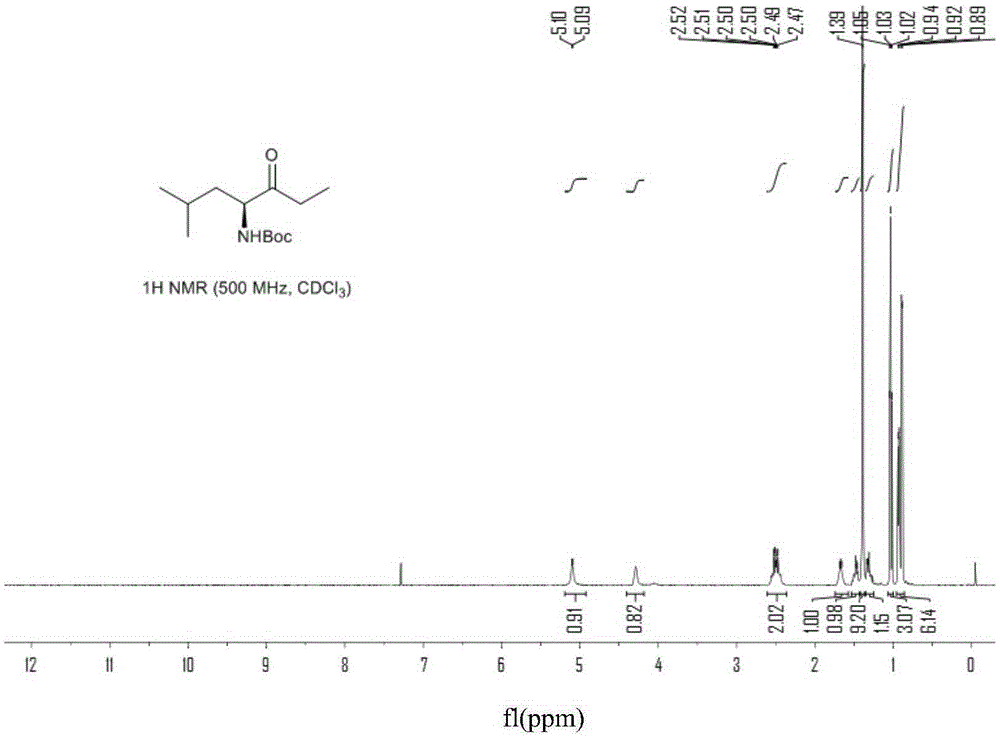
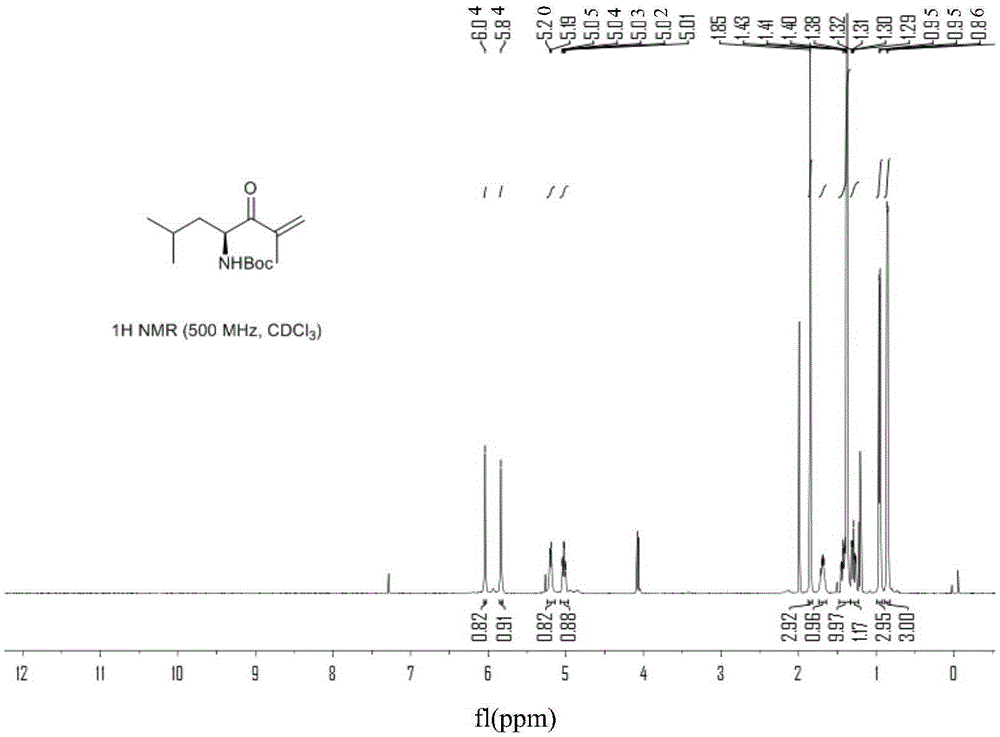
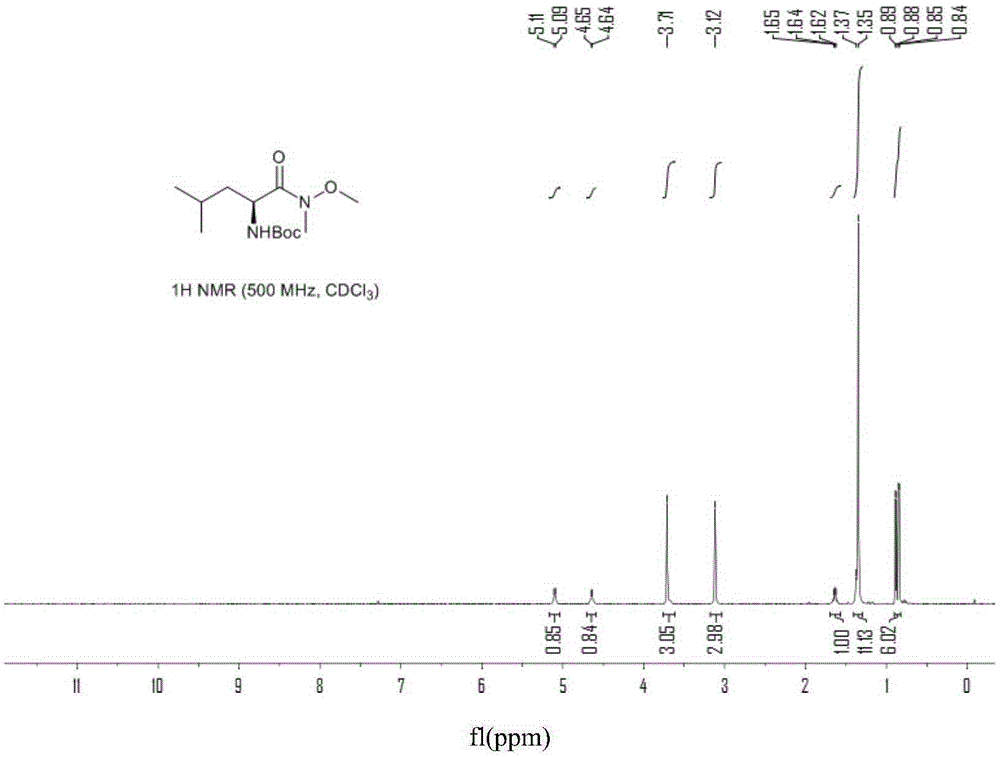
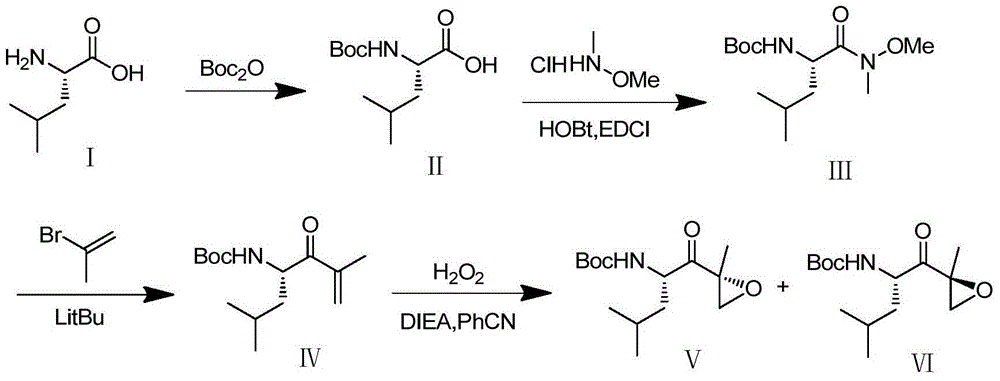
Chiral preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate
ActiveCN104672180ARaw materials are easy to getSimple reaction conditionsOrganic chemistryCarbamateCarfilzomib
The invention relates to a chiral preparation method of a carfilzomib intermediate: [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate. The preparation method comprises the step that a compound (III) carries out catalytic reaction with a phase transfer catalyst (PTC) and hydrogen peroxide under alkaline conditions. The compound (III) is shown in a formula in the specification. The carfilzomib intermediate (I) can be synthesized with a starting material (III) by adopting the preparation method. The preparation method is available in used raw materials and simple in reaction conditions, is simple and convenient to operate, is simple in aftertreatment, has good selectivity, is good in yield and is suitable for industrial production.
