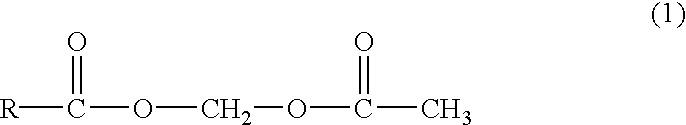
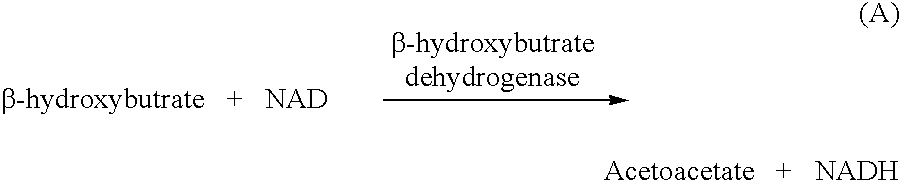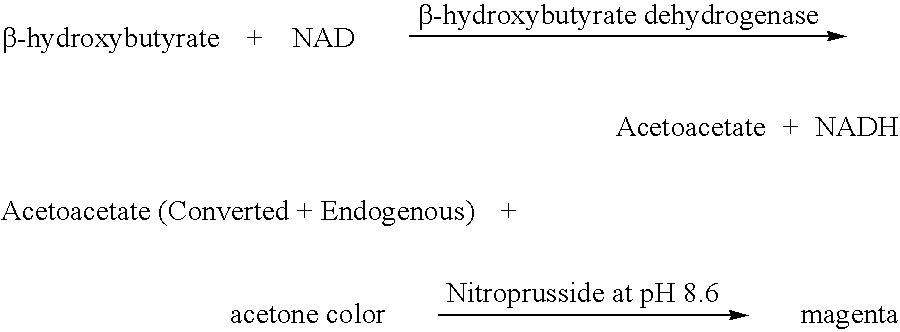Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1093 results about "Aceglatone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
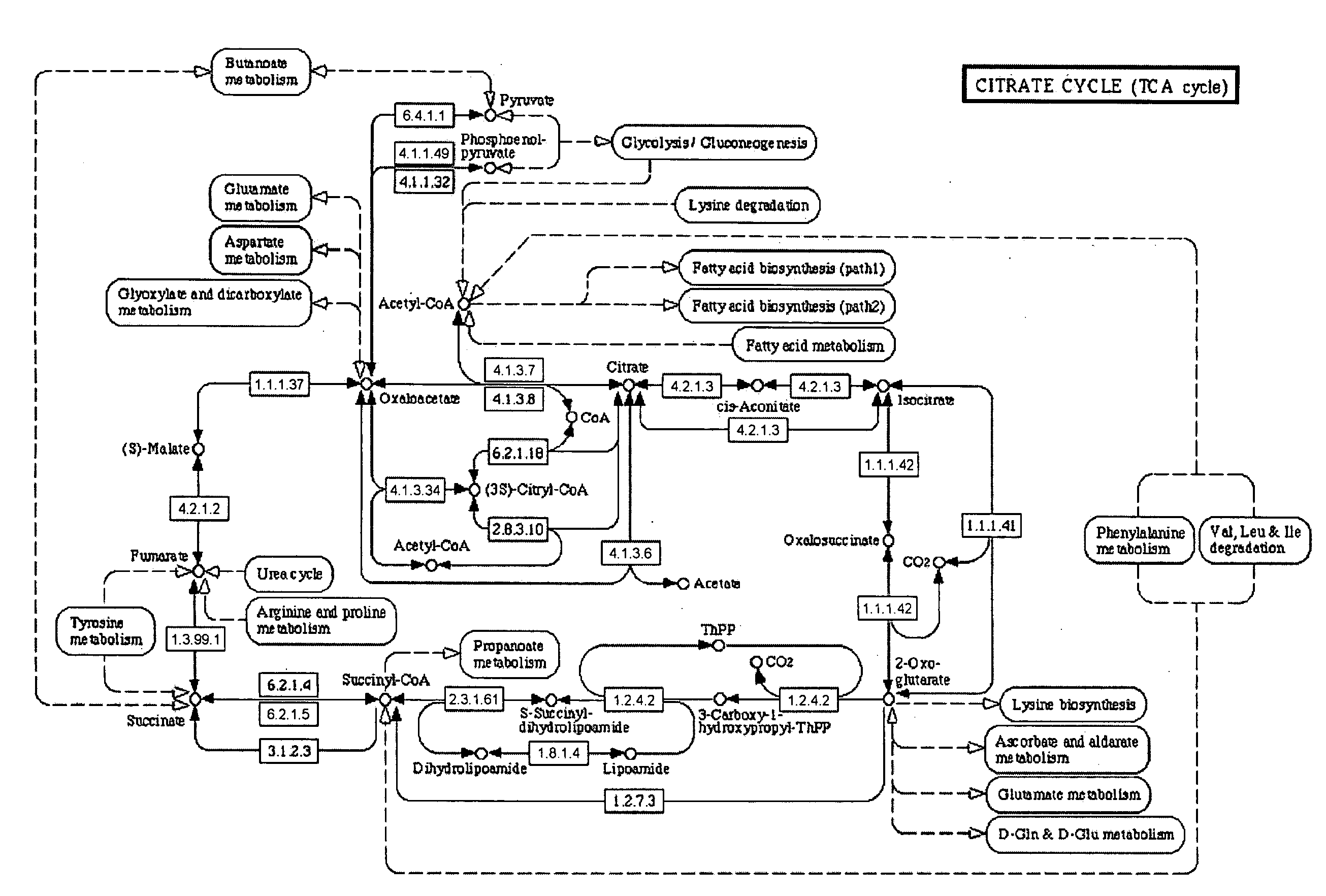
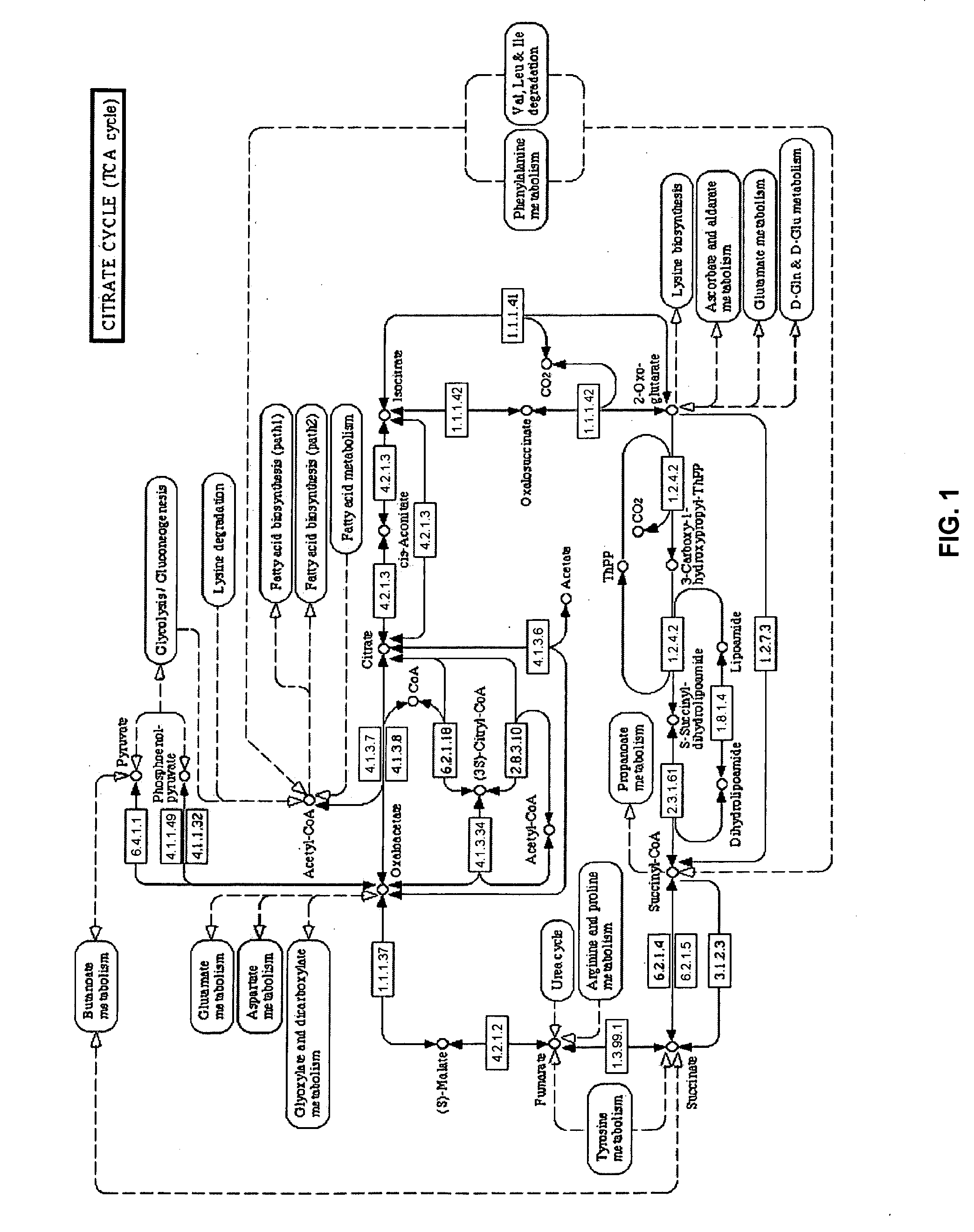
Aceglatone (Glucaron) is an antineoplastic drug available in Japan. It is an inhibitor of the enzyme β-glucuronidase.
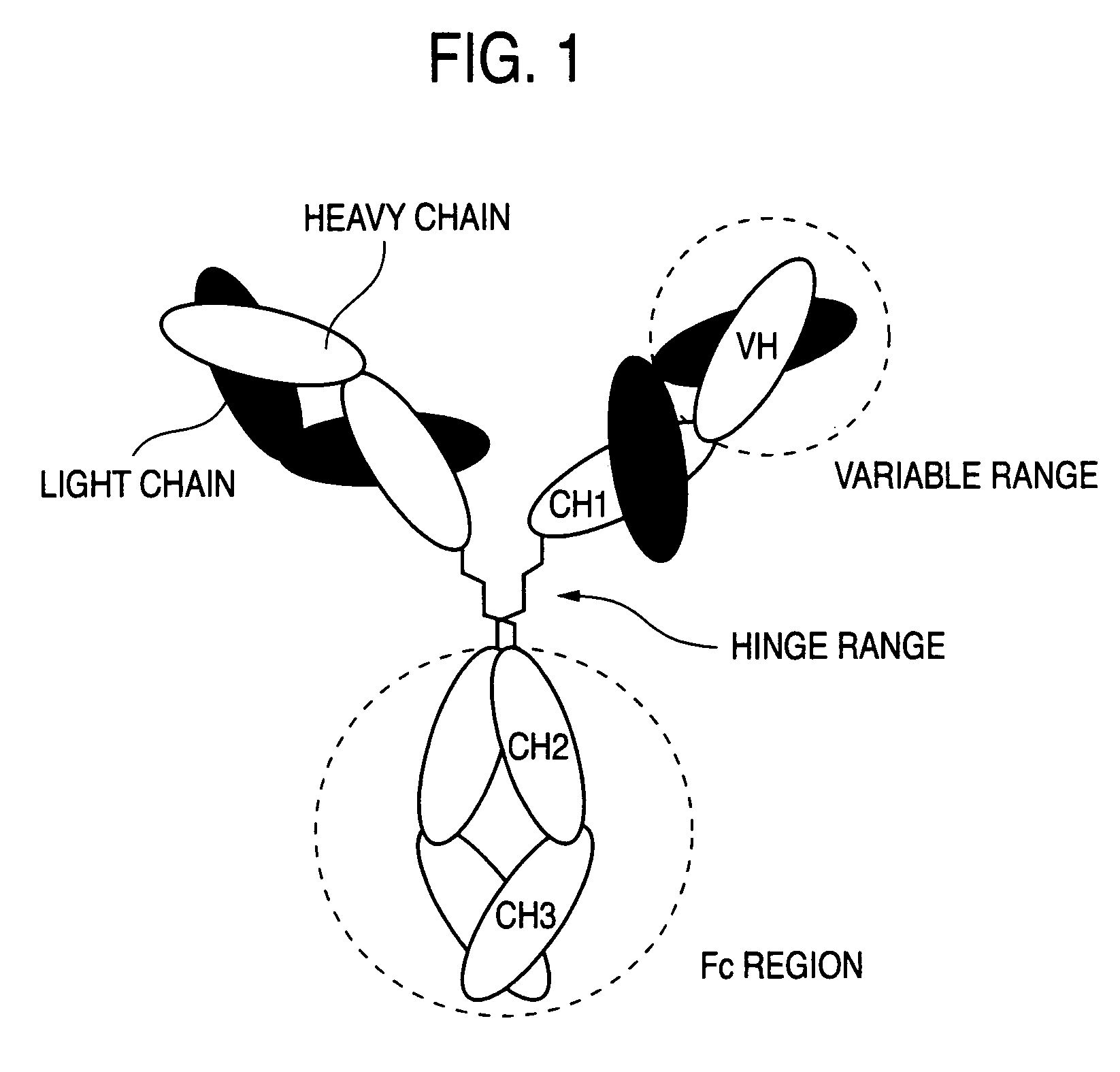
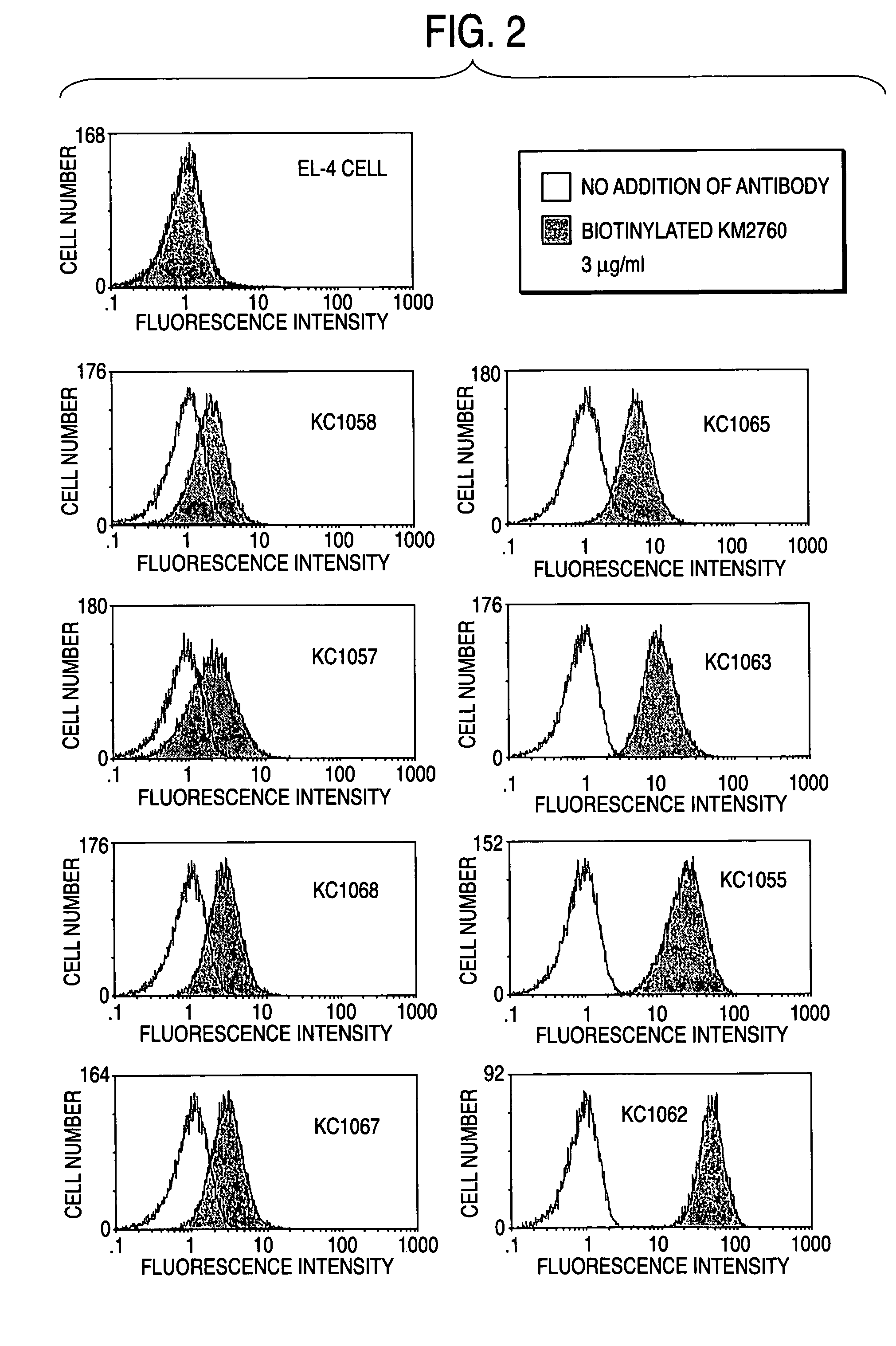
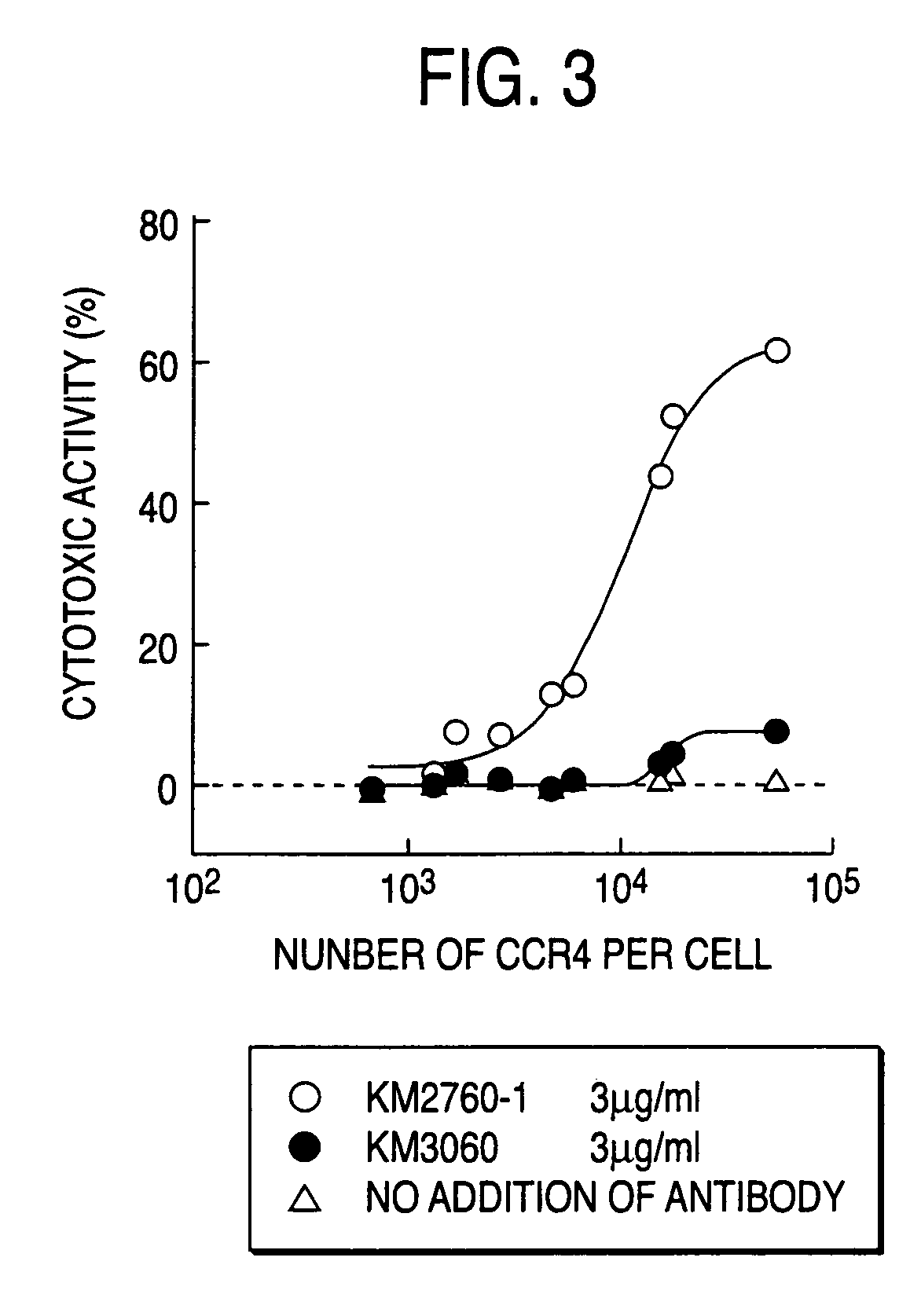
Method of enhancing of binding activity of antibody composition to Fcgamma receptor IIIa
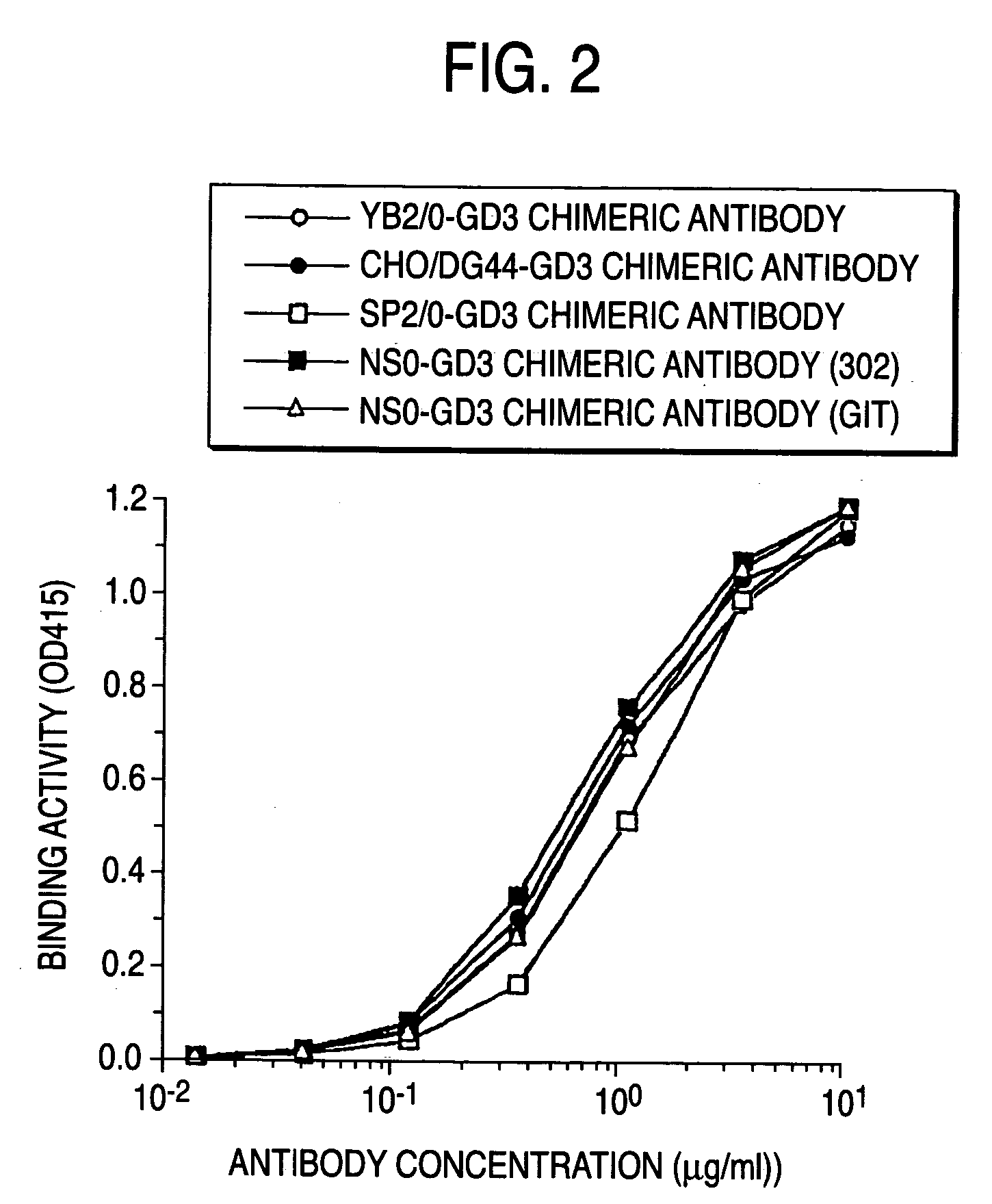
A method for enhancing a binding activity of an antibody composition to Fcgamma receptor IIIa, which comprises modifying a complex N-glycoside-linked sugar chain which is bound to the Fc region of an antibody molecule; a method for enhancing an antibody-dependent cell-mediated cytotoxic activity of an antibody composition; a process for producing an antibody composition having an enhanced binding activity to Fcgamma receptor IIIa; a method for detecting the ratio of a sugar chain in which fucose is not bound to N-acetylglucosamine in the reducing end in the sugar chain among total complex N-glycoside-linked sugar chains bound to the Fc region in an antibody composition; an Fc fusion protein composition produced by using a cell resistant to a lectin which recognizes a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through alpha-bond in a complex N-glycoside-linked sugar chain; and a process for producing the same.
