Fucosylated glycosaminoglycan derivative and preparation method thereof
A technology of glycosaminoglycan carboxylate and glycosylamine, which is applied in the field of fucosylated glycosaminoglycan carboxylate and its preparation, can solve the problems of large individual differences and slow onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
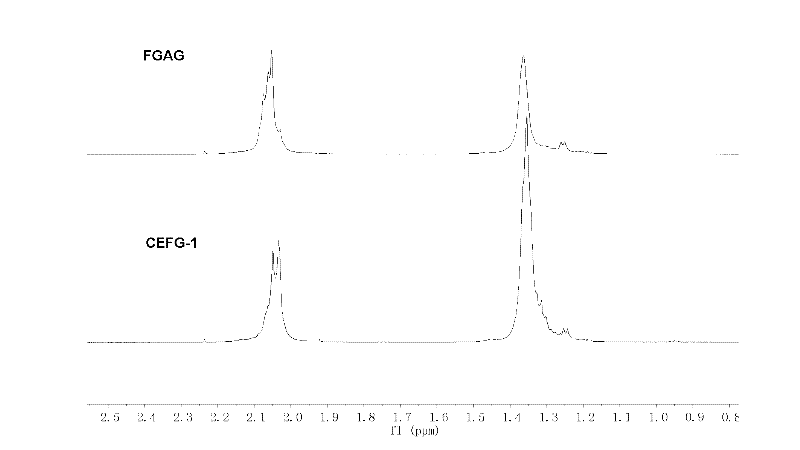
Image
Examples
Embodiment 1
[0053] [Example 1] Preparation of FGAG carboxyethyl esterification product
[0054] 1.1 Materials
[0055] Prunus ginseng (Thelenata ananas Jaeger), commercially available, eviscerated and dried body wall;
[0056] h 2 o 2 , CH 3 COONa·3H 2 O, NaCl, NaOH, Cu(CH 3 COO) 2 ·H 2 O, tetrabutylammonium hydroxide, ethyl bromide, N, N-dimethylformamide, sodium hydroxide, sodium chloride and ethanol were commercially available analytical reagents.
[0057] 1.2 Method
[0058] (1) Extraction and preparation of fucosylated glycosaminoglycans (FGAG): Take the dried body wall of Echinodermata ginseng, and prepare FGAG according to the literature method (J Biol Chem, 1991, 266(21): 13530-6). The yield 0.75%, purity 98% (HPGPC, area normalization method), weight average molecular weight (Mw), 65,960.
[0059] (2) Preparation of low-molecular-weight fucosylated glycosaminoglycan (FGAG): 5.0 g of FGAG obtained in step (1) was prepared according to the method of patent CN 201110114860...
Embodiment 2
[0070] [Example 2] Preparation of FGAG-carboxyallyl esterification product
[0071] 2.1 Materials
[0072] Haitian melon (Acaudiina molpadioides Sepmper) is commercially available, the internal organs are removed and the body wall is dried; allyl bromide is a commercially available analytical reagent, and the rest of the reagents are the same as in Example 1.
[0073] 2.2 Method
[0074] (1) Preparation of FGAG quaternary ammonium salt
[0075] According to the preparation of steps (1) to (3) in the method of [Example 1], 253.1 mg of ammonium salt was obtained.
[0076] (2) Preparation of FGAG-carboxyallyl esterification product
[0077] Get the quaternary ammonium salt 253.1mg that step (1) gained is placed in the reaction test tube, add 3ml DMF to dissolve, add reactant propylene bromide 120 μ l, in N 2 Protected, protected from light, reacted at 30°C for 24h under stirring (450r / min). After the reaction, add 3ml of 0.5M NaCl to the reaction solution, add 15ml of absolu...
Embodiment 3
[0078] [Example 3] Preparation of FGAG-carboxy n-butyl esterification product
[0079] 3.1 Materials
[0080] Sea cucumber (Apostichopus japonicus Selenka, 1867), commercially available, eviscerated and dried body wall; bromo-n-butane and tributylamine are commercially available reagents of analytical grade, and the rest of the reagents are the same as [Example 1].
[0081] 3.2 Method
[0082] (1) Preparation of FGAG quaternary ammonium salt
[0083] According to the method of [Example 1] steps (1) to (3), tributylamine was used to prepare to obtain 166.6 mg of ammonium salt.
[0084] (2) Preparation of FGAG-carboxy n-butyl ester product
[0085] Get the quaternary ammonium salt 166.6mg that step (1) gained is placed in reaction test tube, add 2ml DMSO to dissolve, add reactant n-bromobutane 150 μ l, in N 2 Protected and reacted at 30°C for 20h. After the reaction was completed, 2ml of 0.5M NaCl was added to the reaction solution, and 20ml of absolute ethanol was added, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com