Owner:ZHEJIANG YONGNING PHARMA
Synthetic method of carfilzomib intermediate and carfilzomib intermediate
ActiveCN104557793AReduce usageReduce manufacturing costCarbamic acid derivatives preparationOrganic compound preparationChemical synthesisCarfilzomib
The invention relates to the field of chemical synthesis of medicines, and in particular relates to a synthetic method of a carfilzomib intermediate 1. The synthetic method of the carfilzomib intermediate 1 comprises the steps of ring opening, ring closing, deprotection and the like. The method is simple in routes, high in yield of each reaction step and suitable for industrial production. The invention also provides a new compound which is a key intermediate required for synthesizing the compound 1, and the key intermediate is shown in a formula 4 in the specification. The method also provides a synthetic method of the compound 4; in the method, an expensive reagent 2-bromopropylene is not used, so that the production cost is reduced.
Owner:苏州中科新药篮生物医药科技有限公司
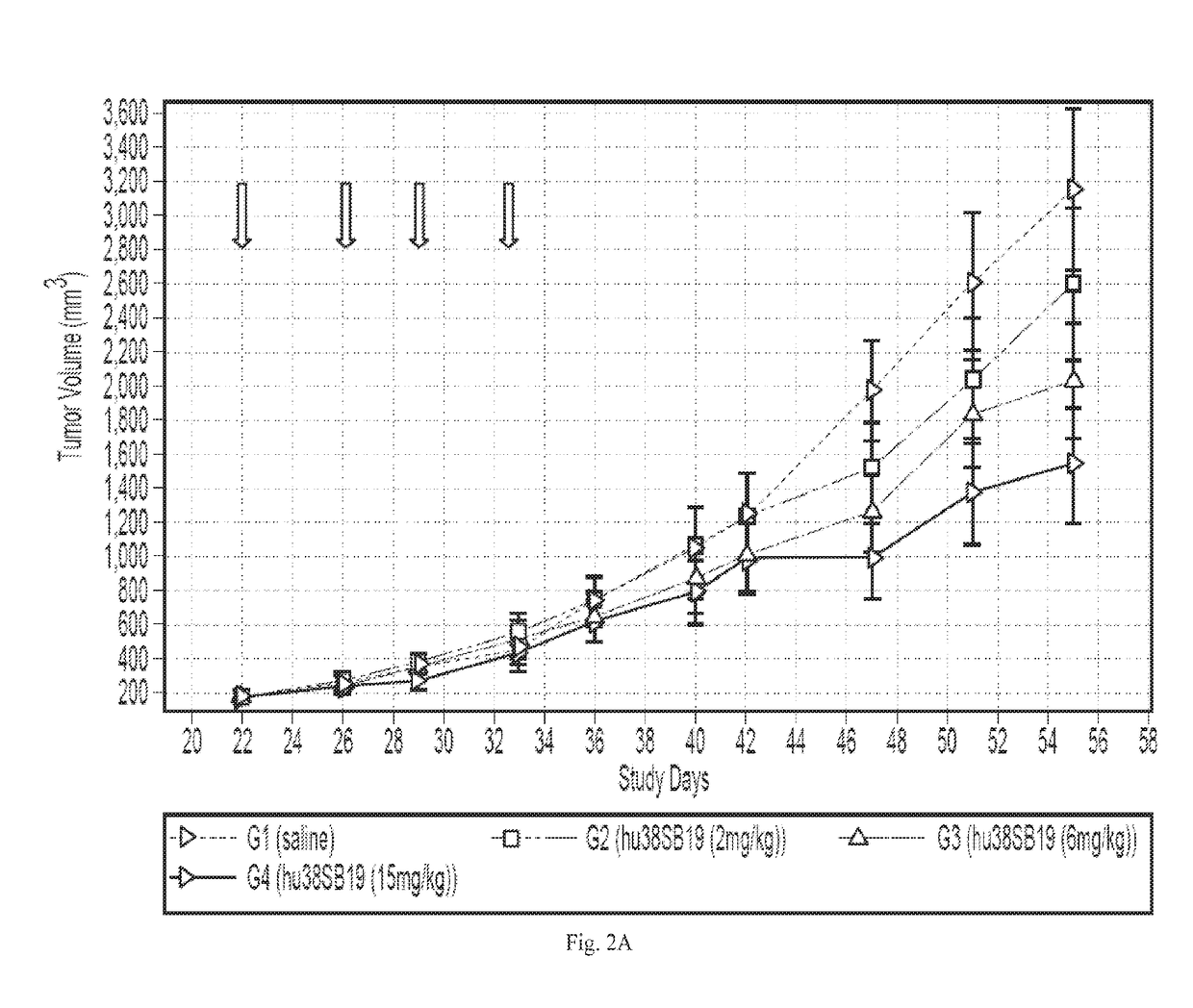
Compositions comprising Anti-cd38 antibodies and carfilzomib
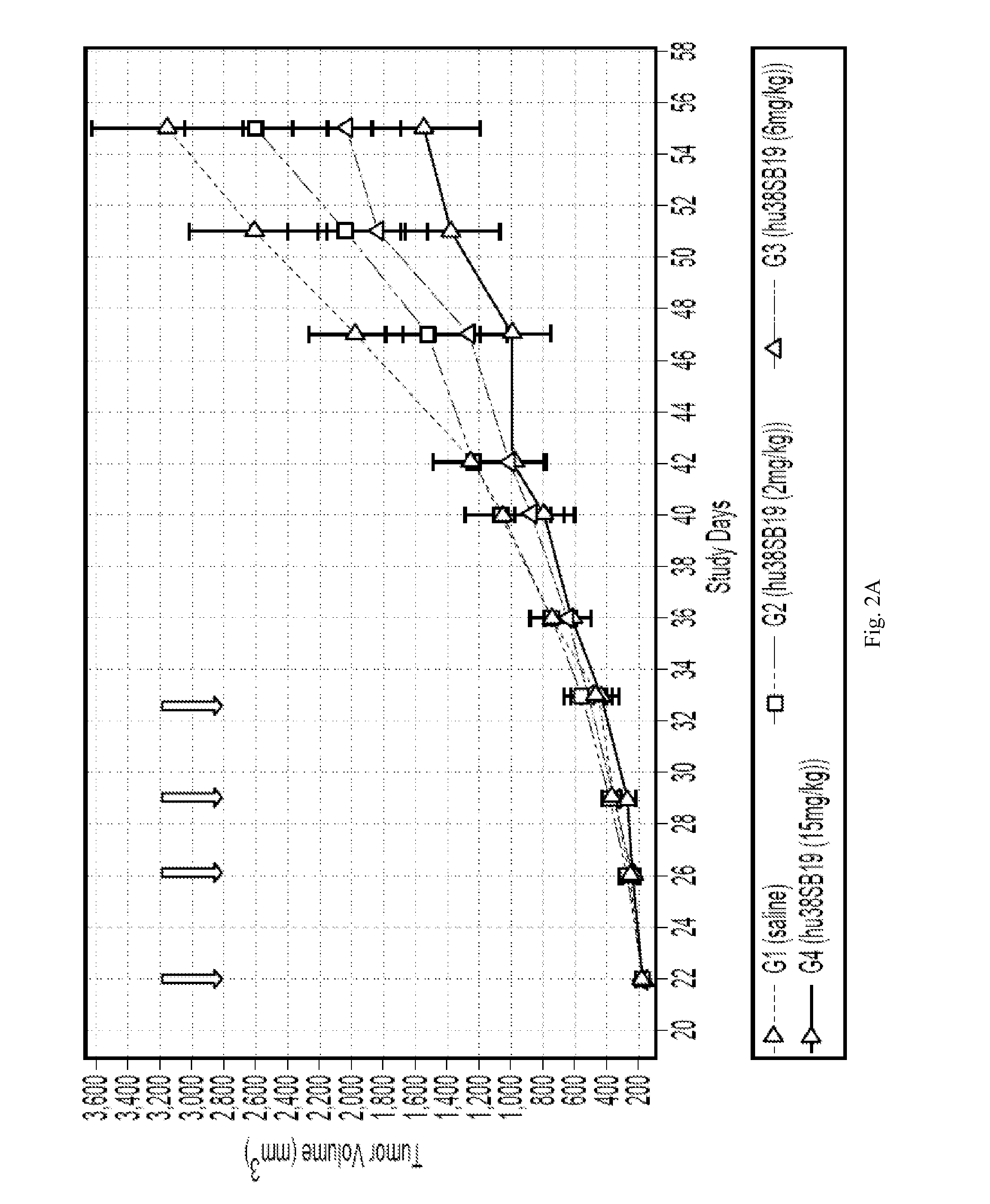
InactiveUS20160022813A1Improve treatment outcomesImprove survivalOrganic active ingredientsTetrapeptide ingredientsAntiendomysial antibodiesCarfilzomib
Disclosed herein are compositions and kits which comprise anti-CD38 antibodies and carfilzomib compounds. Also disclosed are methods for treating cancers, such as multiple myeloma, in subjects with the compositions and kits.