Owner:KYOWA HAKKO KIRIN CO LTD
Structural modification of 19-norprogesterone I: 17-α-substituted-11-β-substituted-4-aryl and 21-substituted 19-norpregnadienedione as new antiprogestational agents
The present invention relates, inter alia, to compounds having the general formula: in which: R1 is a member selected from the group consisting of —OCH3, —SCH3, —N(CH3)2, —NHCH3, —NC4H8, —NC5H10, —NC4H8O, —CHO, —CH(OH)CH3, —C(O)CH3, —O(CH2)2N(CH3)2, and —O(CH2)2NC5H10; R2 is a member selected from the group consisting of hydrogen, halogen, alkyl, acyl, hydroxy, alkoxy (e.g., methoxy, ethoxy, vinyloxy, ethynyloxy, cyclopropyloxy, etc.), acyloxy (e.g., acetoxy, glycinate, etc.), alkylcarbonate, cypionyloxy, S-alkyl, —SCN, S-acyl and —OC(O)R6, wherein R6 is a functional group including, but not limited to, alkyl (e.g., methyl, ethyl, etc.), alkoxy ester (e.g., —CH2OCH3) and alkoxy (—OCH3); R3 is a member selected from the group consisting of alkyl, hydroxy, alkoxy and acyloxy; R4 is a member selected from the group consisting of hydrogen and alkyl; and X is a member selected from the group consisting of ═O and ═N—OR5, wherein R5 is a member selected from the group consisting of hydrogen and alkyl.In addition to providing the compounds of Formula I, the present invention provides methods wherein the compounds of Formula I are advantageously used, inter alia, to antagonize endogenous progesterone; to induce menses; to treat endometriosis; to treat dysmenorrhea; to treat endocrine hormone-dependent tumors; to treat meningiomas; to treat uterine leiomyomas; to treat uterine fibroids; to inhibit uterine endometrial proliferation; to induce cervical ripening; to induce labor; and for contraception.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF
Use of phosphoketolase for producing useful metabolites
Owner:AJINOMOTO CO INC
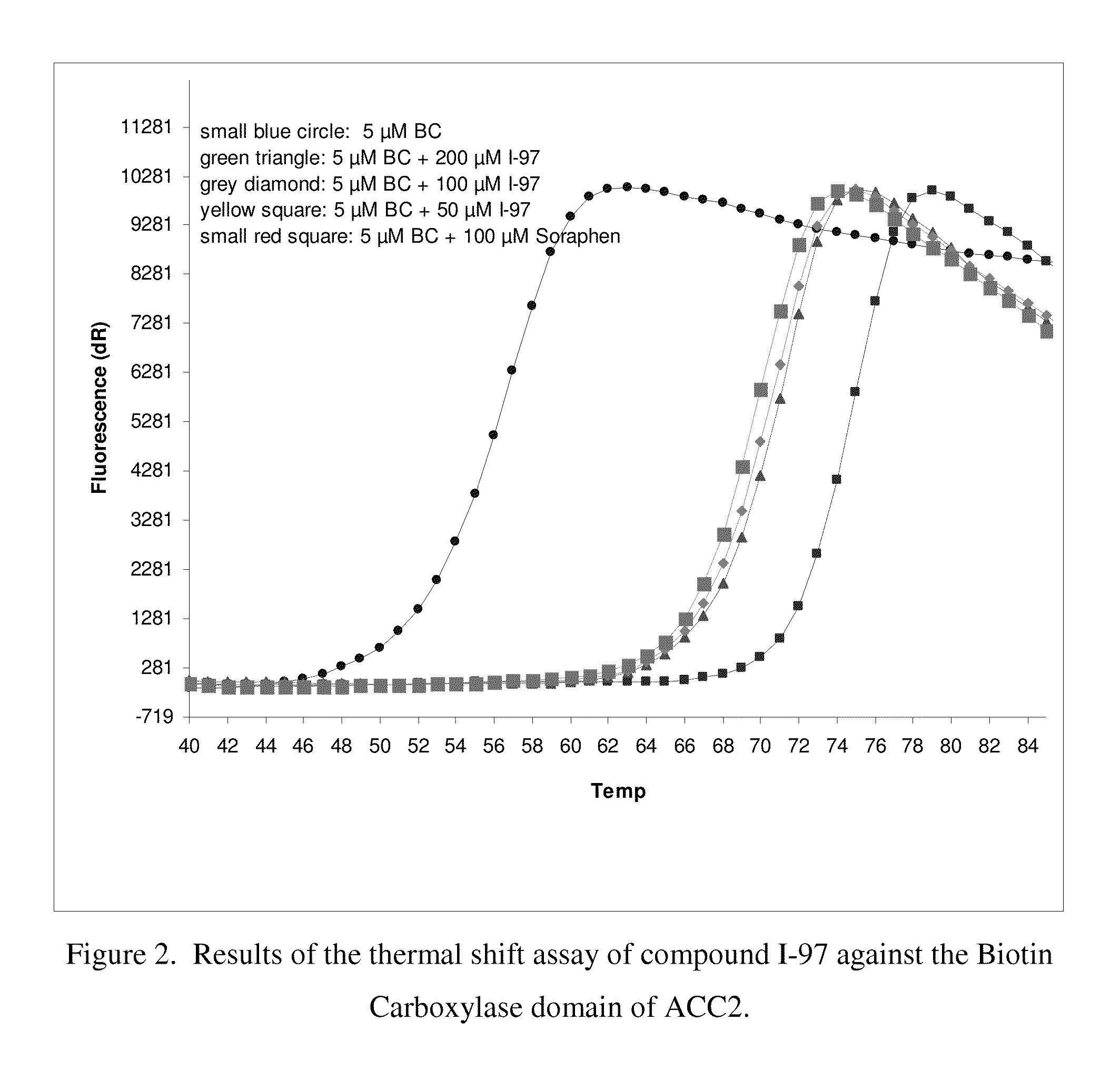
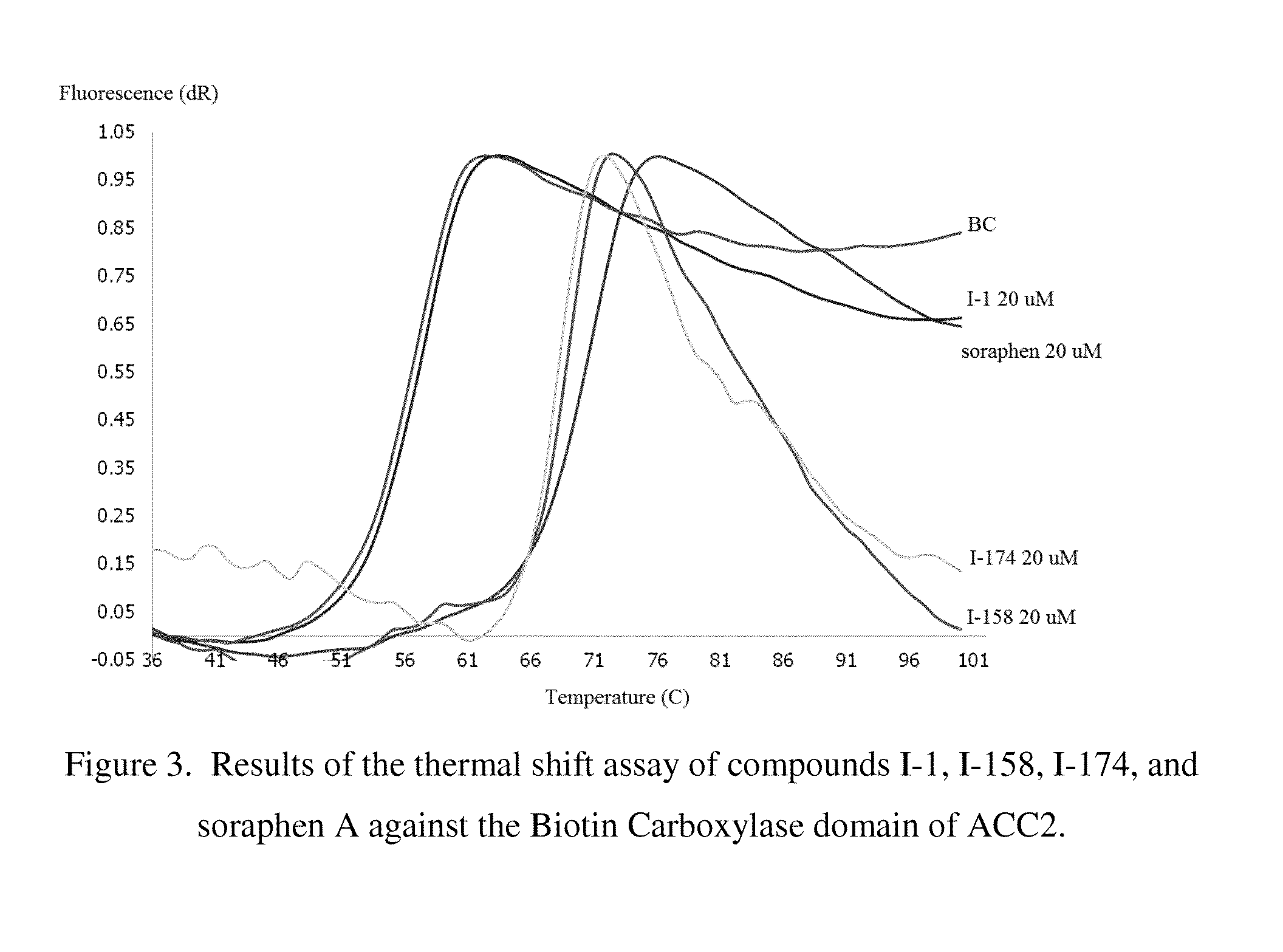
Acetyl-CoA carboxylase inhibitors
Acetyl Coenzyme A Carboxylase inhibitors, pharmaceutical compositions containing such compounds and the use of such compounds to treat for example, Metabolic Syndrome including atherosclerosis, diabetes and obesity.
Owner:PFIZER INC +1
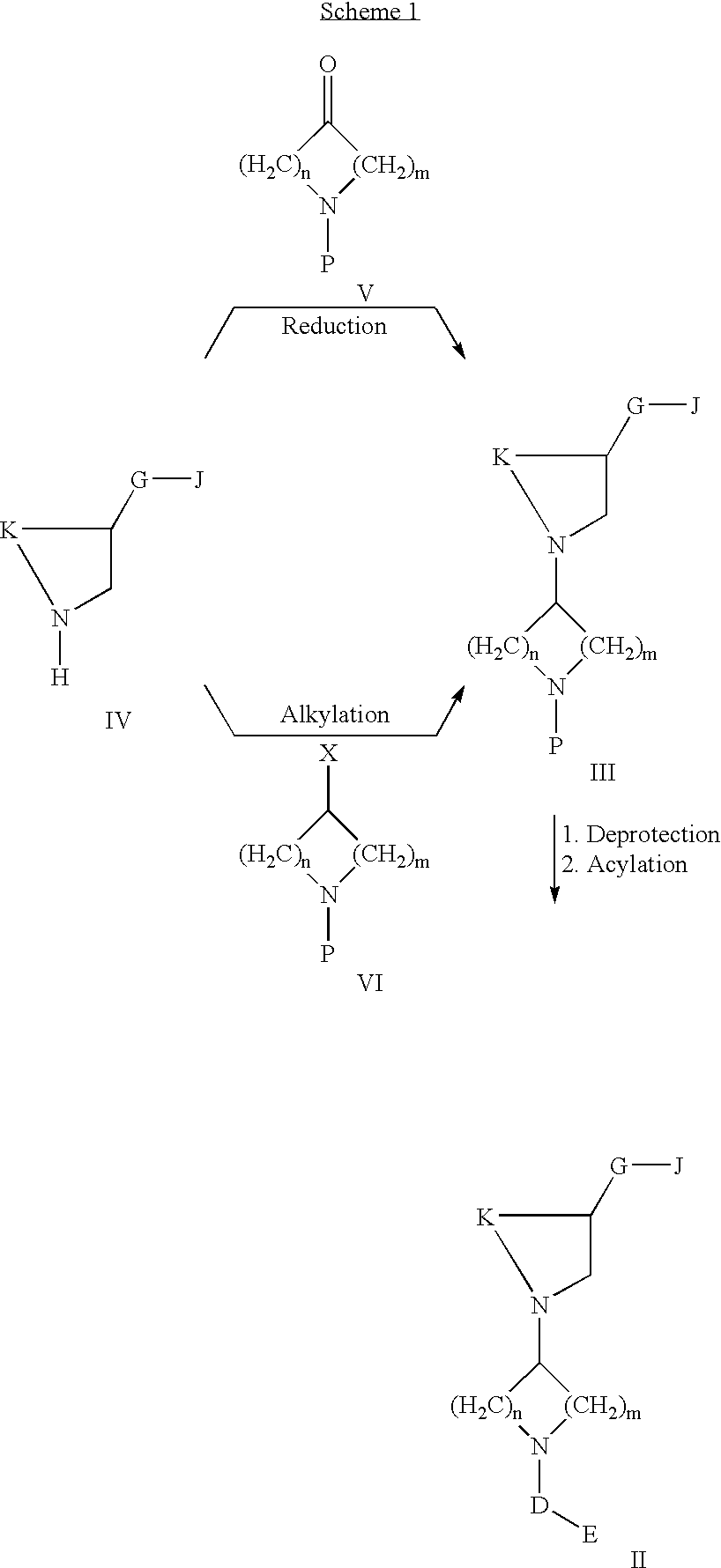
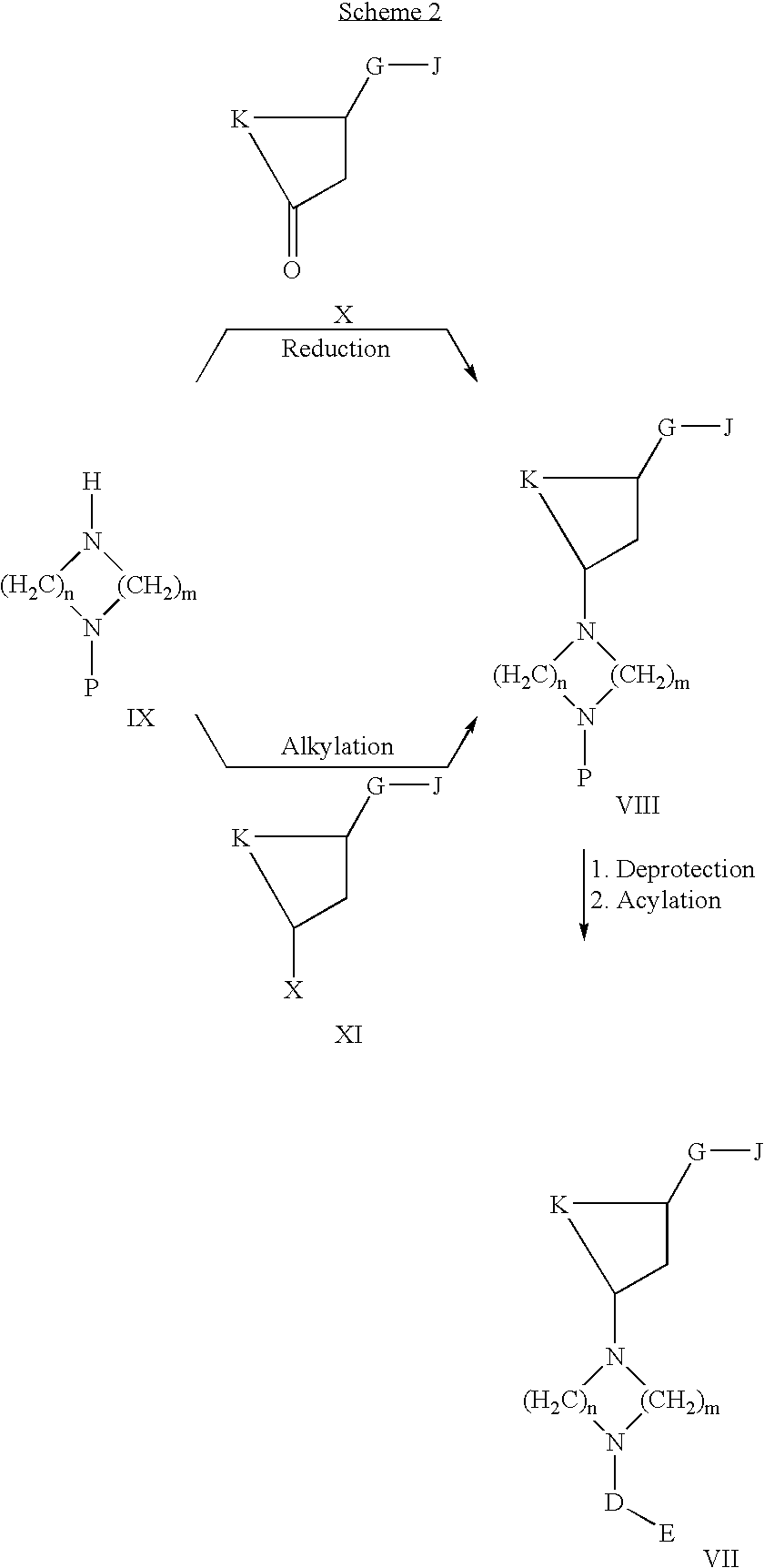
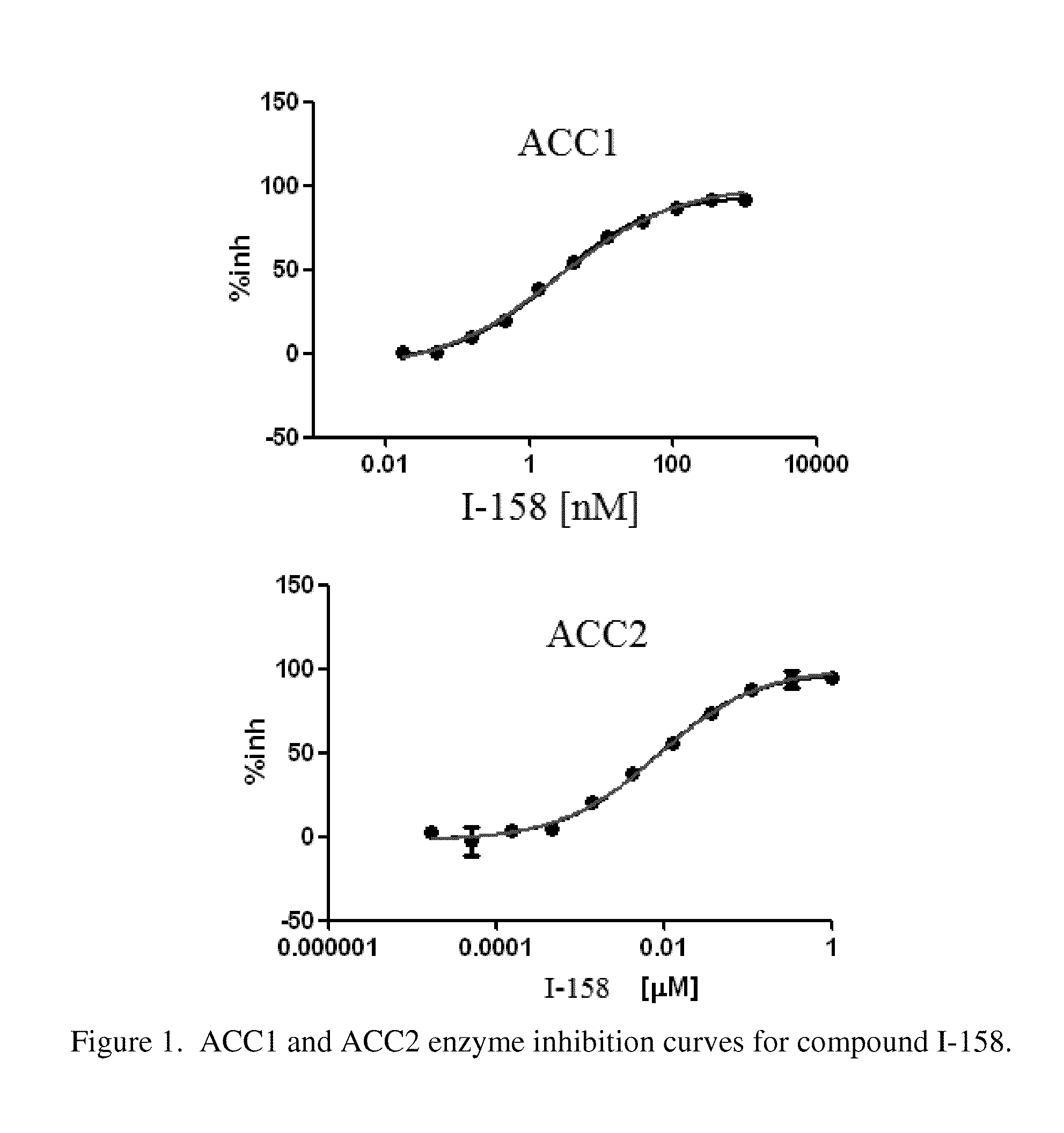
Acc inhibitors and uses thereof
The present invention provides compounds useful as inhibitors of Acetyl CoA Carboxylase (ACC), compositions thereof, and methods of using the same.
Owner:NIMBUS DISCOVERY INC +1
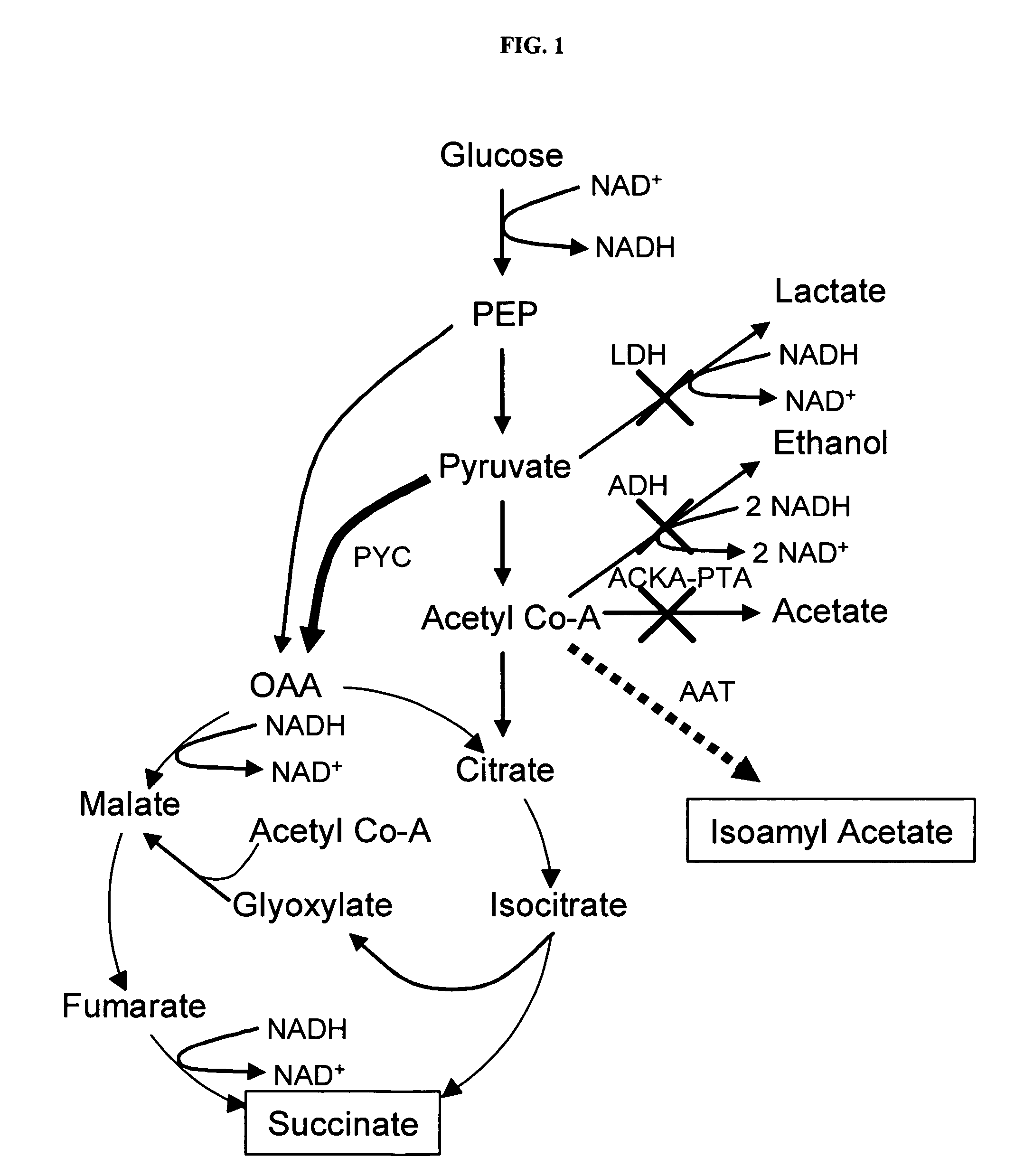
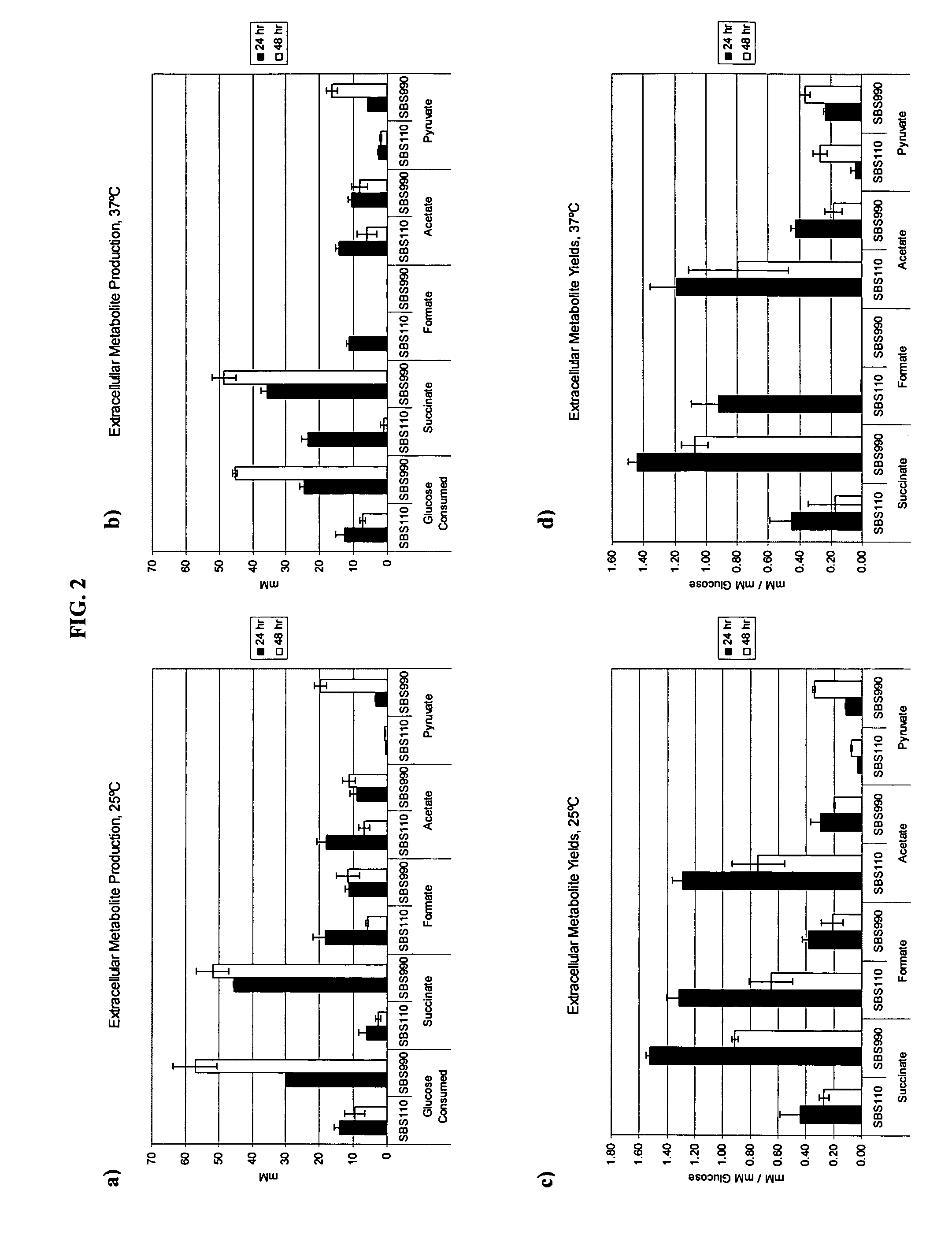
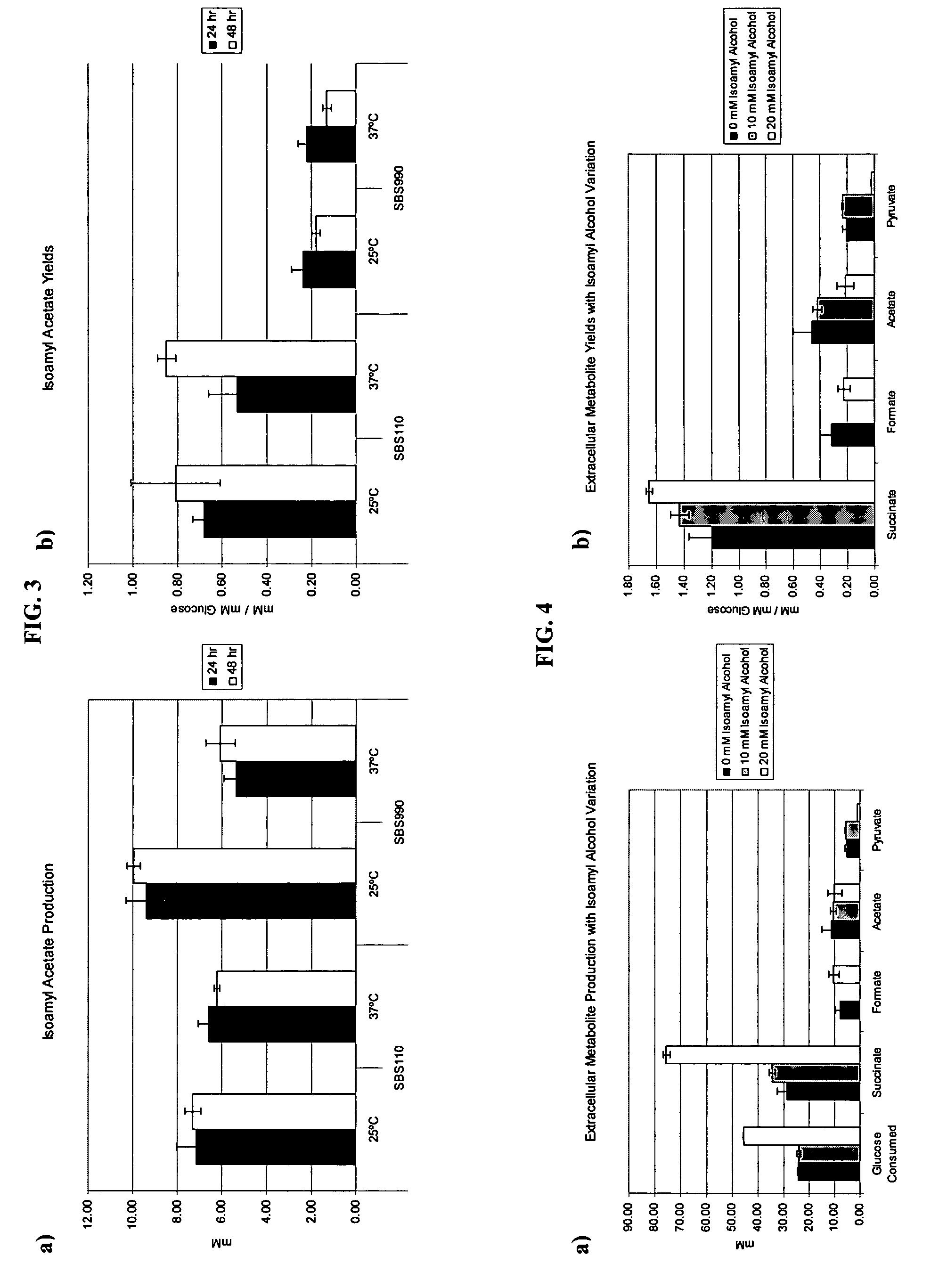
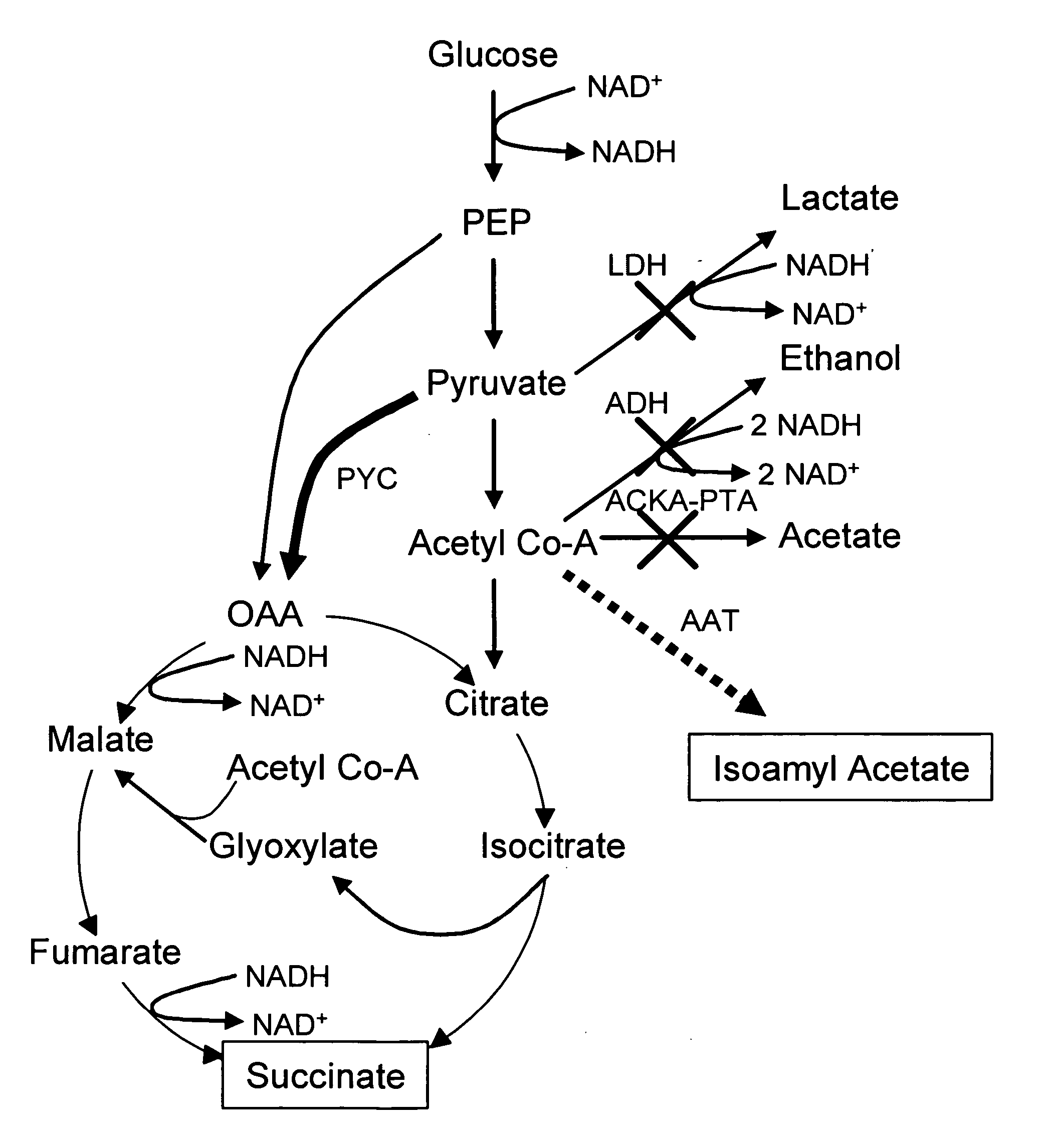
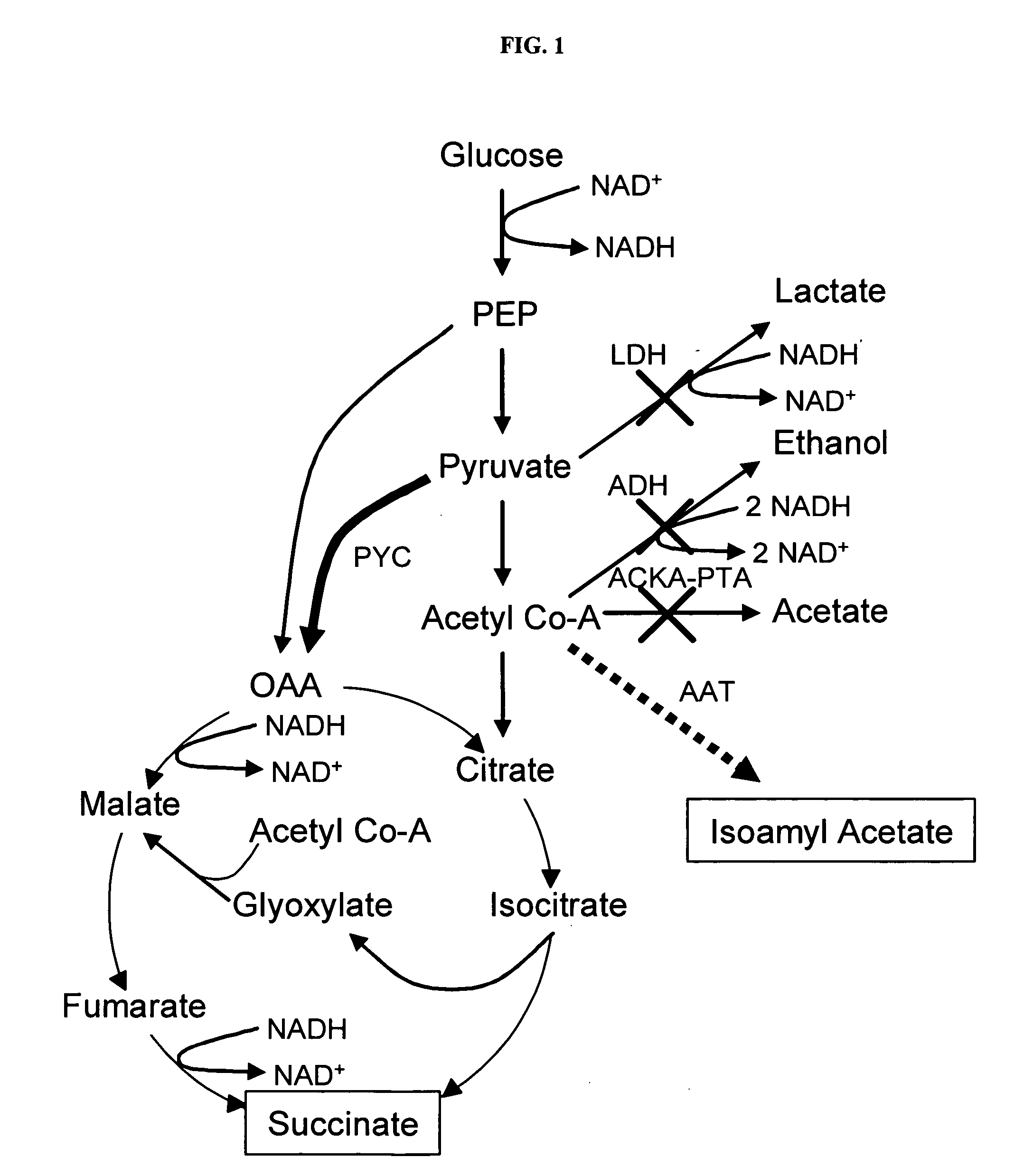
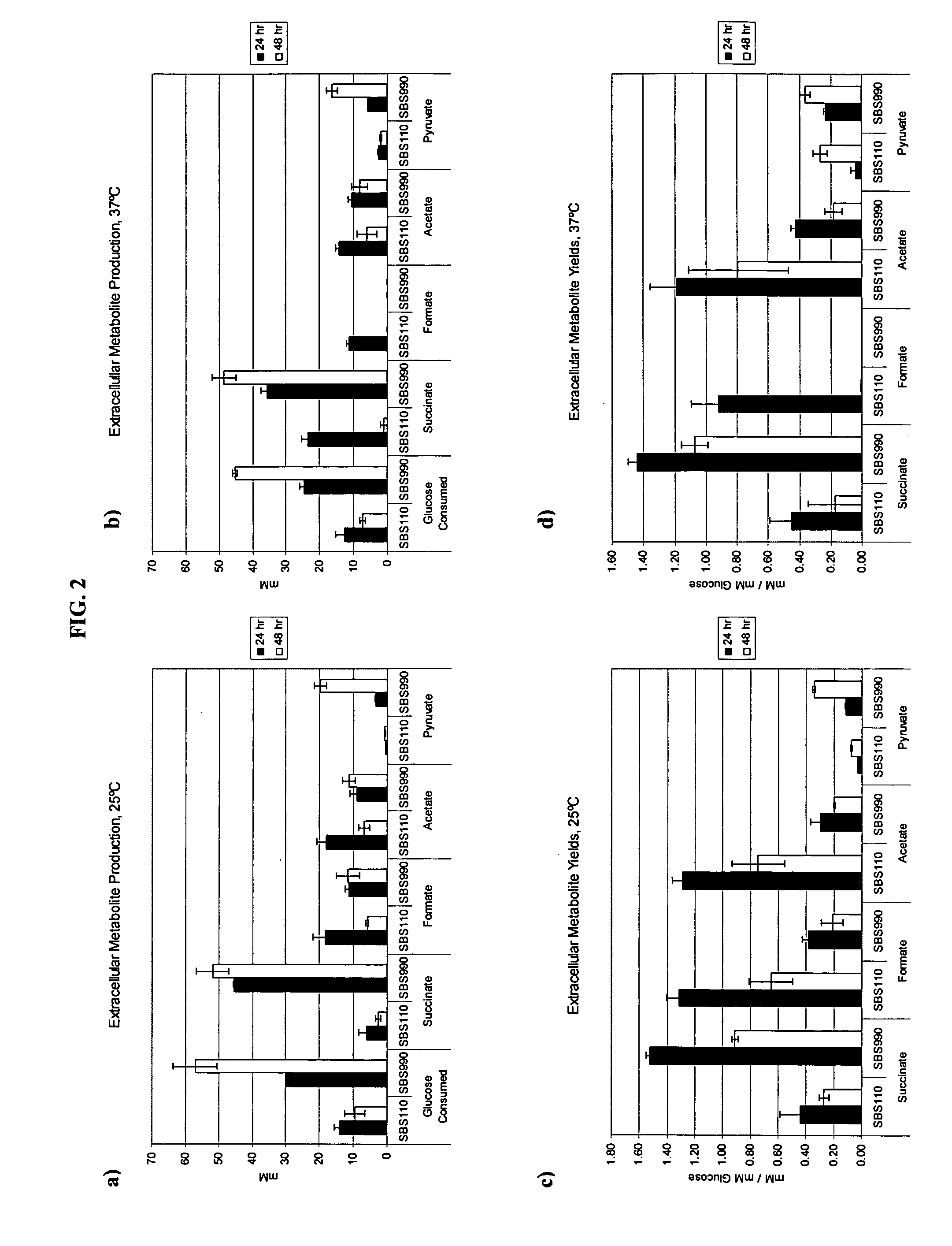
Simultaneous anaerobic production of isoamyl acetate and succinic acid
In vivo method of producing esters from acetyle coA, such as isoamyl acetate and succinate, has been developed by producing null mutants in pathways that use acetyl coA and by overexpressing products that use NADH and in order to maintain the proper redox balance between NADH and NAD+. The method is exemplified with null mutations in ldhA, adhE, ackA-pta and overexpression of pyruvate carboxylase and alcohol acetyltransferase. This strain produces higher levels of both isoamyl acetate and succinate.