Owner:RGT UNIV OF CALIFORNIA +1
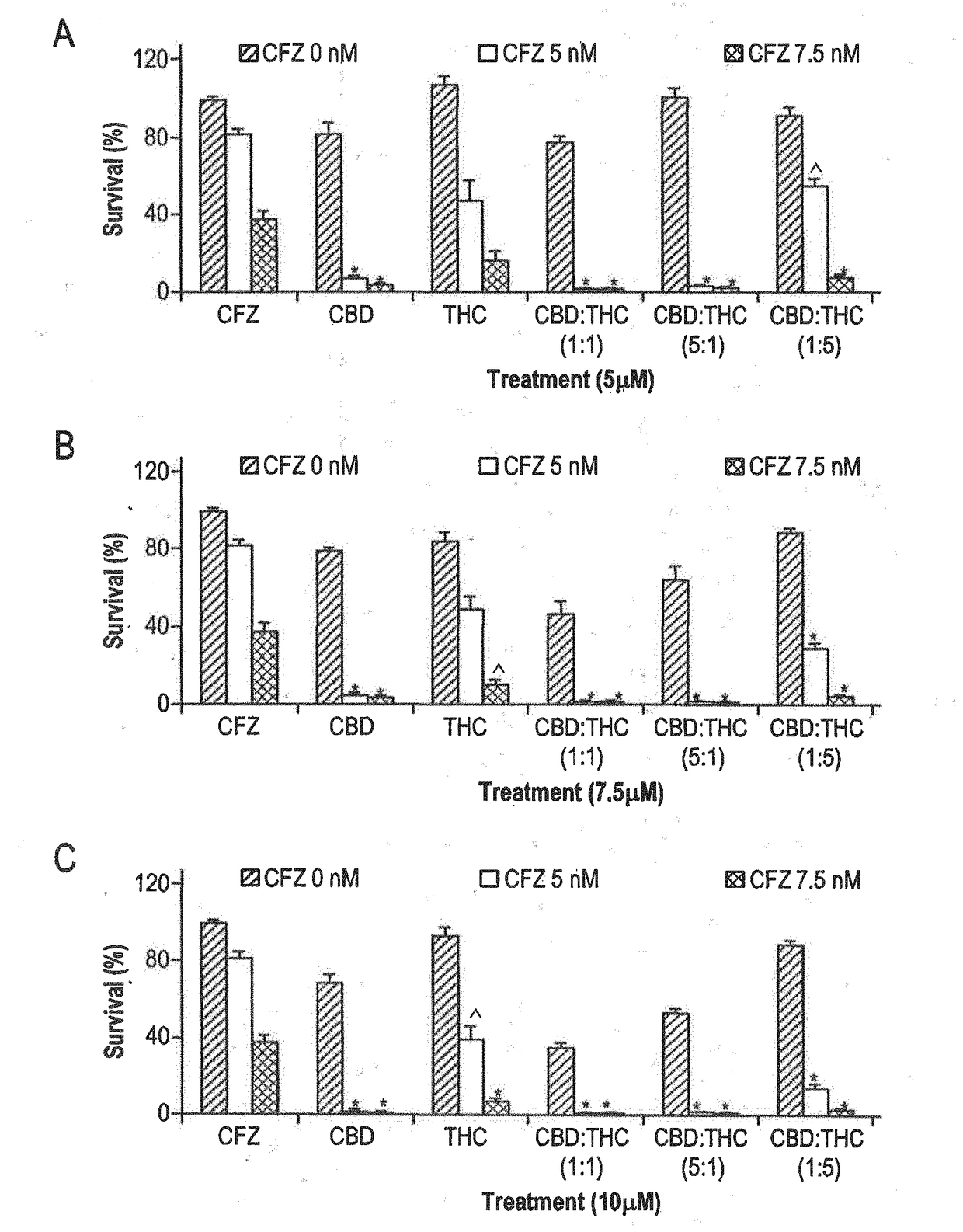
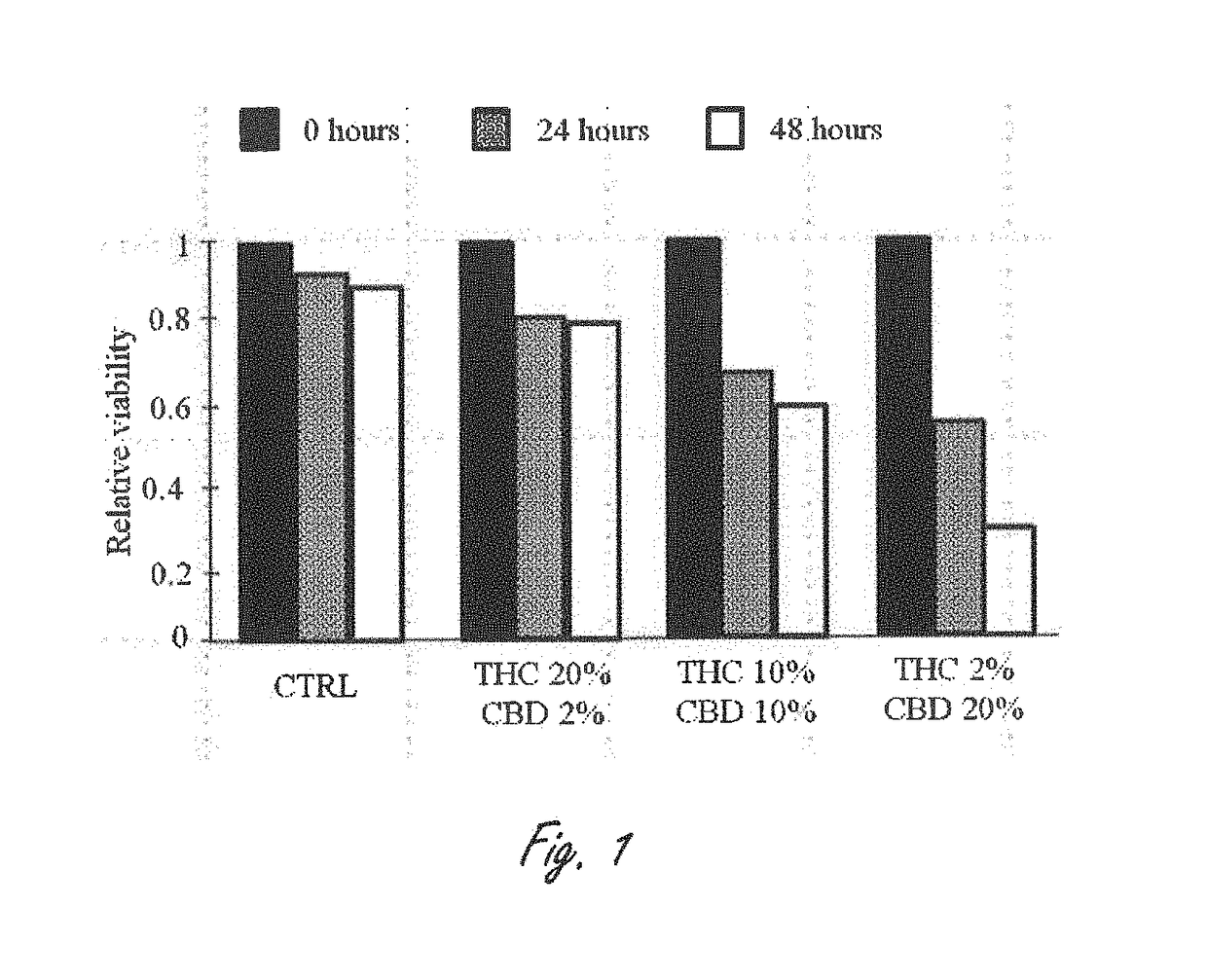
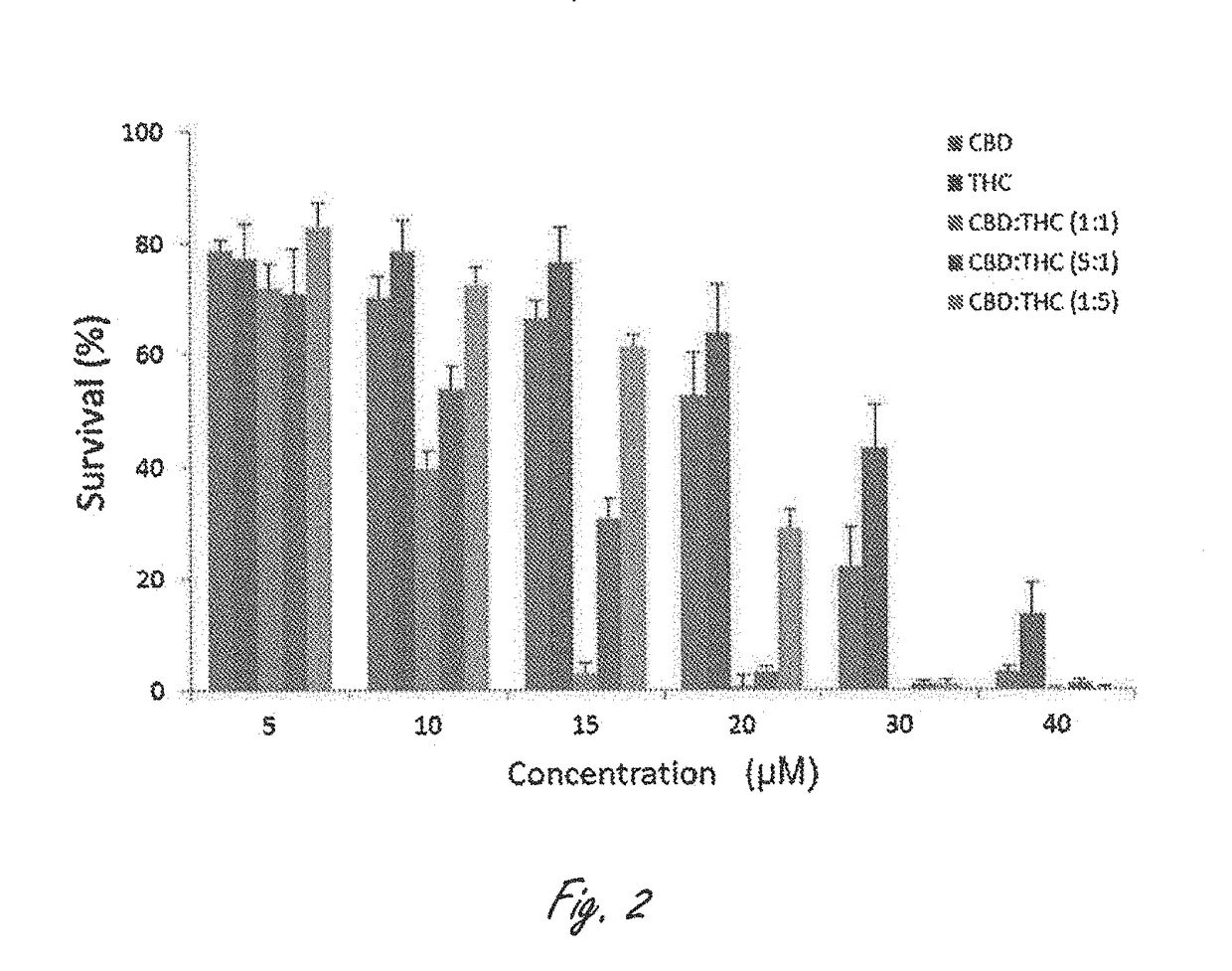
Novel cannabinoid combination therapies for multiple myeloma (MM)
InactiveUS20180185324A1Strong cytotoxicityReduced viabilityHydroxy compound active ingredientsBoron compound active ingredientsCannabinoidCytotoxicity
The present invention discloses a cytotoxic cocktail comprising; (a) a therapeutically effective amount of at least one cannabinoid selected from the group consisting of: cannabidiol (CBD) or a derivative thereof, Tetrahydrocannabinol (THC) or a derivative thereof, and any combination thereof; and (b) at least one therapeutic agent selected from the group consisting of: bortezomib (BTZ), carflizomib (CFZ), lenalidomide (LEN), dexamethasone (DEX), melphalan (MEL) and doxorubicin (DOXO). In a core embodiment the cocktail is conferring a synergistic effect with respect to inhibition or cytotoxicity of multiple myeloma (MM) cells, relative to said at least one therapeutic agent selected from the group consisting of: BTZ, CFZ, LEN, DEX, MEL, DOXO and said CBD and THC, administered separately in a similar concentration.
Owner:ONE WORLD CANNABIS LTD