Owner:RICE UNIV
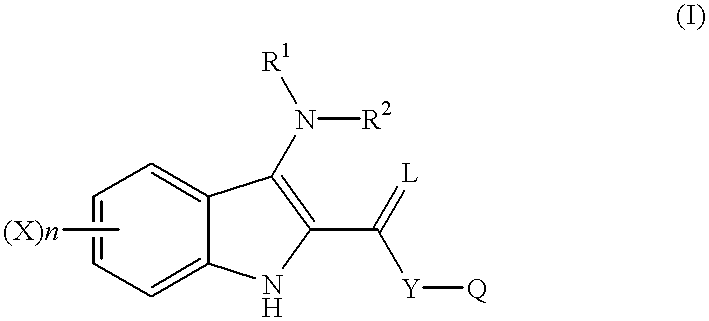
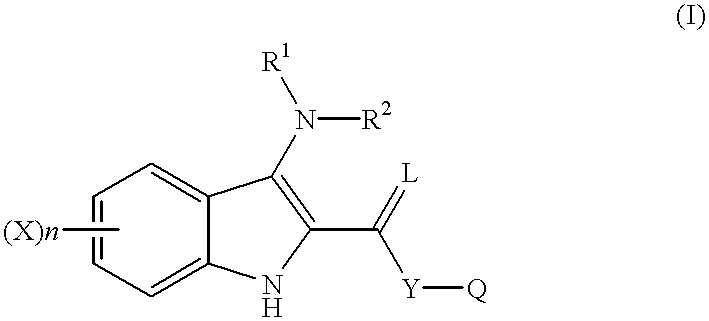
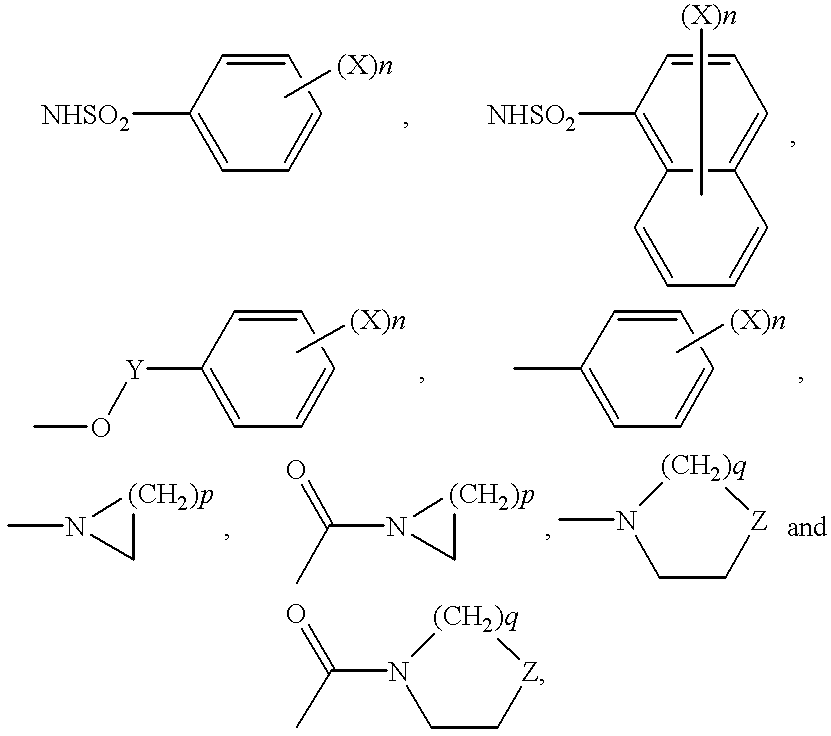
Indole compounds as COX-2 inhibitors
InactiveUS6300363B1Inhibit prostaniod-induced smooth muscle contractionInhibit synthesisBiocideOrganic chemistryDiseaseEthyl group
This invention provides a compound of the following formula:and the pharmaceutically acceptable salts thereof, wherein L is oxygen or sulfur; Y is a direct bond or C1-4 alkylidene; Q is C1-6 alkyl, C3-7 cycloalkyl, phenyl, naphthyl, heteroaryl or the like; R1 is hydrogen, C1-6 alkyl or the like; R2 is hydrogen, C1-4 alkyl, C(O)R5 wherein R5 is C1-22 alkyl or C2-22 alkenyl, halosubstituted C1-8 alkyl, halosubstituted C2-8alkenyl, -Y-C3-7 cycloalkyl, -Y-C3-7 cycloalkenyl, phenyl, naphthyl, heteroaryl or the like; X is halo, C1-4 alkyl, hydroxy, C1-4 alkoxy or the like; and n is 0, 1, 2 or 3, with the proviso that a group of formula -Y-Q is not methyl or ethyl when X is hydrogen; L is oxygen; R1 is hydrogen; and R2 is acetyl.This invention also provides a pharmaceutical composition useful for the treatment of a medical condition in which prostaglandins are implicated as pathogens.
Owner:PFIZER INC +1
Simultaneous anaerobic production of isoamyl acetate and succinic acid
InactiveUS20060141594A1Maximizing isoamyl acetate productionEasy to separateBacteriaTransferasesSuccinic acidPyruvate synthesis
In vivo method of producing esters from acetyle coA, such as isoamyl acetate and succinate, has been developed by producing null mutants in pathways that use acetyl coA and by overexpressing products that use NADH and in order to maintain the proper redox balance between NADH and NAD+. The method is exemplified with null mutations in ldhA, adhE, ackA-pta and overexpression of pyruvate carboxylase and alcohol acetyltransferase. This strain produces higher levels of both isoamyl acetate and succinate.
Owner:RICE UNIV
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
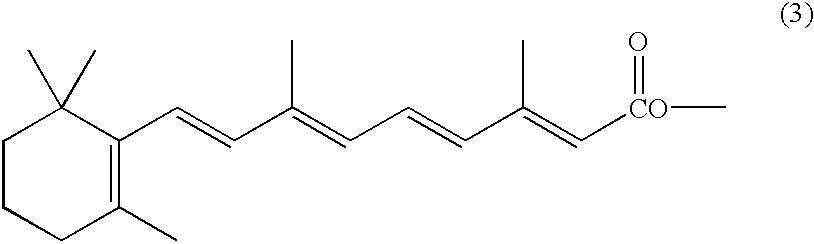
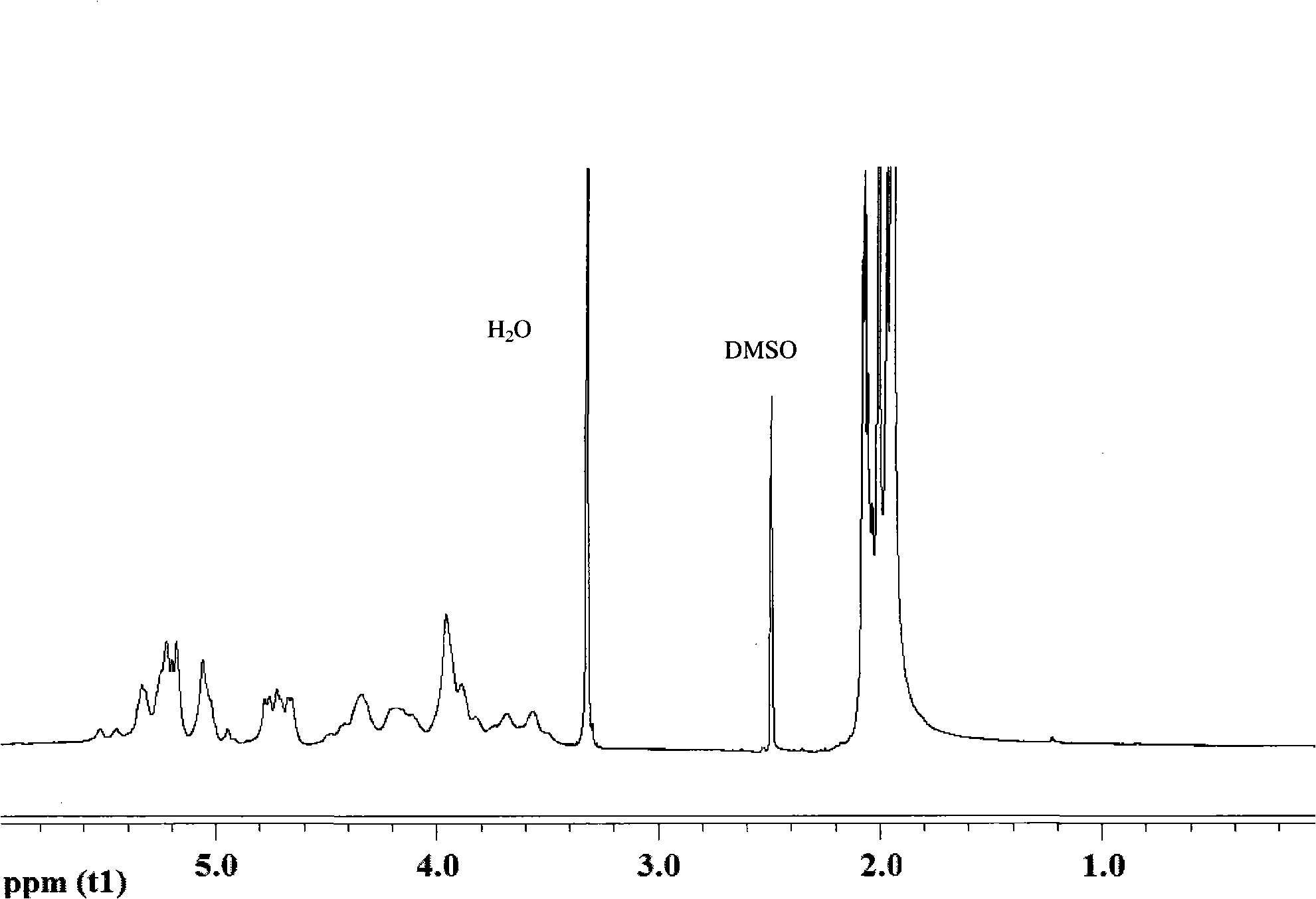
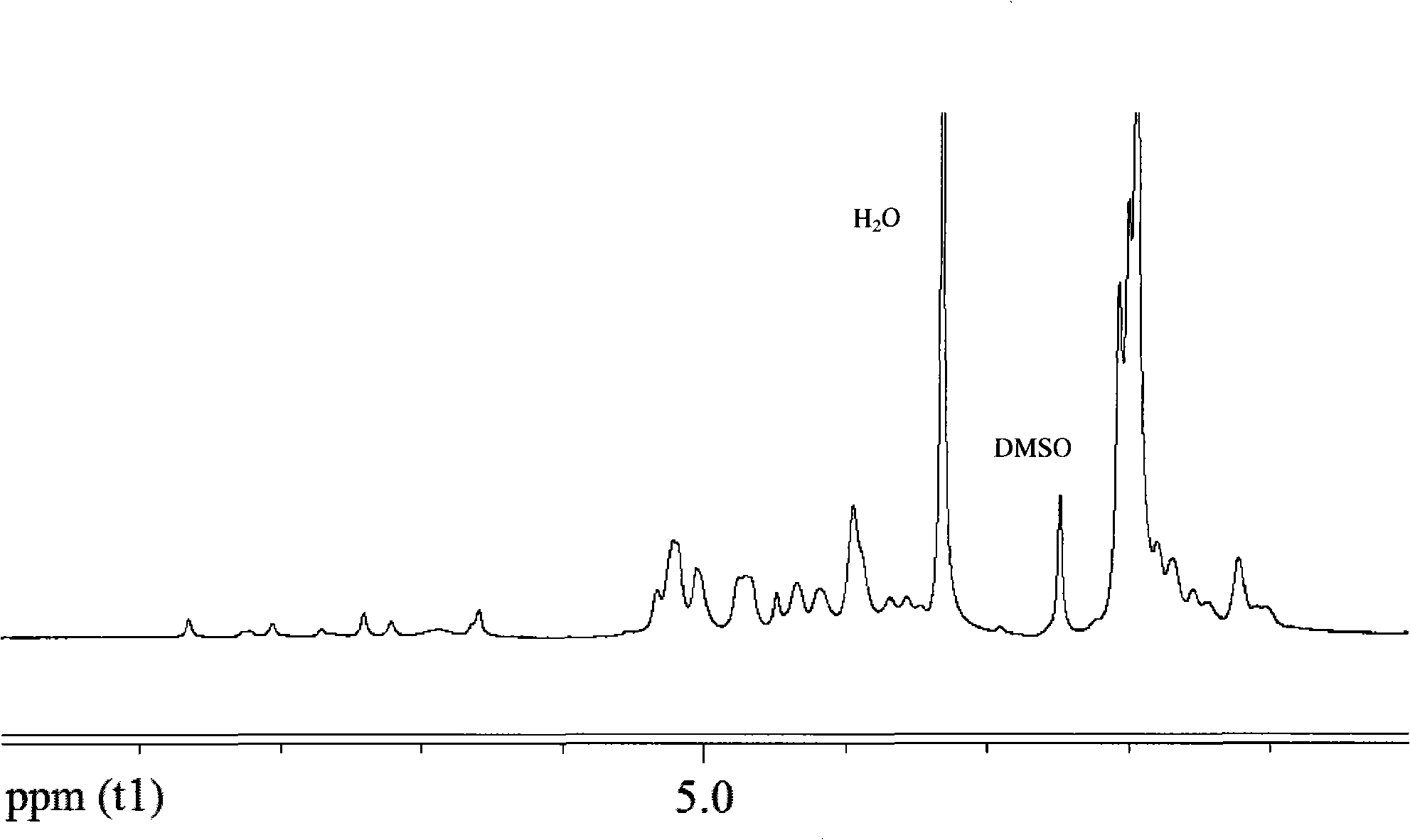
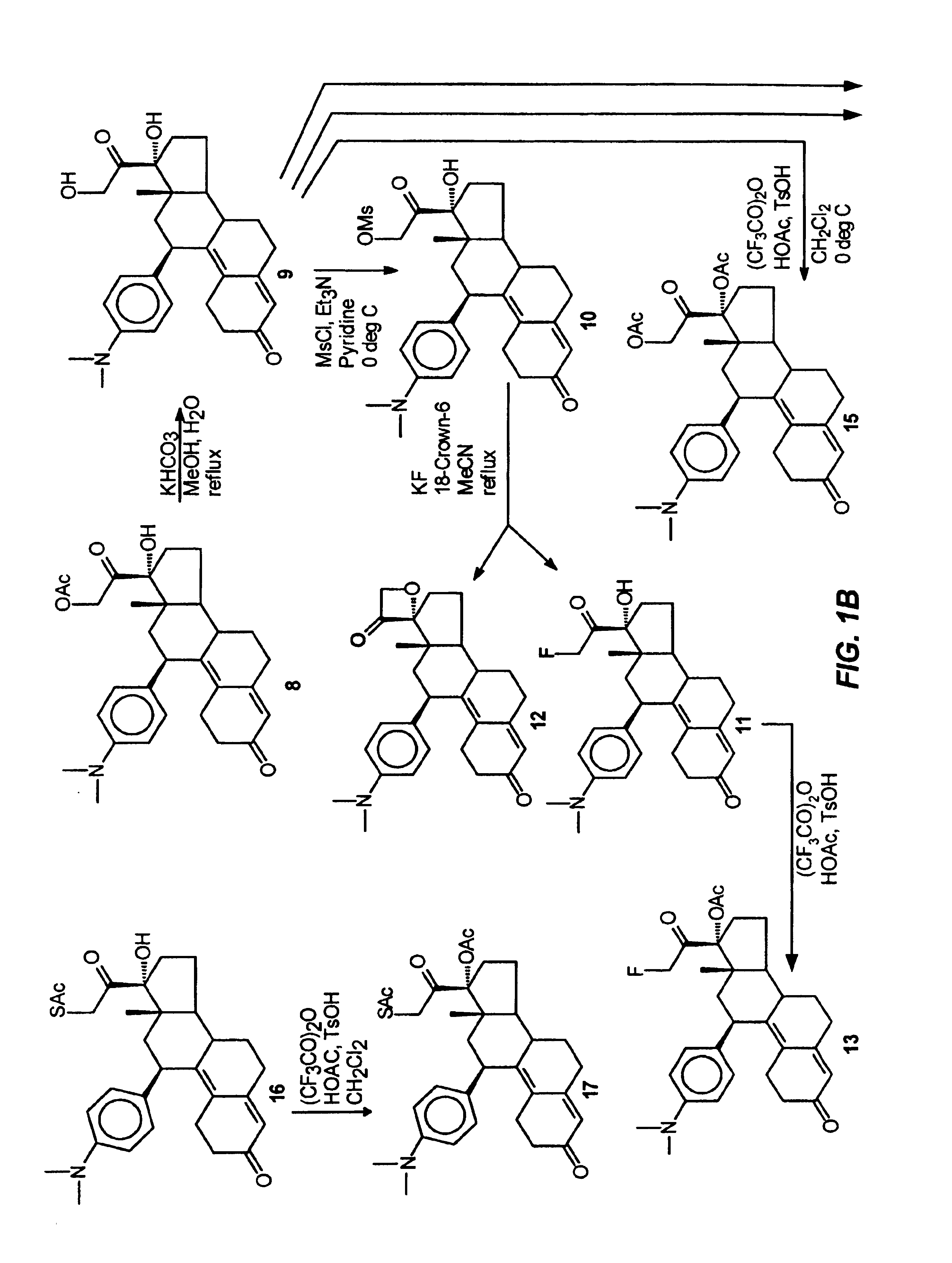
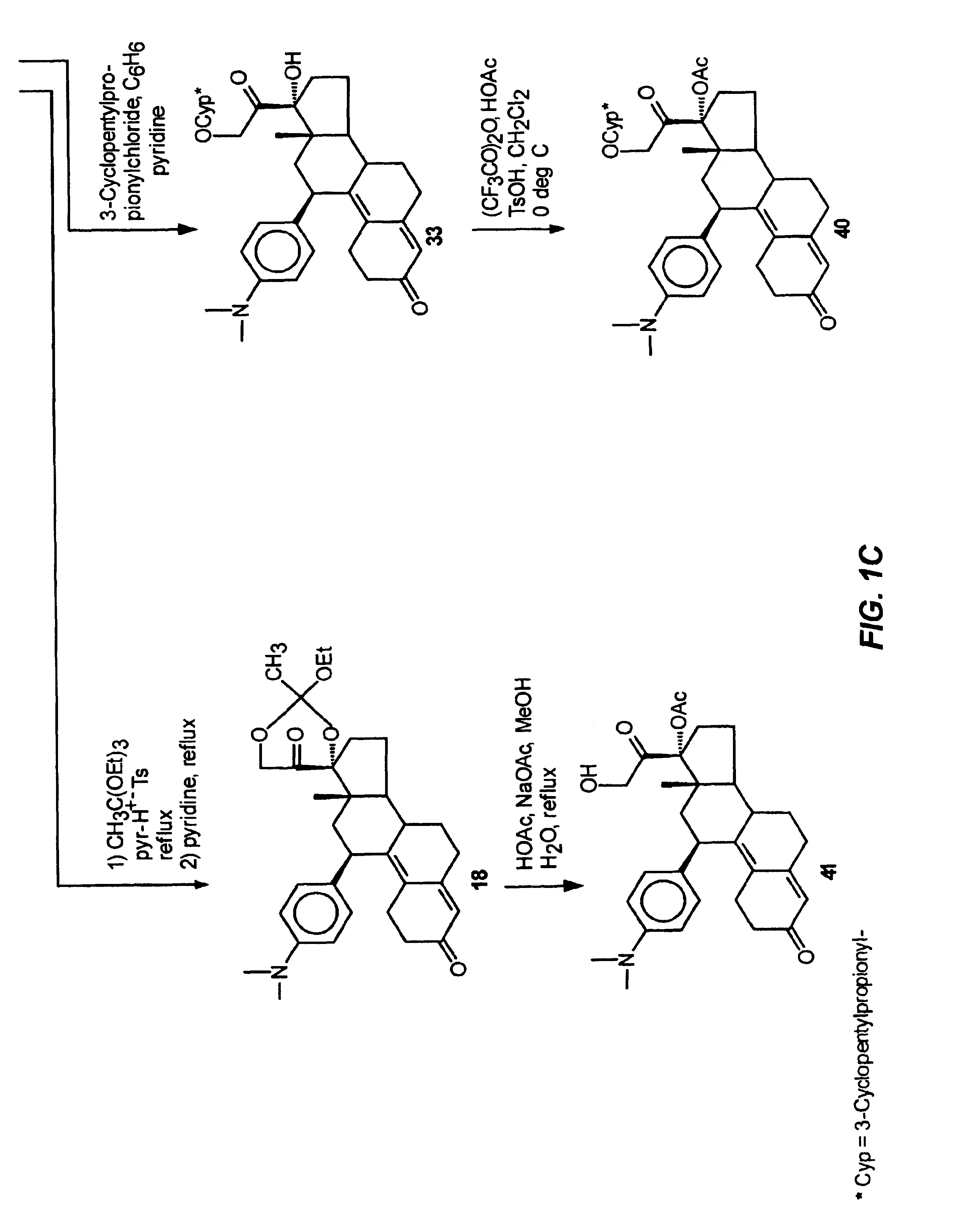
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Process for producing antibody composition by using rna inhibiting the function of alpha1,6-fucosyltransferase
InactiveUS20070134759A1High effector functionHighly functionalFungiSugar derivativesGlycosideAntiendomysial antibodies
The present invention provides a process for producing an antibody composition using a cell, which comprises using a cell into which an RNA having activity of suppressing the function of an enzyme relating to the modification of a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through α-bond in a complex type N-glycoside-linked sugar chain is introduced; the RNA used in the production process; a DNA corresponding to the RNA; a cell in which the RNA or DNA is introduced or expressed; a process for producing the cell; and a method for suppressing the enzyme.
Owner:KYOWA HAKKO KIRIN CO LTD
N1-pyrazolospiroketone acetyl-CoA carboxylase inhibitors
The invention provides a compound of Formula (I) Z N N O N O A1R2 R1 R3R 3 L A2 (I) or a pharmaceutically acceptable salt of the compound, wherein R1, R2, R3,Z, A1, L and A 5 2 are as described herein; pharmaceutical compositions thereof; and the use thereof in treating diseases, conditions or disorders modulated by the inhibition of an acetyl-CoA carboxylase enzyme(s) in an animal.
Owner:PFIZER INC
O-linked glycosylation using n-acetylglucosaminyl transferases
InactiveUS20110177029A1Time and cost-efficient production routePeptide/protein ingredientsAntibody mimetics/scaffoldsTransferaseWater soluble polymers
The present invention provides covalent conjugates between a polypeptide and a modifying group, such as a water-soluble polymer (e.g., PEG). The amino acid sequence of the polypeptide includes one or more O-linked glycosylation sequence, each being a substrate for a GIcNAc transferase. The modifying group is covalently linked to the polypeptide via a glycosyl-linking group interposed between and covalently linked to both the polypeptide and the modifying group. In one embodiment, a glucosamine linking group is directly attached to an amino acid residue of the O-linked glycosylation sequence. The invention further provides methods of making polypeptide conjugates. The present invention also provides non-naturally occurring polypeptides that include at least one O-linked linked glycosylation sequence of the invention, wherein each glycosylation sequence is a substrate for a GIcNAc transferase. The invention further provides pharmaceutical compositions that include a polypeptide conjugate of the invention.