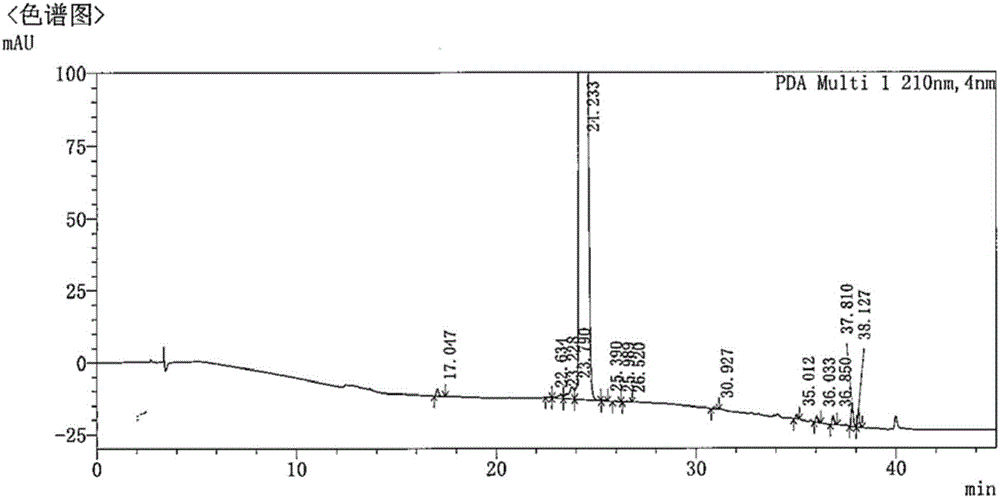
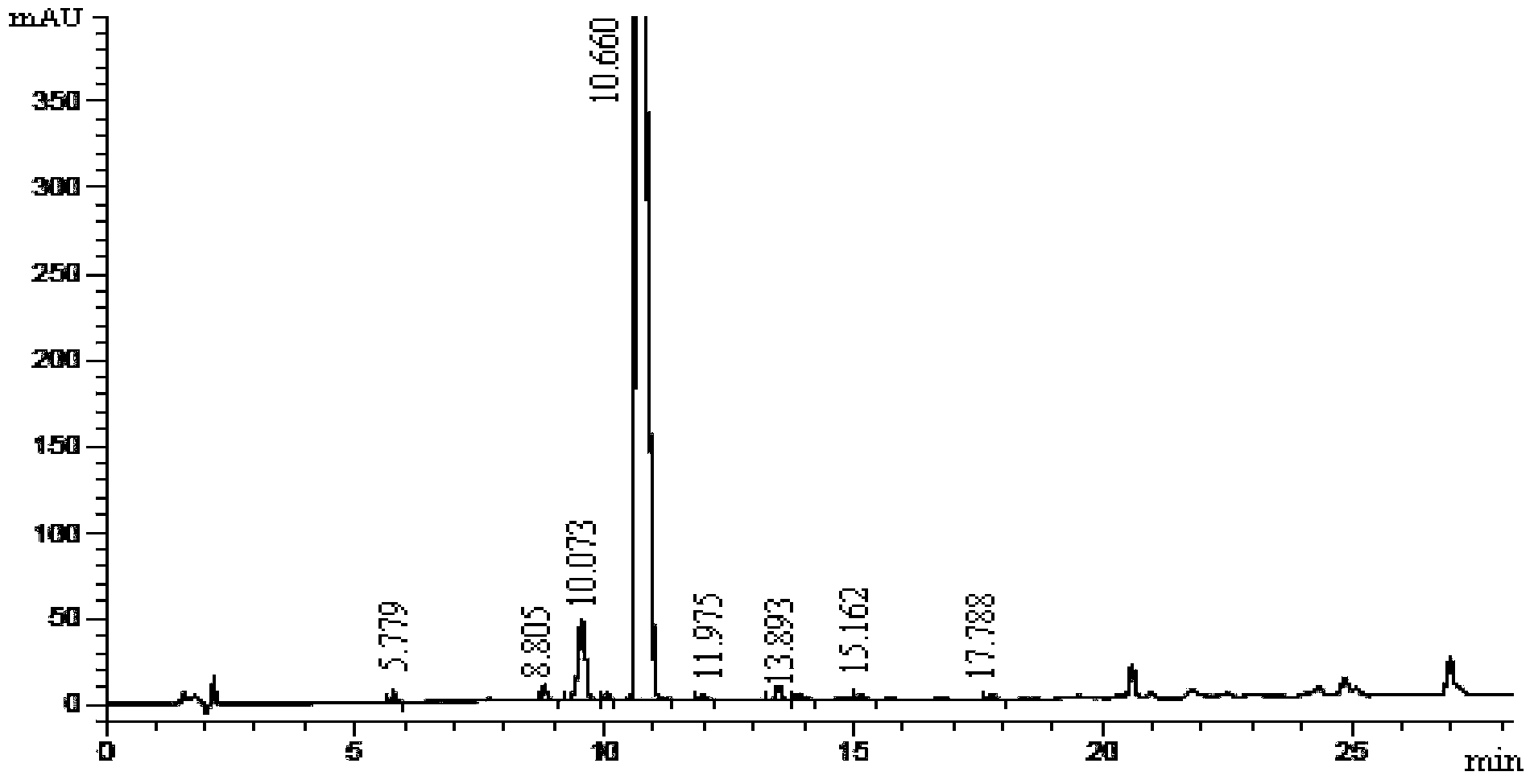
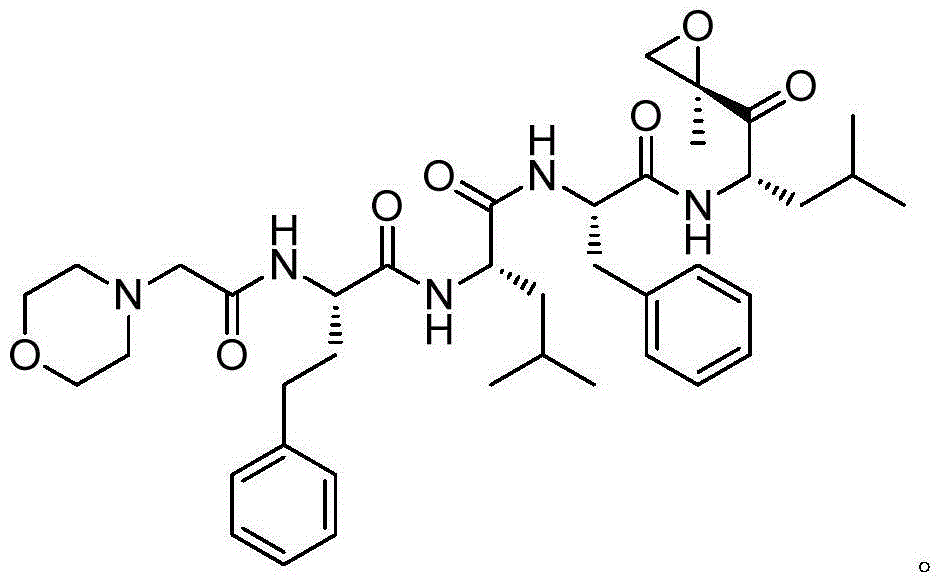
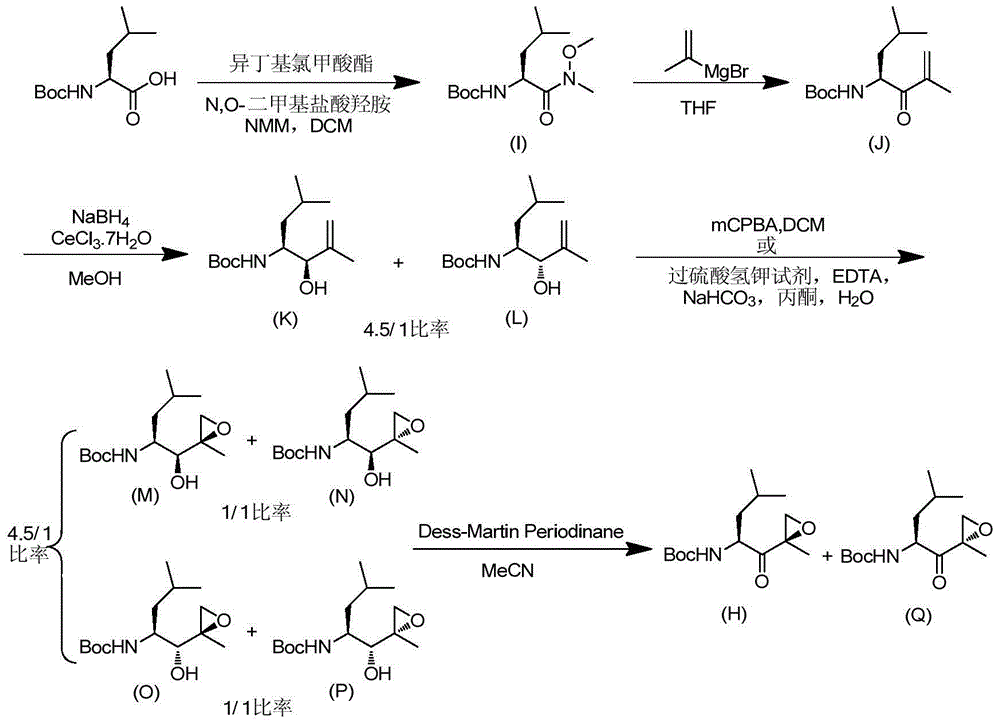
Preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate
The invention relates to a preparation method of a carfilzomib intermediate: [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate. The preparation method comprises the following step: adding triphenylphosphine and tert-butyl alcohol hydroperoxide to a compound (III) to carry out catalytic reaction in the presence of an asymmetric chiral catalyst (R)-La-BINOL. The compound (III) is shown in a formula in the specification. The carfilzomib intermediate (I) can be synthesized with a starting material (III) by adopting the preparation method. The preparation method is accessible in used raw materials and simple in reaction conditions, is simple and convenient to operate, is simple in aftertreatment, has good selectivity, is good in yield and is suitable for industrial production.
Owner:ZHEJIANG YONGNING PHARMA
Pharmaceutical composition containing carfilzomib and preparation method thereof
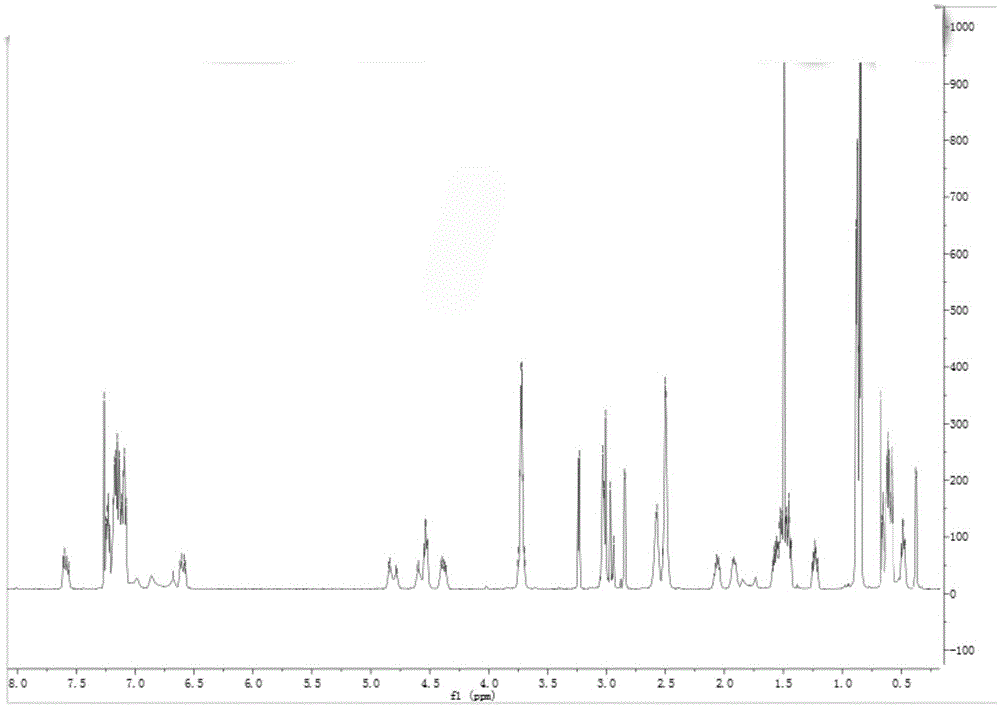
ActiveCN106310221AEnsure safetyGood chemical stabilityPowder deliveryPeptide/protein ingredientsZeta potentialFreeze-drying
The present invention provides a pharmaceutical composition containing carfilzomib and a preparation method thereof. The pharmaceutical composition comprises carfilzomib as an active ingredient, protein and pH regulator. The pH value ranges from 2 to 5, the average particle size is less than 200nm, the Zeta potential is -20 to -30mV, and the mass ratio of carfilzomib to protein is 1:6-12. The invention aims to provide the pharmaceutical composition containing carfilzomib which is better used for the in-vivo delivery of carfilzomib, the pharmaceutical composition does not contain the sulfobutylether-beta-cyclodextrin in currently listed carfilzomib injection, so as to avoid the occurrence of allergic reactions and prevent vasospasm and phlebitis caused by the clinical use of the pharmaceutical composition. The freeze-dried preparation of pharmaceutical composition can be prepared by high-pressure homogenization, and can be transformed into blocks or powder which can re-disperse. After re-dispersing in an aqueous medium, compared with the products in current market, the stability of the pharmaceutical composition is increased significantly, and the safety of medication in clinical application is enhanced.