Owner:NOVO NORDISK AS
Application of chitosanoligosaccharide and derivatives thereof in plant cold resisting
InactiveCN102771476AWide variety of sourcesLow pricePlant growth regulatorsBiocideGuanidine derivativesO-Phosphoric Acid
The invention discloses a wide-spectrum plant cold-resisting agent for plants such as food crops, economic crops, vegetables, fruit trees, economic trees, flowers, and lawns. The active component of the agent is chitosanoligosaccharide or a derivative thereof. The chitosanoligosaccharide is oligosaccharide of glucosamine linked through beta-1,4-glucosidic bond, and has a molecular weight of 300-10000Da, and a deacetylation degree of 50-100%. The chitosanoligosaccharide derivatives are chitosanoligosaccharide sulfuric acid derivative, chitosanoligosaccharide hydrochloric acid derivative, chitosanoligosaccharide phosphoric acid derivative, chitosanoligosaccharide sulfonamide derivative, chitosanoligosaccharide acyl isothiocyanate derivative, chitosanoligosaccharide phosphoric derivative, chitosanoligosaccharide guanidine derivative, chitosanoligosaccharide nicotinicacyl isothiocyanate derivative, chitosanoligosaccharide-cerium (IV) complex, and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Composition of amino acid
ActiveCN101049500AImprove metabolic disordersNutritional support therapy works wellOrganic active ingredientsDipeptide ingredientsNutrition supportTryptophan
An amino acid composition with high nutrition supporting effect is composed of acetylcysteine, acetyltyrosine and tryptophan in weight ratio of (0.81-2): (0.81-2): (0.9-1.6).
Owner:BEIJING SHIQIAO BIOPHAM
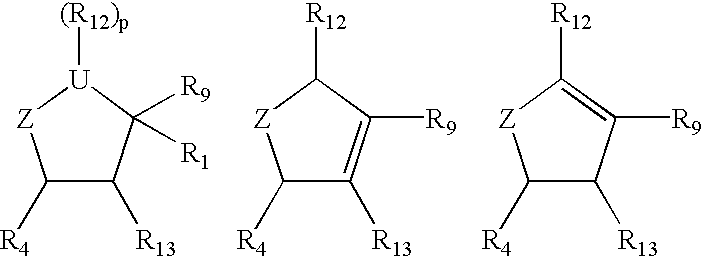
Substituted cyclopentane and cyclopentene compounds useful as neuraminidase inhibitors
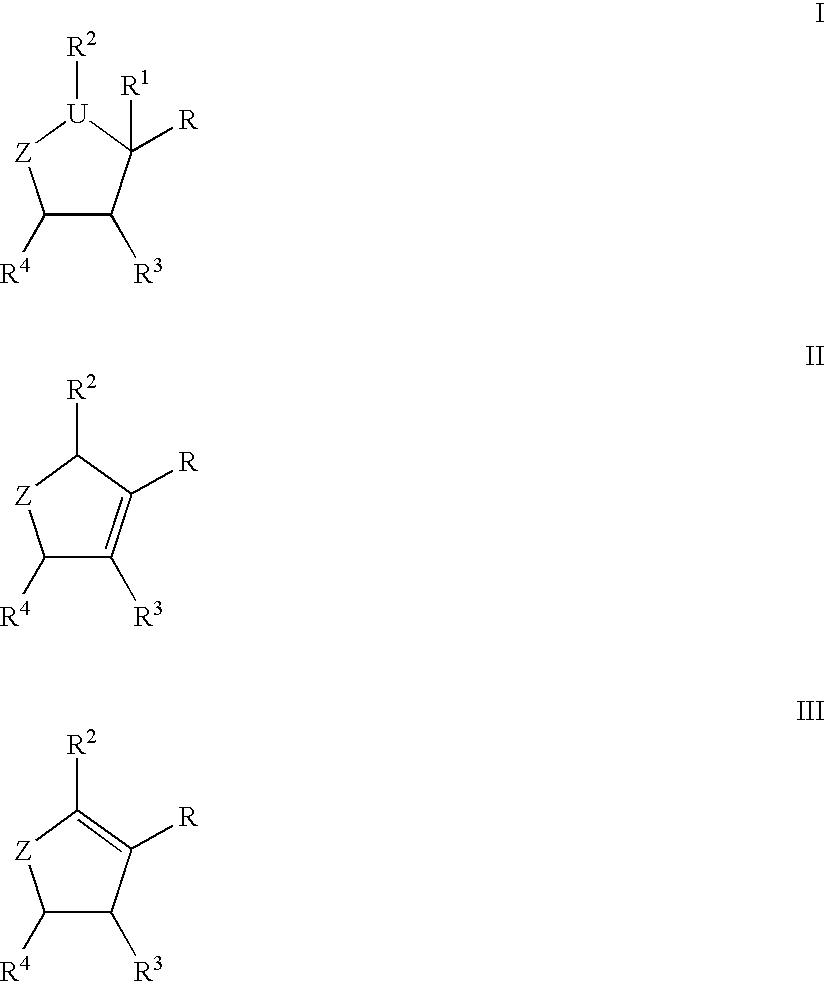
Compounds I-III wherein U is CH, O, or S; Z is mono- or di-substituted carbon; R is (CH2)nCO2H, (CH2)nSO3H, (CH2)nPO3H2, (CH2)nNO2, CH(SCH3)3, esters; R1 is H, hydroxyalkyl, aminoalkyl, alkoxyalkyl; RR1 is O; n is 0-4; R2, R3 is H, hydroxyalkyl, aminoalkyl, alkoxyalkyl, haloalkyl; R4 is (CH2)nOH, (CH2)nNH2, substituted alkyl were prepd. as neuraminidase inhibitors. Thus, (1R,3R,4R,1'S)-(-)-(1'-acetylamino-2 '-ethyl)butyl-4-(aminoimino)methylaminocyclopentan-1-carboxylic acid was prepd. and tested in vitro as neuraminidase inhibitor (IC50<1.mu.M).
Owner:BIOCRYST PHARM INC
Antibody composition-containing medicament
A medicament for treating a patient who cannot be cured with a medicament comprising as an active ingredient an antibody composition produced by a cell unresistant to a lectin which recognizes a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through α-bond in a complex N-glycoside-linked sugar chain, which comprises as an active ingredient an antibody composition produced by a cell resistant to a lectin which recognizes a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through α-bond in a complex N-glycoside-linked sugar chain, and a method for screening the patient by using the medicament.
Owner:KYOWA HAKKO KIRIN CO LTD
Noninvasive Measurement and Identification of Biomarkers in Disease State
ActiveUS20090104596A1Quick measurementQuickly and reliably and inexpensively identify disease stateMicrobiological testing/measurementDisease diagnosisArginineTyrosine
The invention is methods and related kits for diagnosing a disease state of cachexia by measuring biomarker profiles from a biological sample. Rapid measurement of early onset or progression of the disease in a subject is determined by measuring biomarker levels from the subject and optionally comparing the biomarker levels to a standard biomarker profile or metabolome phase portrait for the disease. The biomarkers measured in the assay and related kit for cachexia progression include biomarkers selected from the group consisting of lactate, citrate, formate, acetoacetate, 3-hydroxy butrate, alanine, glutamine, glutamate, valine, isoleucine leucine, thrionine, lysine, arginine, tyrosine, phenyl alanine, histidine and tryptophan.