Owner:QILU PHARMA HAINAN
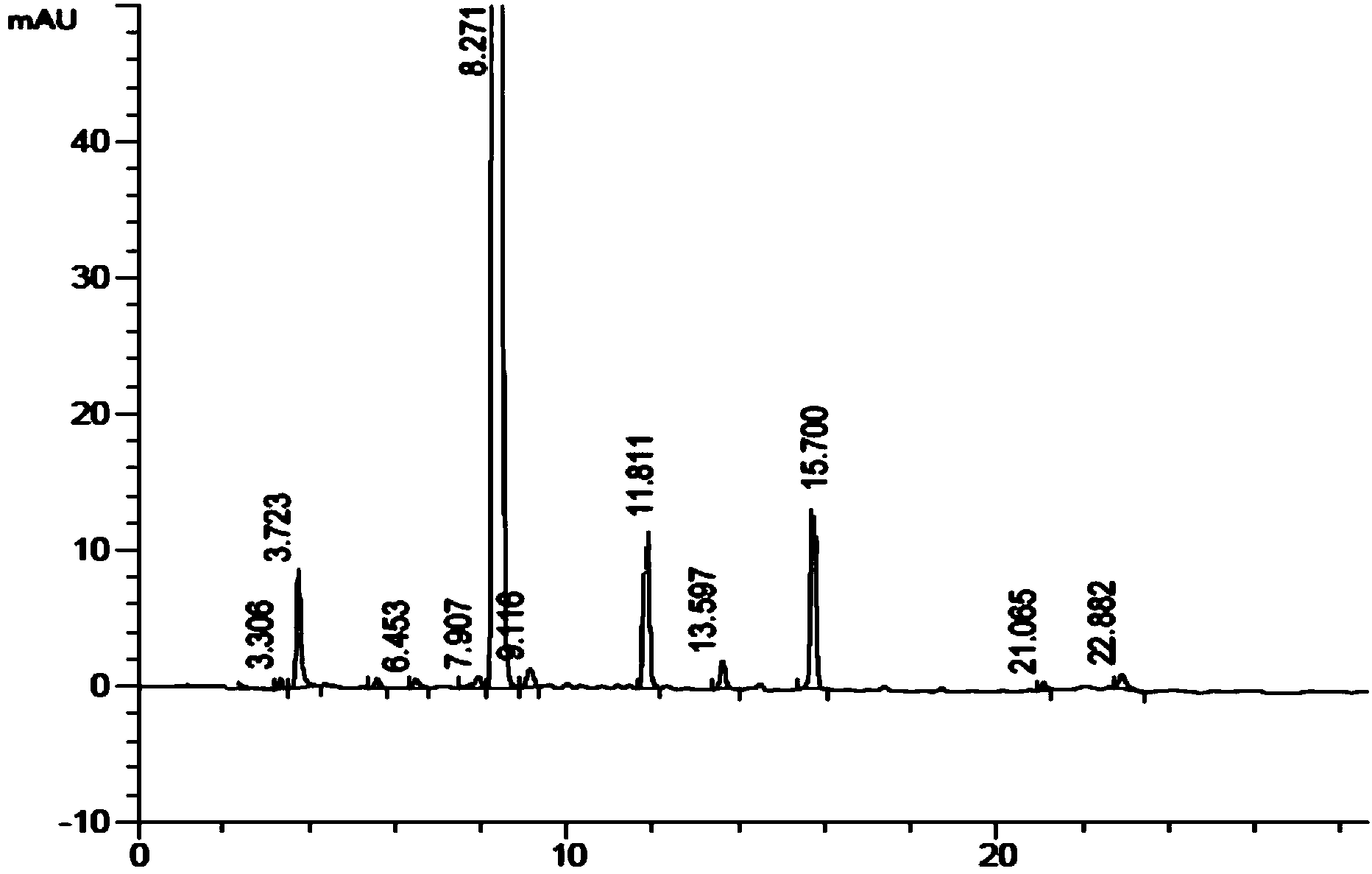
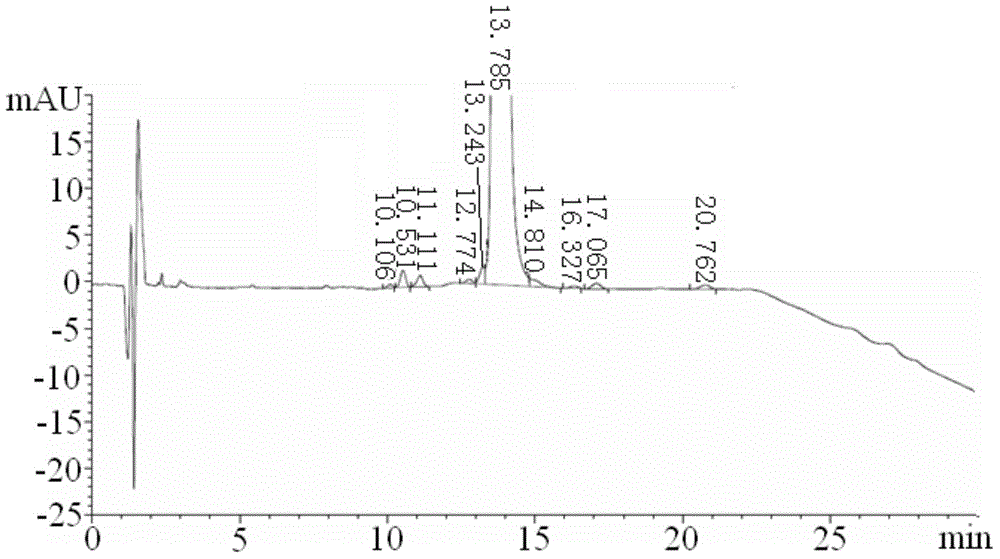
A process for purification of carfilzomib
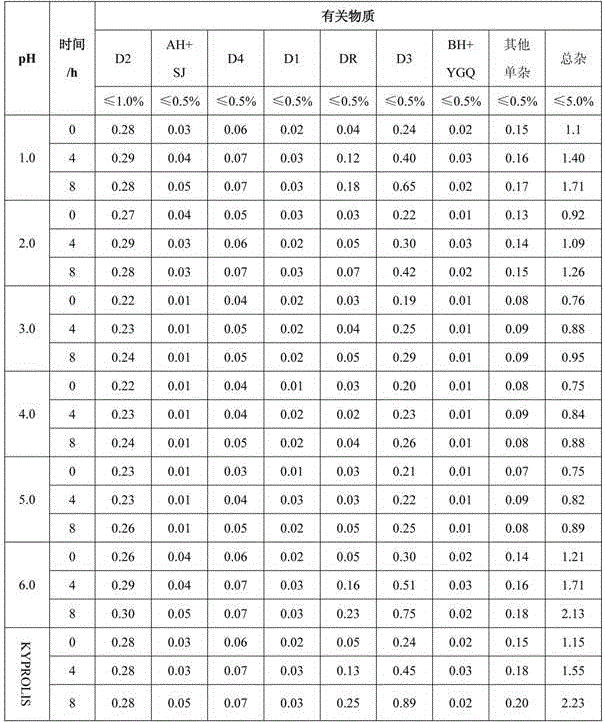
The present invention relates to a process for the purification of Carfiizomib of Formula II that reduces the level of an acetaniide impurity of Formula II preferably below 0.10 wt%. Formula I FormulaII.
Owner:FRESENIUS KABI ONCOLOGY LTD
Injecting carfilzomib and preparation method thereof
InactiveCN107802606ASolve insolubleSolution is oxidizedPowder deliveryPeptide/protein ingredientsAntioxidantFreeze-drying
The invention discloses an injecting carfilzomib freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection is prepared from active components of carfilzomib, lactose, an antioxidant, a surfactant and a pharmaceutically acceptable auxiliary material, wherein the surfactant is preferably polyethylene glycol-12-hydroxystearate. Compared with the prior art, theinjecting carfilzomib freeze-dried powder injection provided by the invention is used for overcoming the defect that a main medicine is difficult to dissolve; further, a cyclodextrin inclusion technique is not used, so that a potential safety hazard brought by cyclodextrin is avoided; meanwhile, through adding the antioxidant, raw materials are prevented from being oxidized. The overall productionprocess is simple and the requirements of production and clinical medication can be effectively met.
Owner:QILU PHARMA HAINAN +1
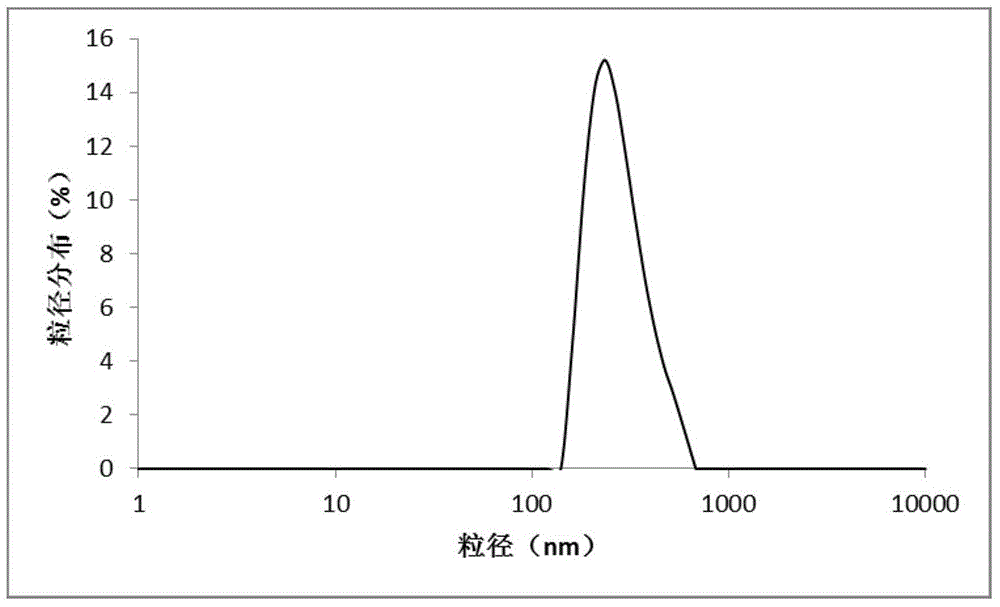
Nanoparticles preparation encapsulated with carfilzomib, and preparation method thereof
InactiveCN105919972ALow impurity contentGood water solubilityPowder deliveryPeptide/protein ingredientsSolubilityPEG-PLGA-PEG
The invention provides a nanoparticles preparation loaded with carfilzomib. The preparation includes, by weight, 1-20 parts of carfilzomib, 0.5-10 parts of lecithin, 10-100 parts of polyethylene glycol copolymer, which is a PEG-PLGA-PEG triblock copolymer with number-average molecular weight of 5000-50000 and / or a PEG-PLGA block copolymer with the number-average molecular weight of 5000-50000. The invention selects the specific polyethylene glycol copolymer, and uses lecithin as a surfactant. The prepared nanoparticles encapsulated with carfilzomib have water solubility better than carfilzomib encapsulated with cyclodextrin, have high water solubility, uniform particle size, and lower impurity content.