Owner:WISCONSIN ALUMNI RES FOUND
Nutrient compositions and methods for enhanced effectiveness of the immune system
InactiveUS20050142124A1Reduce the burden onTimelyOrganic active ingredientsAntimycoticsDiseaseBeta-Carotene
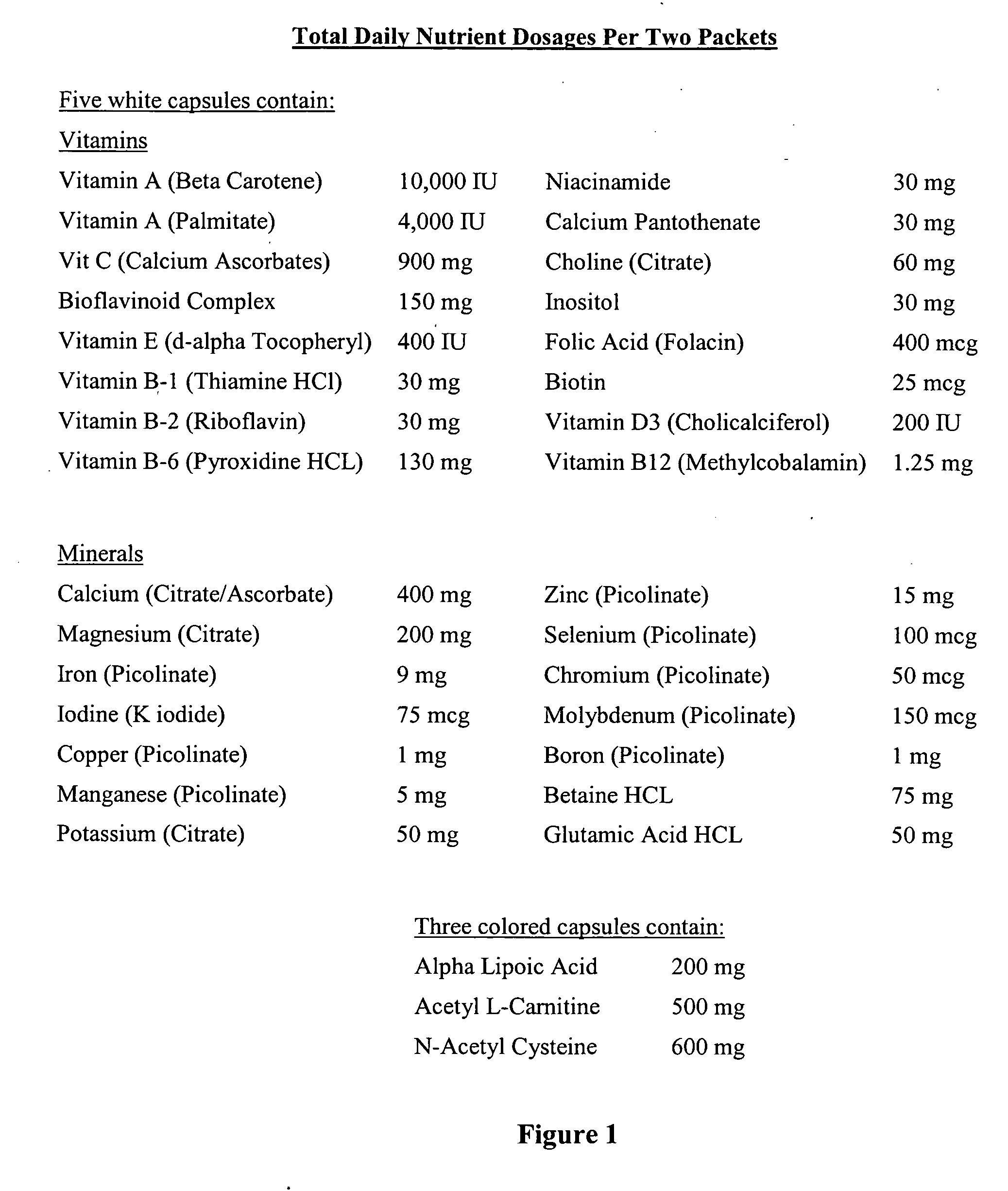
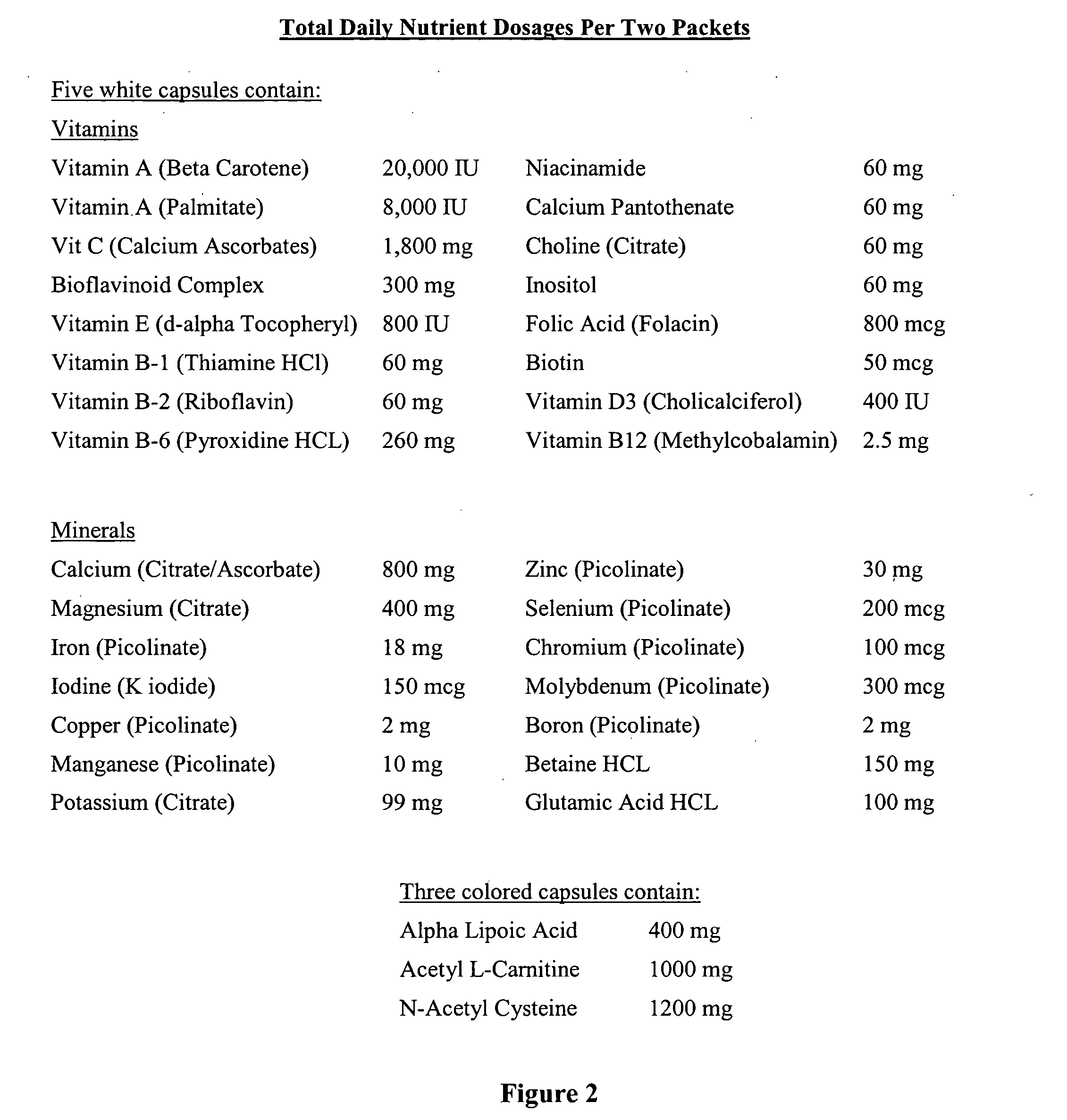
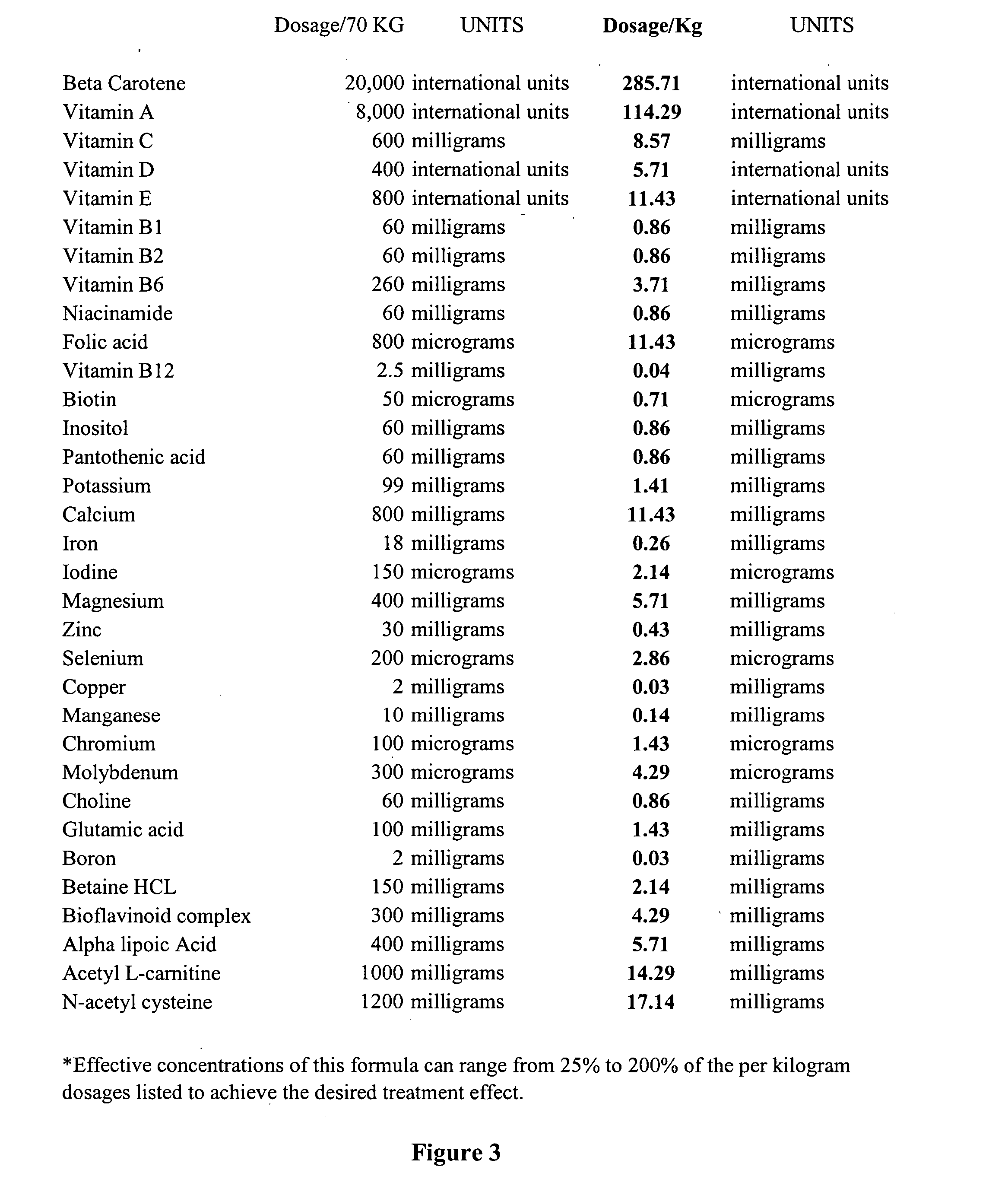
The invention provides a nutrient composition for augmenting immune strength or physiological detoxification. The nutrient composition consists of an optimal combination of an effective amount of at least one vitamin antioxidant, at least one mineral antioxidant and a highly saturable amount of at least three high potency antioxidants. The at least one vitamin antioxidant can be vitamin C, bioflavonoid complex, vitamin E, vitamin B6 or beta-carotene and the at least one mineral antioxidant can be zinc or selenium. The at least three high potency antioxidants can be alpha lipoic acid, acetyl L-carnitine, N-acetyl-cysteine, co-enzyme Q10 or glutathione. Also provided is a nutrient composition for augmenting immune strength or physiological detoxification that consists of an optimal combination of an effective amount of at least three vitamin antioxidants, at least two mineral antioxidants and a highly saturable amount of at least three high potency antioxidants. Further provided is a method of stimulating immune system function or a method of augmenting a therapeutic treatment of a disease. The method consists of administering to an individual a nutrient composition of the invention one or more times a day over a period of about 5-7 weeks, the immune system function being stimulated to result in an increase of CD4+ cells of at least about 15% compared to pre-administration levels. A method of stimulating a physiological detoxification function of an individual or a method of augmenting a therapeutic treatment of a disease is also provided. The method consists of administering to an individual a nutrient composition of the invention one or more times a day over a period of about 5-7 weeks, the physiological detoxification function being stimulated to result in a decrease of one or more free radical markers by about 20% compared to pre-administration levels.
Owner:INTEGRATIVE HEALTH CONSULTING INC
Novel acetyloxymethyl esters and methods for using the same
Novel acetyloxymethyl esters are disclosed. Methods of treating an illness, including cancer, hemological disorders and inherited metabolic disorders, and treating or ameliorating other conditions using these compounds are also disclosed. The compounds are effective in the inhibition of histone deacetylase.
Owner:ERRANT GENE THERAPEUTICS
Betaine compositions
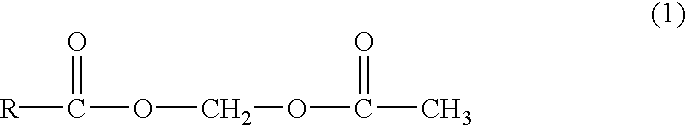
The invention refers to the pharmaceutical combination including at least: a first compound selected among the group consisting of acetylsalicylic acid, salicylic acid, pharmaceutical derivatives thereof, and a second compound selected from the group consisting of lipidic betaines, betaines lipids, betaines of Formula (CH3)3N+(CH2)nCOO− with n an integer from 1 to 5, pharmaceutically acceptable salts thereof, esters thereof, precursors thereof, and mixtures thereof, with the proviso that the second compound is different from the first compound and in an amount by weight at least three times the amount of first compound.
Owner:BIO ETHIC
Genetically engineered bacteria for efficiently producing N-acetylglucosamine
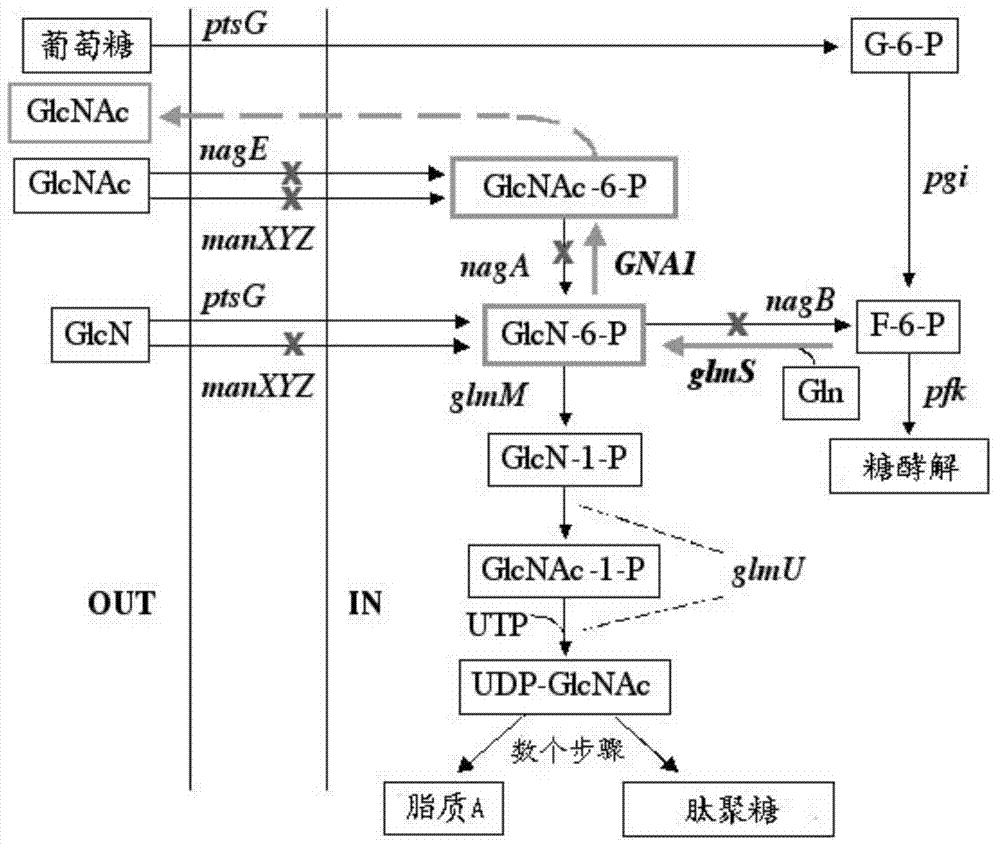
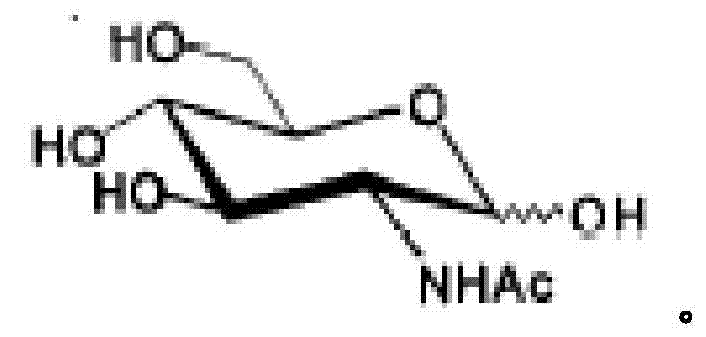
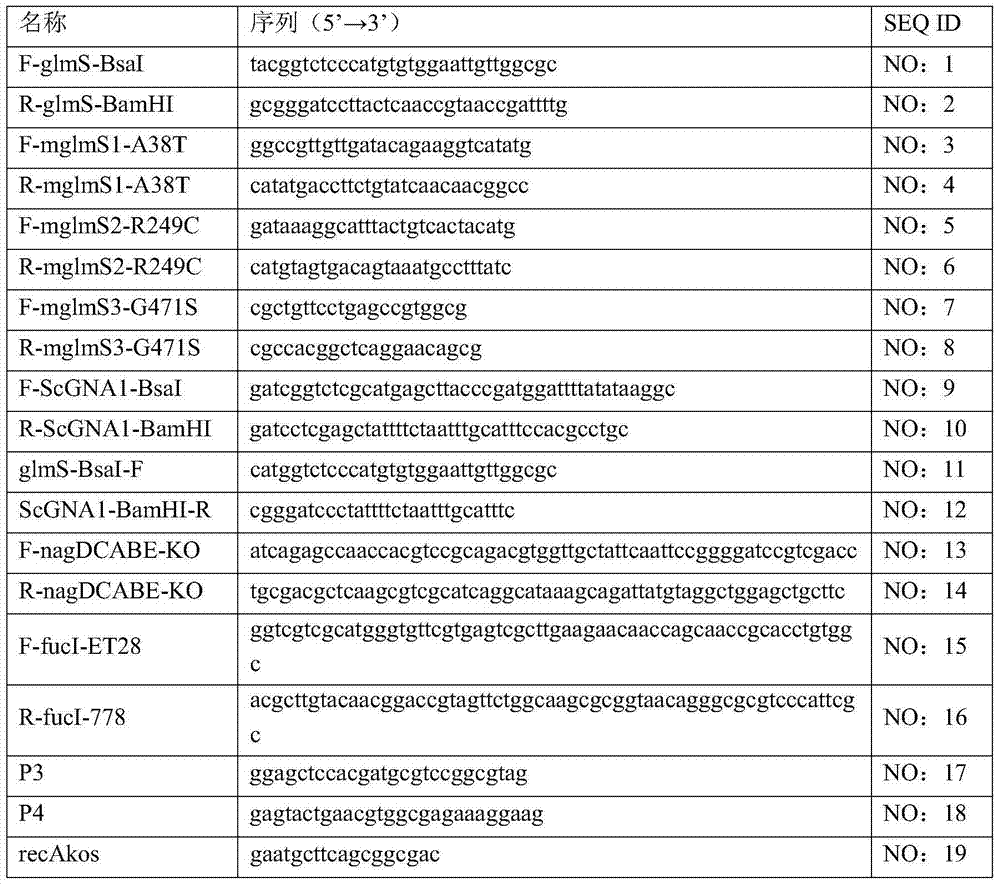
InactiveCN104293724AImprove fermentation yieldImprove stabilityBacteriaHydrolasesEscherichia coliBiotechnology
The invention provides genetically engineered bacteria for efficiently producing N-acetylglucosamine. The genetically engineered bacteria are prepared by the following steps: integrating chromosome deficiency nag DCABE gene clusters of Escherichia coli, respectively connecting 6-glucosamine phosphate synthetase mutant genes and N-acetylglucosamine transferase genes which are respectively mediated by a T7 promoter and a Trc promoter with a gene expression cassette in series, wherein the 6-glucosamine phosphate synthetase mutant genes are obtained by mutating wild 6-glucosamine phosphate synthetase genes from an Escherichia coli W3110 strain source into A38T / R249C / G471S mutants. The genetically engineered bacteria constructed by the invention have the advantages of high N-acetylglucosamine fermentation yield and high strain stability and have wide industrial application prospects.
Owner:SHANGHAI RES & DEV CENT OF INDAL BIOTECH +1
Recombinant corynebacterium crenatum for over expression of N-acetylglutamate kinase and application thereof
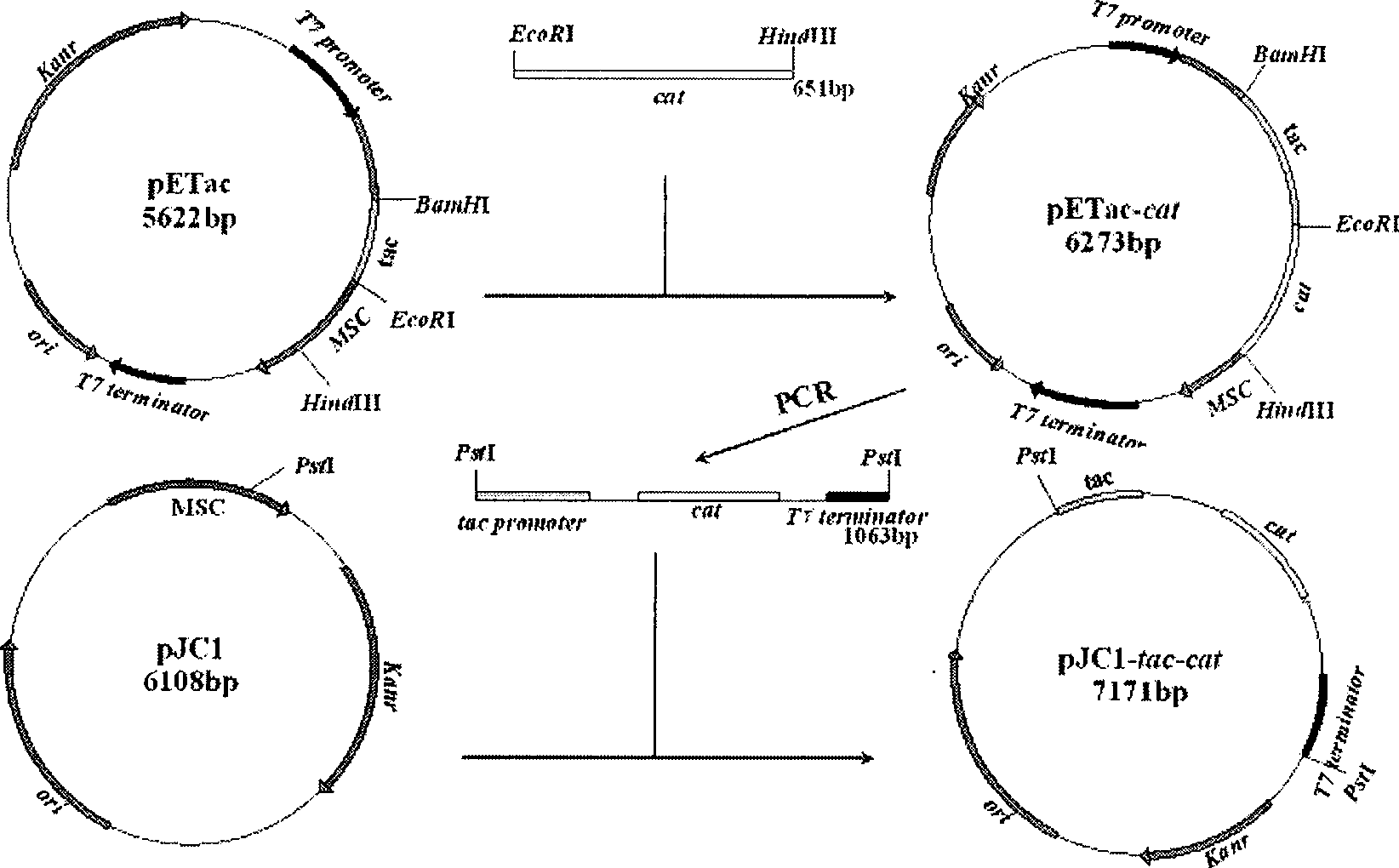
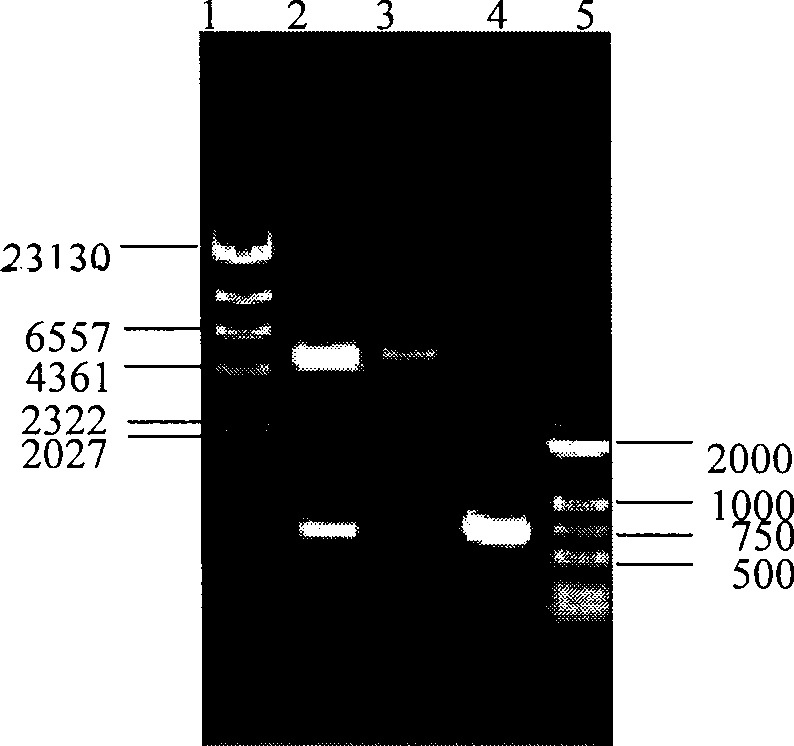
The invention relates to a recombinant corynebacterium crenatum to enhance the expression of N-acetyl glutamic acid kinase and the application thereof, which belongs to the technical field of gene engineering. The classification and nomenclature of the recombinant corynebacterium crenatum to enhance the expression of N-acetyl glutamic acid kinase is corynebacterium crenatum SYPA / pJC1-tac-argB with an access number of CCTCC NO: M 208133; The application is as follows: using a key enzyme N-acetyl glutamic acid kinase from corynebacterium crenatum pathway arginine to construct a corynebacterium crenatum expression vector pJC1-tac-argB; introducing the corynebacterium crenatum expression vector pJC1-tac-argB into corynebacterium crenatum SYPA by electrotransformation to obtain the recombinant corynebacterium crenatum SYPA / pJC1-tac-argB; and excessively expressing N-acetyl glutamic acid kinase, which greatly enhances the utilization rate of a precursor glutamic acid and allows the metabolic flow to flow to the arginine synthesis pathway, weakens the synthesis of proline, achieves the purpose of improving the output of arginine and the output of arginine is increased by 23.4 percent than C. crenatum SYPA.
Owner:JIANGNAN UNIV
Method for preparing konjac glucomannan adsorbing material
InactiveCN101884909ABiodegradableSimple methodOther chemical processesWater/sewage treatment by sorptionWater bathsHigh concentration
The invention discloses a method for preparing a konjac glucomannan adsorbing material. Water-resistant konjac glucomannan which keeps acetyl functional groups is prepared by a high concentration swelling method. The method comprises the following steps of: purifying refined konjac powder to obtain high-purity konjac glucomannan; swelling the high-purity konjac glucomannan in water for 10 minutes, adding a small amount of dimethyl sulfoxide and fully swelling at the temperature of between 40 and 70 DEG C; extruding the fully swelled konjac glucomannan after taking out to form filaments with the diameter of 0.5 to 1.0mm; adding the filaments into 70 to 95 volume percent solution of dimethyl sulfoxide, and putting the solution into a boiling water bath for 30 minutes; and shearing the product after taking out to form particles with the diameter of 0.5 to 1.0mm and the height of 0.5 to 1.0mm, and drying the particles to constant weight. Compared with the chemical modification method, the method has the advantages of simple preparation process and low energy consumption; and the obtained product has high water resistance, can be used as an adsorbing material and has good application prospect in the aspect of dyeing and finishing wastewater.
Owner:湖北李泽园食品有限公司
Fucosylated glycosaminoglycan derivative and preparation method thereof
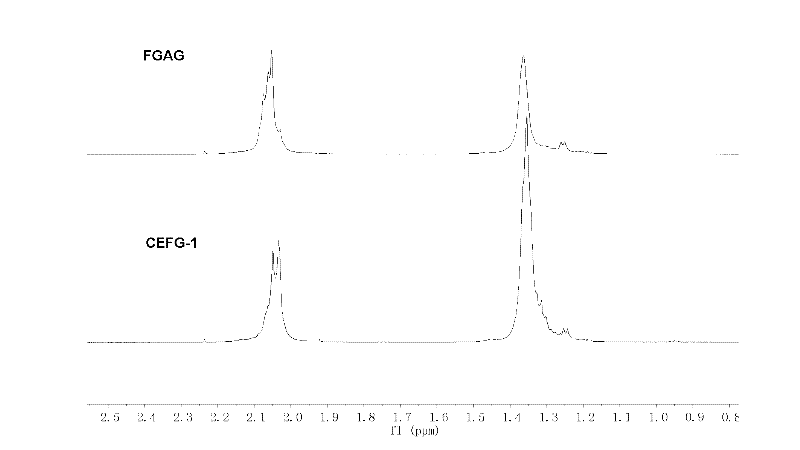
InactiveCN102329397APotent anticoagulant activityOrganic active ingredientsBlood disorderOrganosulfateCarboxylic ester
The invention discloses a carboxylic ester of fucosylated glycosaminoglycan (CEFG) with anticoagulation activity, a pharmaceutically acceptable salt thereof, a preparation method of the CEFG and the pharmaceutically acceptable salt thereof, a pharmaceutical composition containing the CEFG or the salt thereof, and application of the pharmaceutical composition in preparation of anticoagulants. The monosaccharides for preparing the CEFG comprise D-glucuronic acid or D-glucuronate (D-GlcU), D-2-deoxy-2-acetyl galactosamine sulfate (D-GalNAcS) and L-fucose sulfate (L-FucS), wherein the molar ratio of D-GlcU to D-GalNAc to L-Fuc to -OSO3<-> is 1:(1+ / -0.3):(1+ / -0.3):(3.5+ / -0.5); the esterification degree of the D-GlcU is not lower than 20%; and the weight average molecular weight of the CEFG is 3000-20000 Da. The glycosylated chondroitin sulfate esterification derivative has strong anticoagulation activity, and can be applied in preparation of drugs for preventing and / or treating thrombotic diseases.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
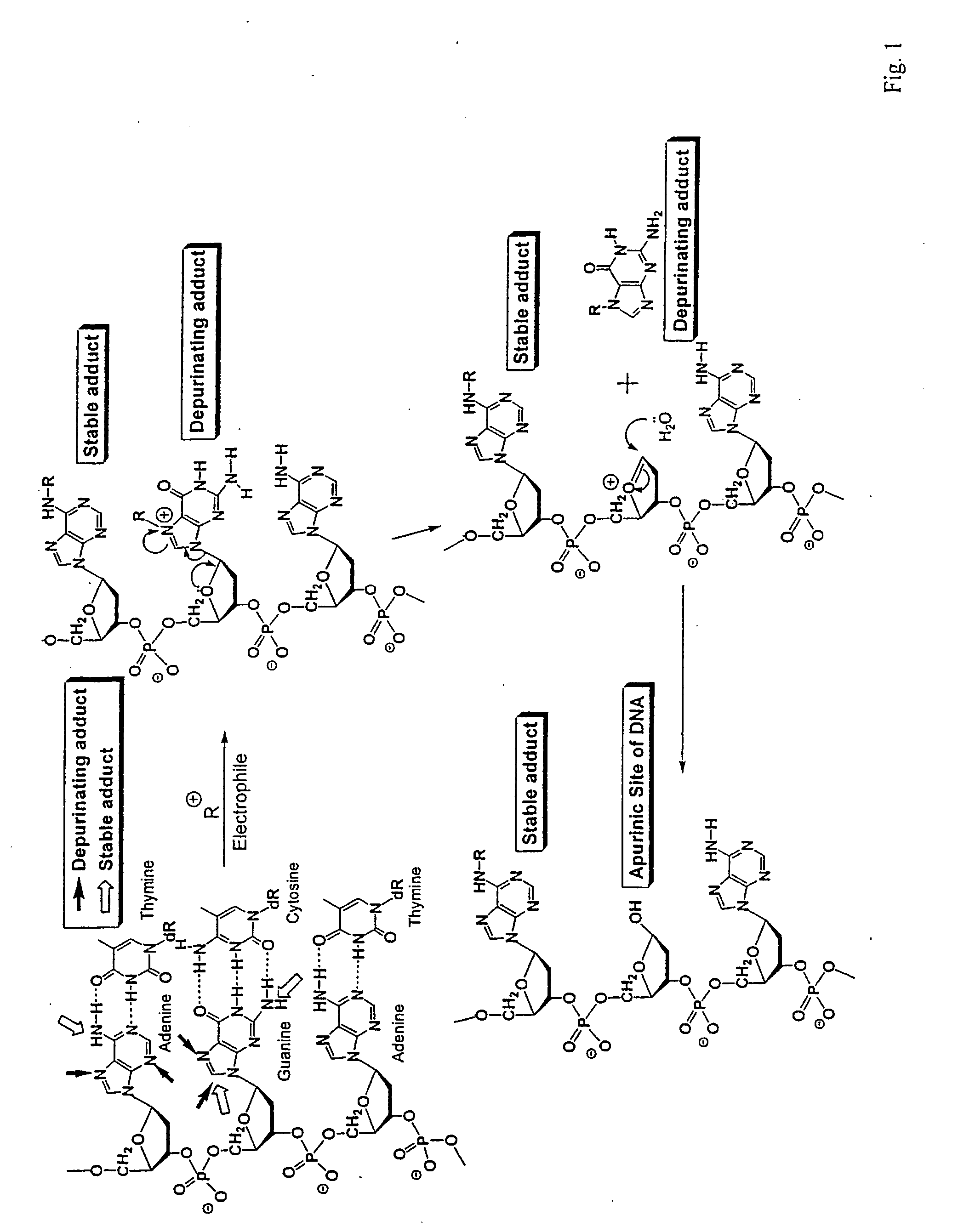
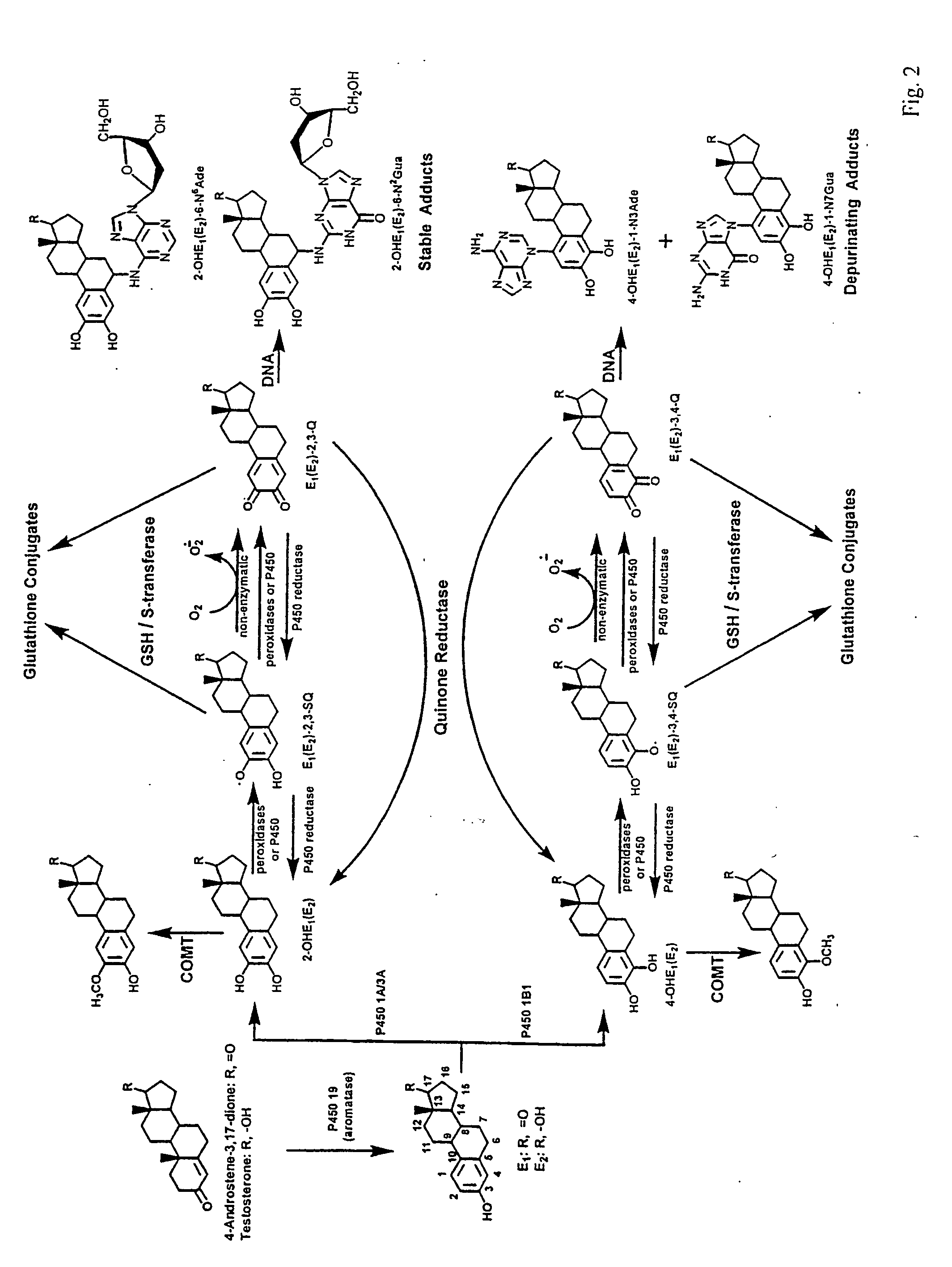
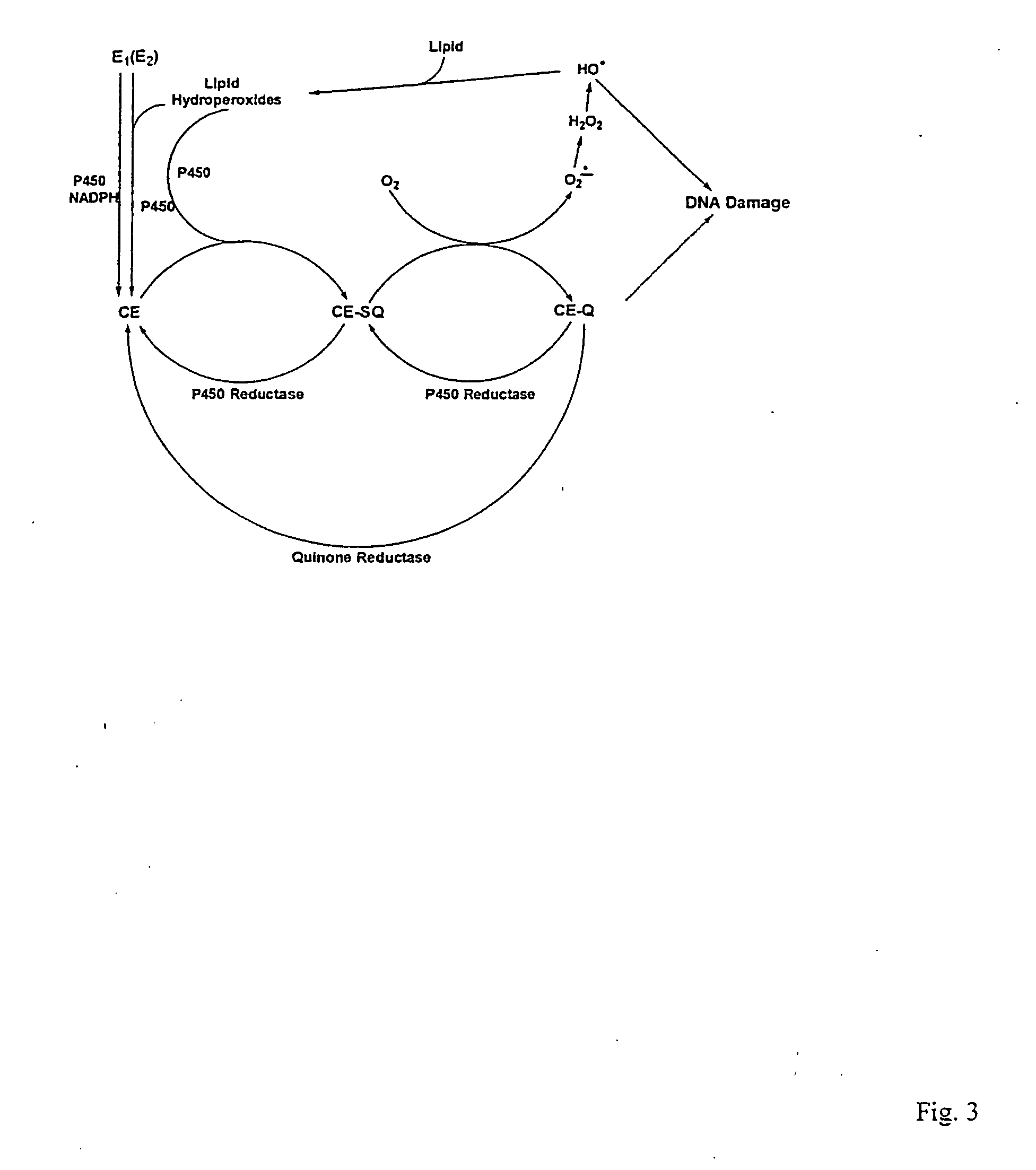
Unifying mechanism and methods to prevent cancer and neurodegenerative diseases
InactiveUS20050164911A1Grow moreInhibition formationBiocideNervous disorderCancer preventionMetabolite
The present invention relates to methods for preventing the development of cancer or neurodegenerative diseases by administering N-Acetylcysteine (NAC), melatonin, or a combination thereof. The present invention also relates to methods for diagnosing cancer and / or neurdegenerative disease by detecting or determining the amount of dopamine metabolites, 4-CE, 2-CE, methylation of CE or CE-Q conjugates.