Owner:LIANGJIANG MEDICINE CO LTD
Ready-to-use carfilzomib compositions
ActiveUS20190351007A1Reduce the risk of oxidationTetrapeptide ingredientsPharmaceutical delivery mechanismReady to useCarfilzomib
The present invention provides a stable, non-aqueous, ready-to-use parenteral composition comprising: carfilzomib or pharmaceutically acceptable salt thereof, acidifying agent, optionally a surfactant, one or more solvents or co-solvents.
Owner:ORBICULAR PHARMA TECH PVT LTD
Purifying method for carfilzomib
The invention specifically relates to a purifying method for carfilzomib, belonging to the field of pharmaceutical chemicals. According to the method, maleic acid is added in the preparation process of carfilzomib to prepare carfilzomib maleate, so isomer impurities are obviously reduced and purity is substantially improved; and then maleate is subjected to dissociation so as to obtain high-yield high-purity carfilzomib. The method is simple in preparation, easy to operate and especially suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Stable carfilzomib pharmaceutical composition
InactiveCN105641676AAvoid degradationKeep dryPeptide/protein ingredientsPharmaceutical non-active ingredientsCarboxymethyl cellulosePharmaceutical drug
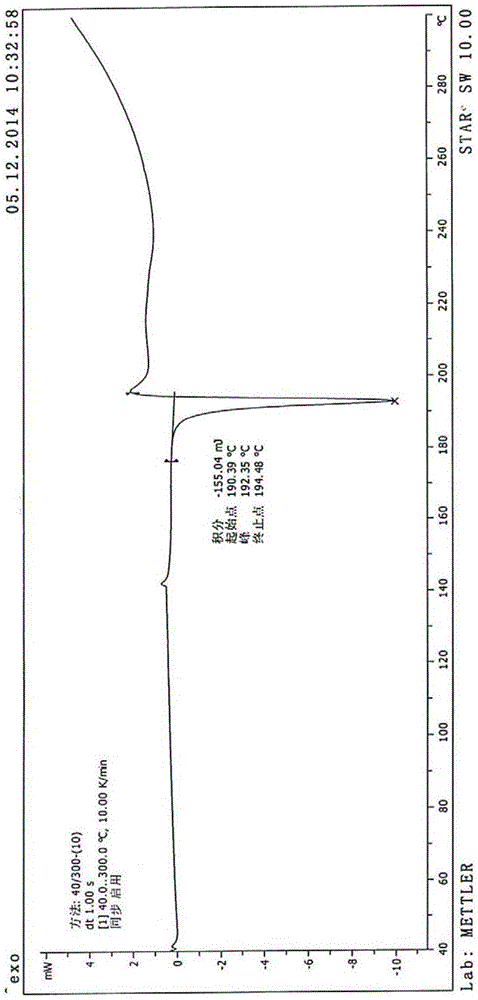
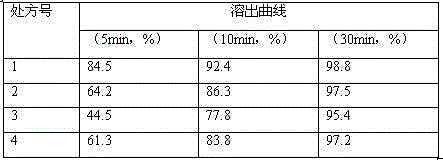
The present invention discloses a stable carfilzomib pharmaceutical composition, which is characterized in that the components in the formula for preparing 1000 tablets of the stable carfilzomib pharmaceutical compositions comprise 30-60 g of carfilzomib, 70-140 g of microcrystalline cellulose, 0.5-1 g of micro-powder silica gel, 50-100 g of cross-linked sodium carboxymethyl cellulose, and a proper amount of a 10% pre-gelatinized starch solution. The present invention further relates to a carfilzomib tablet preparation method. According to the present invention, the carfilzomib prepared by using the formula and the preparation method has characteristics of good dissolution, high bioavailability, and good treatment effect.
Owner:TIANJIN HANKANG PHARMA BIOTECH
An improved processes for the preparation of carfilzomib or pharmaceutically acceptable salts thereof
InactiveUS20190085026A1Carbamic acid derivatives preparationOrganic compound preparationCombinatorial chemistryPharmaceutical medicine
The present invention relates to an improved process for the preparation of carfilzomib or a pharmaceutically acceptable salt thereof. The present invention also relates to a process for the preparation of amorphous form of carfilzomib.