Owner:PREVENTION
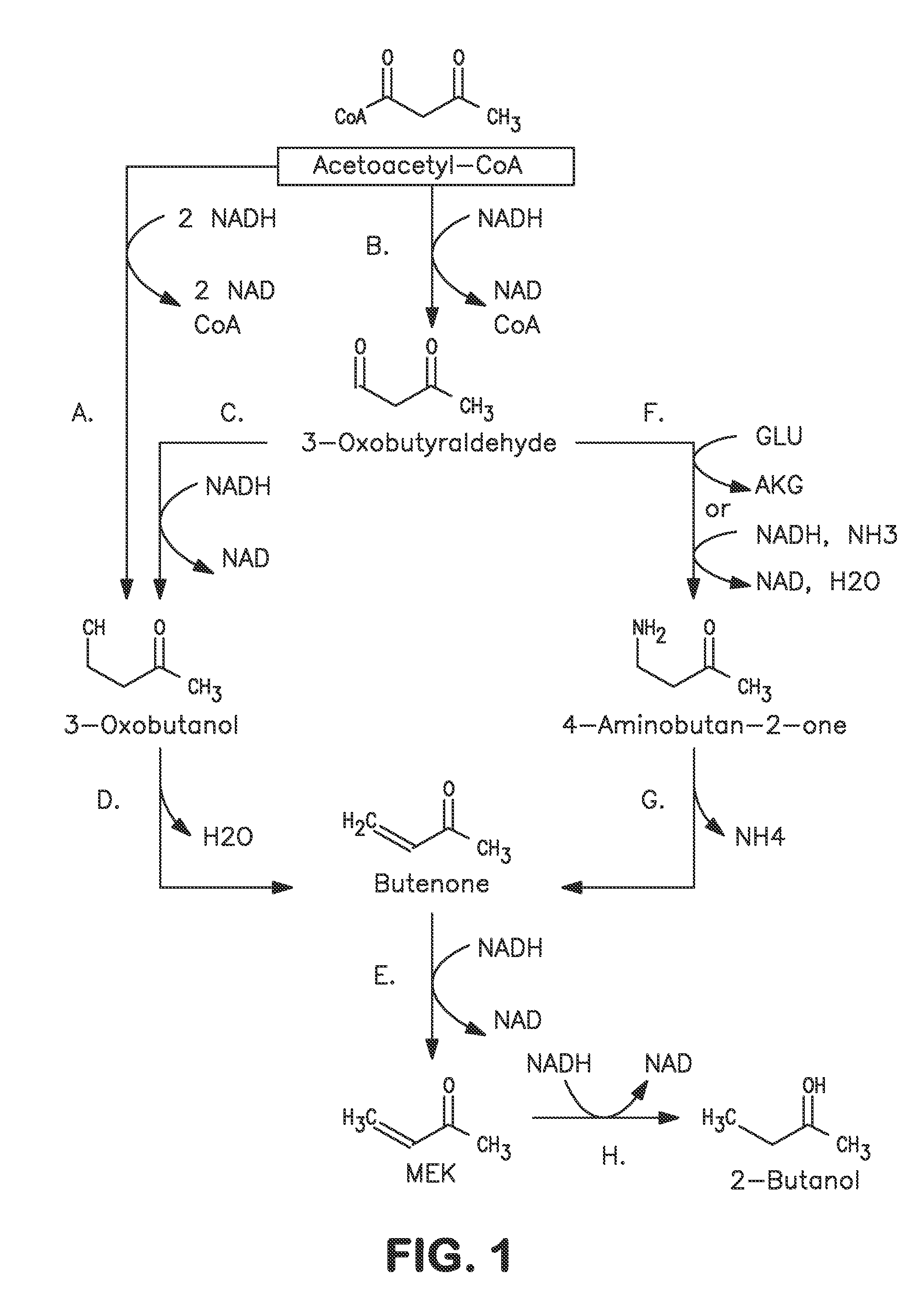
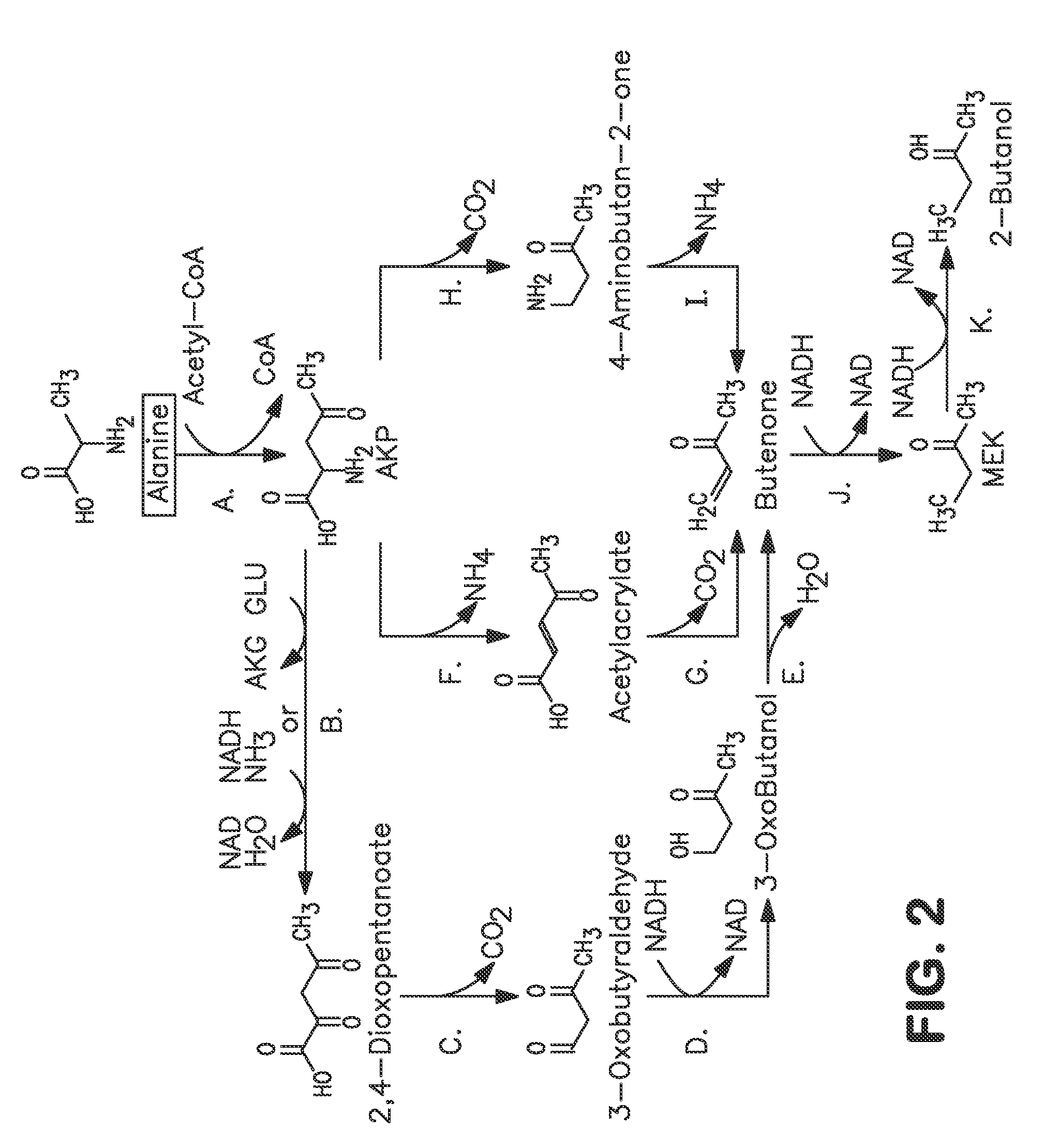
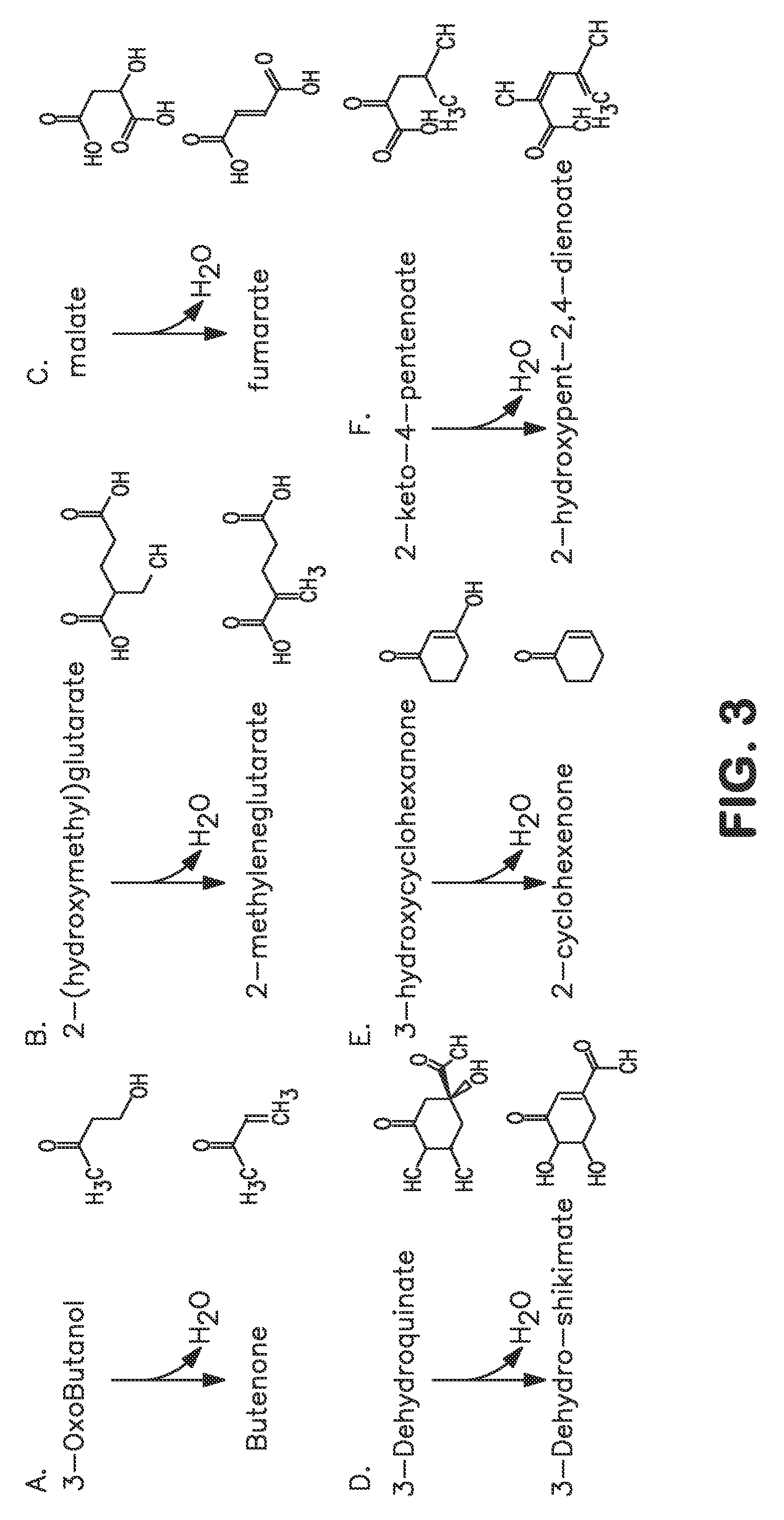
Microorganisms and methods for carbon-efficient biosynthesis of MEK and 2-butanol
A non-naturally occurring microbial organism has at least one exogenous nucleic acid encoding a MEK pathway enzyme expressed in a sufficient amount to produce MEK. The MEK pathway includes an enzyme selected from an acetoacetyl-CoA dehydrogenase (bifunctional), an acetoacetyl-CoA aldehyde dehydrogenase, a 3-oxobutyraldehyde reductase, a 3-oxobutanol dehydratase, an MEK oxidoreductase, a 3-oxobutyraldehyde aminotransferase, a 4-aminobutan-2-one deaminase, a 2-amino-4-ketopentanoate (AKP) thiolase, an AKP aminotransferase, a 2,4-dioxopentanoate decarboxylase, an AKP deaminase, an acetylacrylate decarboxylase, an AKP decarboxylase, a glutamate dehydrogenase, a 3-oxobutyraldehyde oxidoreductase (aminating) and an AKP oxidoreductase (aminating). A 2-butanol pathway further includes an MEK reductase. A method for producing MEK or 2-butanol includes culturing these organisms under conditions and for a sufficient period of time to produce MEK or 2-butanol.
Owner:GENOMATICA INC
Culture media for hepatocyte culture and liver organ preparation
The invention provides a proliferation culture medium and differentiation culture medium for hepatocyte culture and liver organ preparation. The proliferation culture medium and the differentiation medium both take a culture medium for growth of mammalian cells as a basic culture medium, and an agent for supplementing L-glutamine, a pH value modifier for maintaining the pH values of the culture media stable, a primary cell culture antibiotic, a serum substitute, N-acetylcysteine, arbitrary nicotinamide and any one or more of a BMP inhibitor, a Wnt agonist, a growth factor, a Rock signaling pathway inhibitor, a P38 signal path inhibitor, a Notch signal path inhibitor, dexamethasone, BMP7 and a cAMP activator are added into the culture media. By using the culture media for culturing liver cells, functional liver organs can be obtained.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
Folacin receptor mediated targeted acetyl pullulan polysaccharide nano granule and preparation thereof
InactiveCN101254309ASmall particle size distribution rangeImprove bioavailabilityPharmaceutical non-active ingredientsAntineoplastic agentsPullulanHydrophobic polymer
The invention discloses a method for preparing folic acid coupled acetyl pullulan polysaccharide and the nanoparticles thereof, a preparation of drug-loaded nanoparticles which take the compound as the carrier and the role of the drug-loaded nanoparticles on the tumor cells. Firstly, the water soluble pullulan polysaccharide is converted to hydrophobic polymers by acetylation, so as to be conductive to the preparation of the nanoparticles and the loading of a hydrophobic drug, and the tumor cells with the high expression of the folate receptor can be targeted after the coupling of the folic acid by esterification. The drug-loaded nanoparticles are prepared by taking epirubicin as a model drug and adopting the solvent dispersion method, and the role of the drug-loaded nanoparticles on the tumor cells are evaluated by the in vitro cell uptake test. The results show that the method for preparing the folic acid-acetyl pullulan polysaccharide nanoparticles by the solvent dispersion method is simple, the reproducibility is good, the expanded production is easy, the drug-loading ratio is high, and the drug-loaded nanoparticles can be taken into the tumor cells by the route of the folate receptor.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
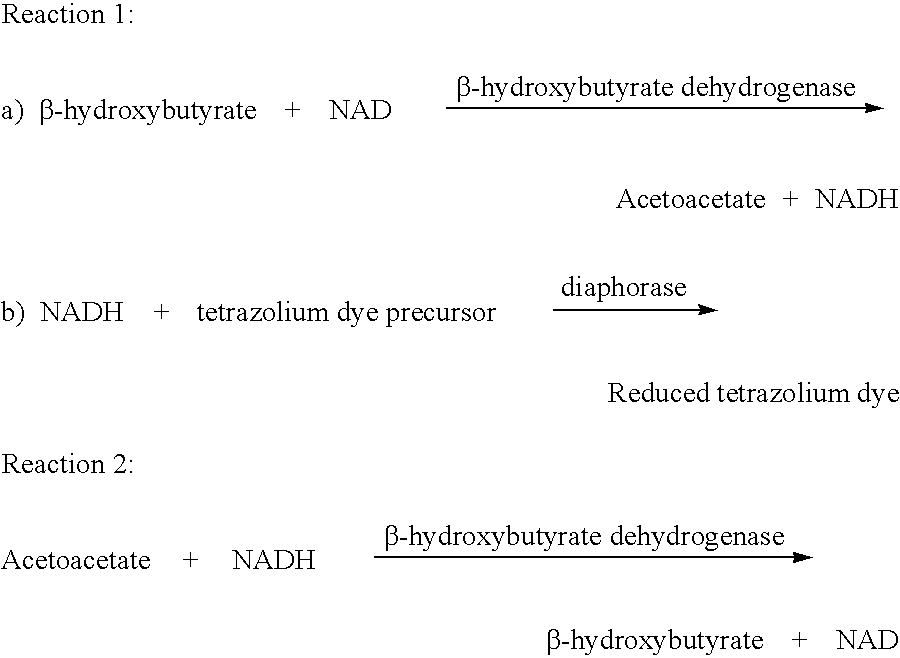
Method and test strips for the measurement of fat loss during weight loss programs
InactiveUS20040043376A1Great advantageEasy to manufactureMicroorganismsMicrobiological testing/measurementPhysiologyAcyl CoA dehydrogenase
Disposable test strips and a wet chemistry method for measuring each of beta-hydroxybutyrate alone, combined beta-hydroxybutyrate and acetoacetate or total ketone bodies (i.e., beta-hydroxybutyrate, acetoacetate and acetone) in human bodily fluid samples, including but not limited to urine, saliva or sweat are described. The test strips need only be dipped in the sample and can be used by anyone in almost any milieu. Measurement can be made electrochemically, spectrophotometrically, fluorometrically or by comparision to a color standard. Combined acetoacetate and beta-hydroxybutyrate which account for 97-98% of total ketone bodies and may be measured in a cyclic reaction that occurs at pH about 7.0 to about 8.3 with beta-hydroxybutyrate dehydrogenase, (beta-HBD), nicotinamide adenine dinucleotide, a tetrazolium dye precursor and an electron mediator. Using this reaction, false positive results obtained from urine samples taken from patients on sulfhydryl drugs are avoided. beta-HBD from some sources was found to cause false negative results in samples (e.g. urine) containing high chloride content due to chloride inhibition of beta-HBD. Using a simple test for chloride inhibition, it was found that beta-HBD from Alcaligenes is not so inhibited. Using either beta-HBD that is not inhibited by chloride or using 10-20 times the normal concentration of this enzyme eliminates false negatives in samples having substantial chloride content, such as urine, both in the reaction described above and in other reactions disclosed for measuring each of beta-hydroxybutyrate alone, combined beta-hydroxybutyrate and acetoacetate and total ketone bodies, all of which reactions occur in the pH range of about 8.6 to about 9.5.
Owner:GUPTA SURENDRA
Methods and compositions for treating cancer
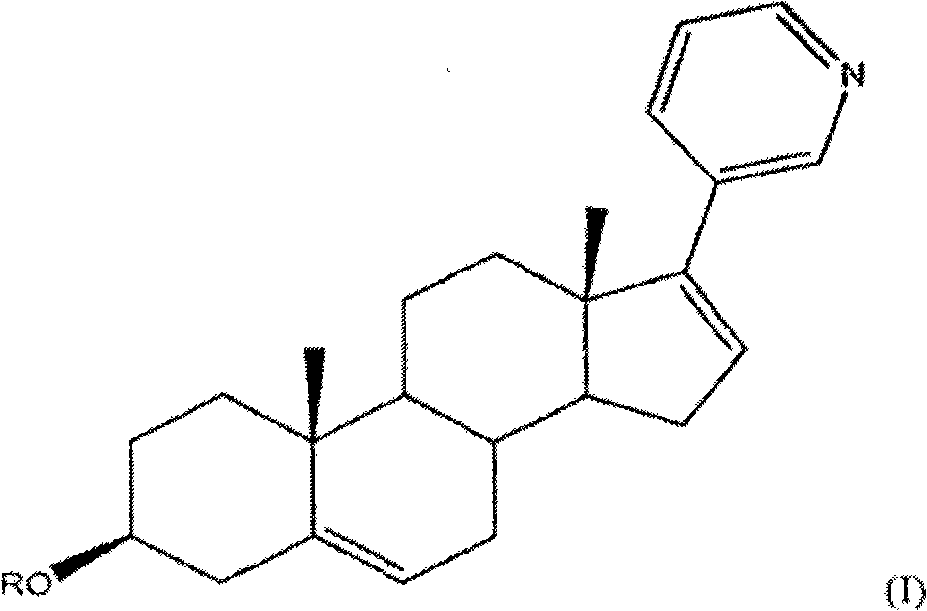
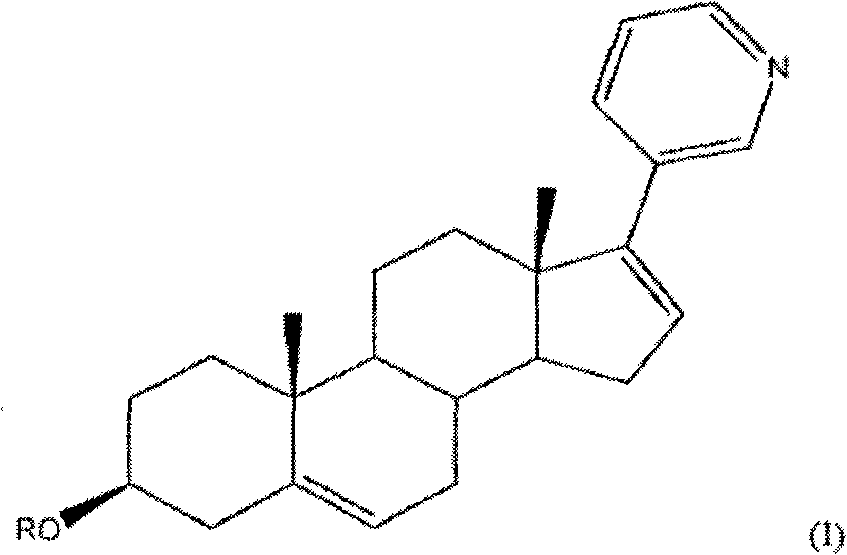
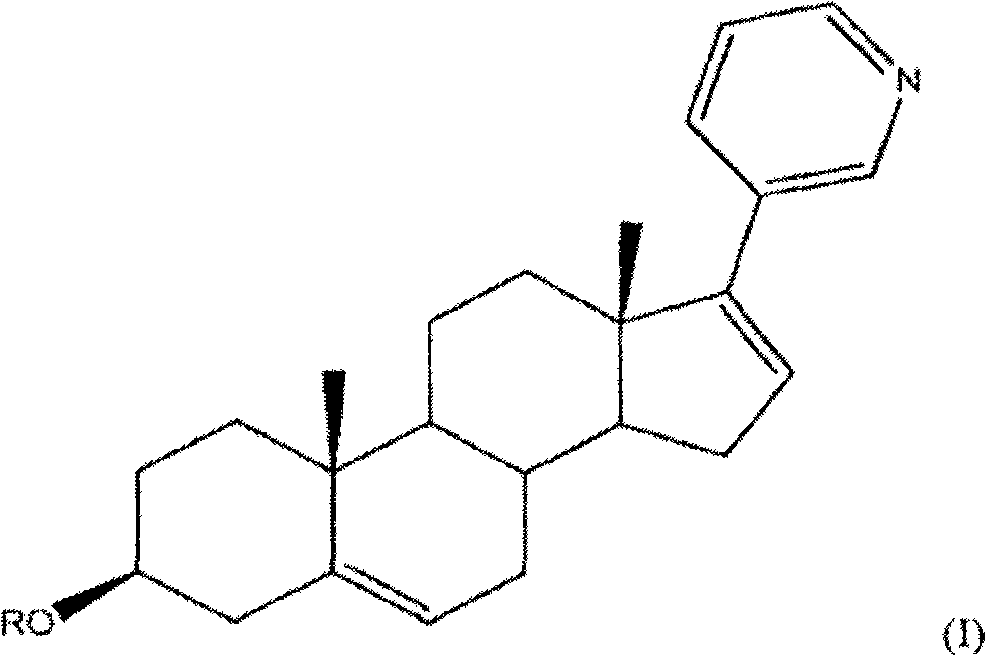
Methods and compositions for treating cancer are described herein. More particularly, the methods for treating cancer comprise administering a 17a-hydroxylase / C17, 20-lyase inhibitor, such as abiraterone acetate (i.e., 3beta-acetoxy-17-(3-pyridyl) androsta-5, 16-diene), in combination with at least one additional therapeutic agent such as an anti-cancer agent or a steroid. Furthermore, disclosed are compositions comprising a 17a- hydroxylase / C17, 20-lyase inhibitor, and at least one additional therapeutic agent, such as an anti-cancer agent or a steroid.
Owner:库伽尔生物科技公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


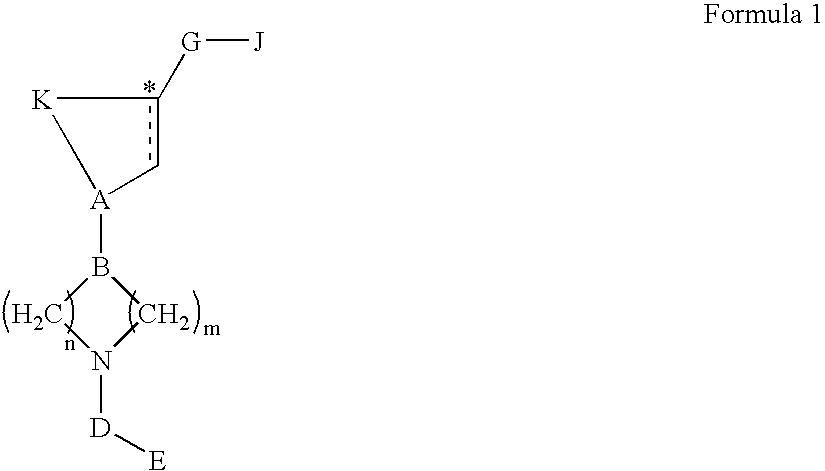
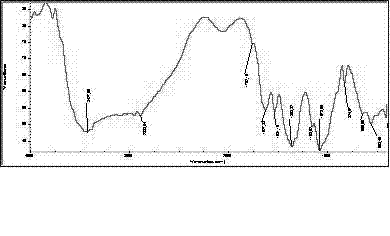
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)