Owner:LAURUS LABS
Synthesis of peptide epoxy ketones
InactiveUS20160215016A1High purityHigh yieldPeptide preparation methodsAntineoplastic agentsKetoneCarfilzomib
It is provided an improved process for preparing peptide epoxy ketones, including novel compounds that can be used as intermediates in the process for preparing Carfilzomib and other peptide epoxy ketones.
Owner:SANDOZ AG
Compositions comprising Anti-cd38 antibodies and carfilzomib
InactiveUS20170106085A1Improve treatment outcomesImprove survivalOrganic active ingredientsTetrapeptide ingredientsAntiendomysial antibodiesCarfilzomib
Disclosed herein are compositions and kits which comprise anti-CD38 antibodies and carfilzomib compounds. Also disclosed are methods for treating cancers, such as multiple myeloma, in subjects with the compositions and kits.
Owner:RGT UNIV OF CALIFORNIA +1
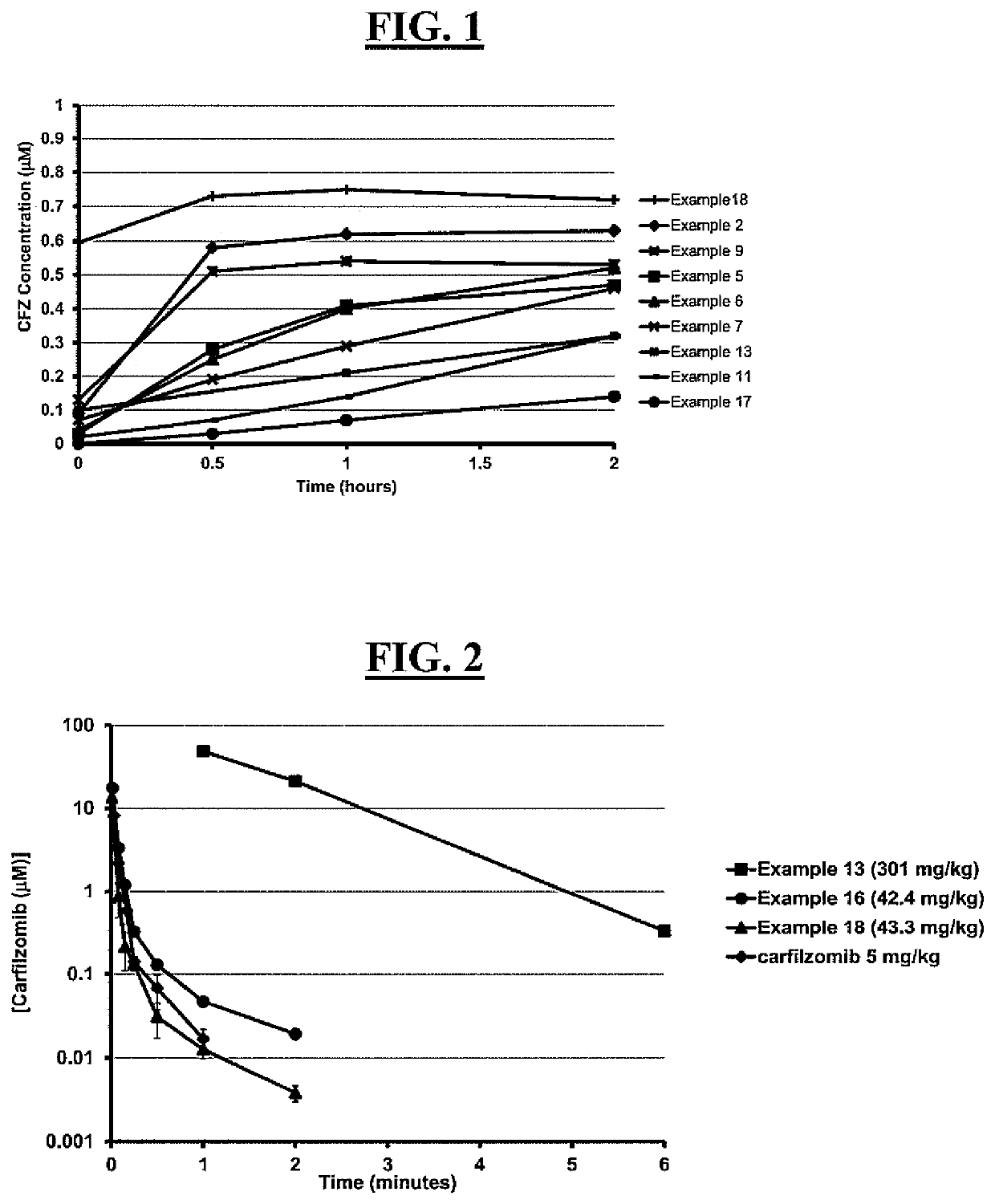
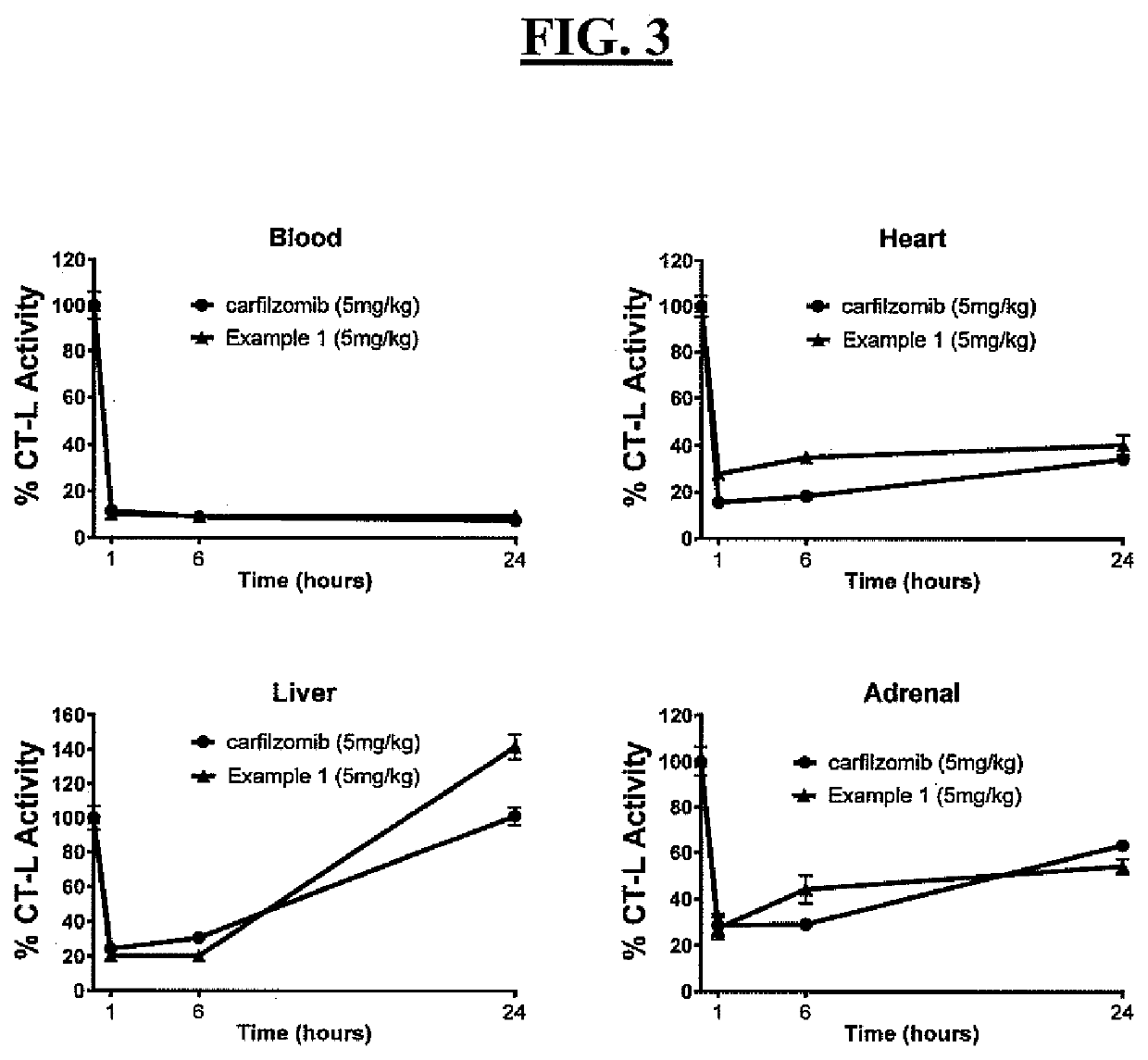
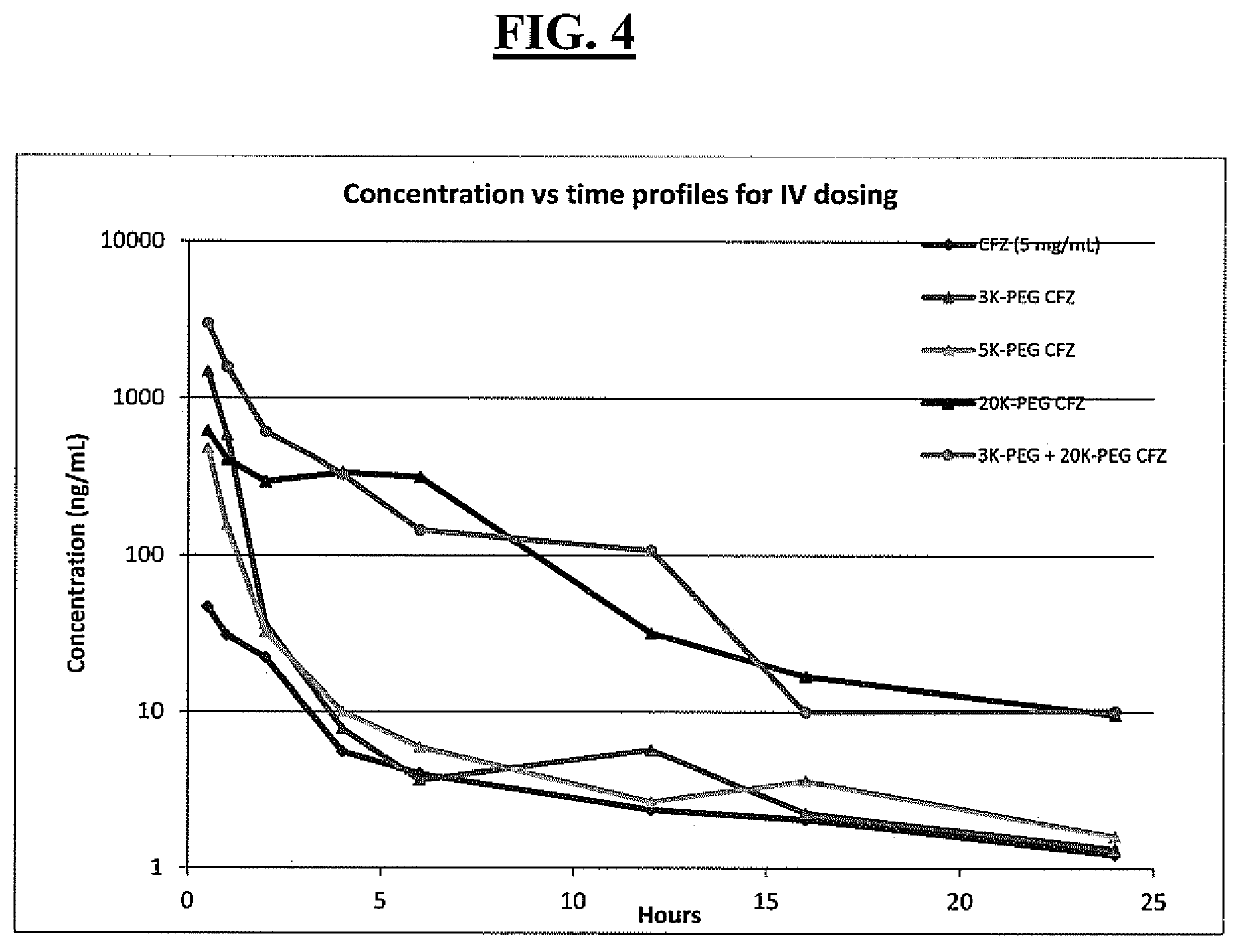
Pegylated carfilzomib compounds
ActiveUS20200069808A1Good water solubilitySuitable bioavailabilityTetrapeptide ingredientsSkeletal disorderPharmaceutical medicineCarfilzomib
Owner:AMGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Chiral preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate Chiral preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f488830-e1d8-4ffa-ae83-6971e614521b/BDA0000673750440000011.PNG)
![Chiral preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate Chiral preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f488830-e1d8-4ffa-ae83-6971e614521b/BDA0000673750440000012.PNG)
![Chiral preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate Chiral preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6f488830-e1d8-4ffa-ae83-6971e614521b/BDA0000673750440000021.PNG)
![Preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate Preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f89a47f3-9754-411c-9717-b5d27f9846eb/BDA0000673800160000011.PNG)
![Preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate Preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f89a47f3-9754-411c-9717-b5d27f9846eb/BDA0000673800160000012.PNG)
![Preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate Preparation method of [(1S)-3-methyl-1-[[(2R)-2-methylepoxyethyl]carbonyl]butyl]tert-butyl carbamate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/f89a47f3-9754-411c-9717-b5d27f9846eb/BDA0000673800160000021.PNG)