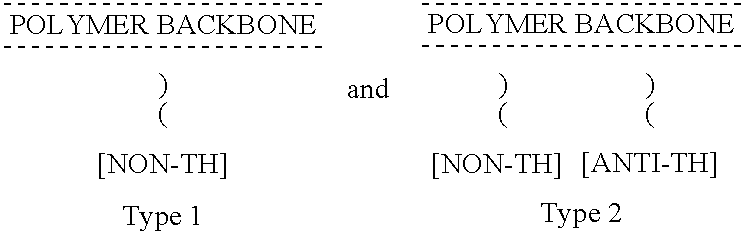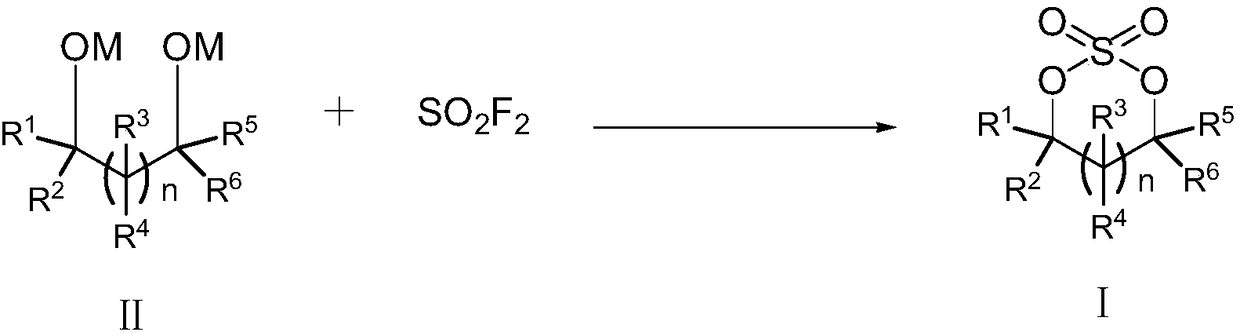Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
112 results about "Organosulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
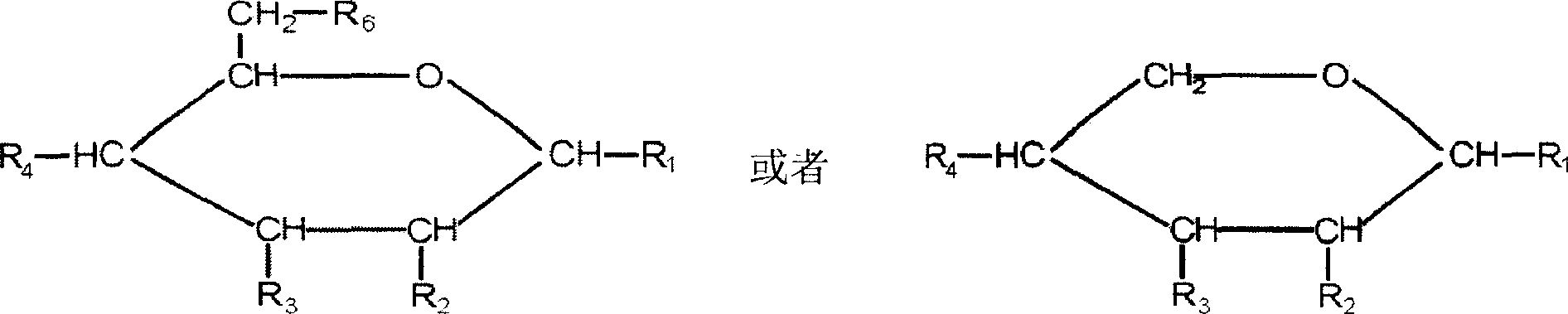
Organosulfates are a class of organic compounds sharing a common functional group commonly with the structure R-O-SO₃⁻. The SO₄ core is a sulfate group and the R group is any organic residue. All organosulfates are formally esters derived from alcohols and sulfuric acid, although many are not prepared in this way. Many sulfate esters are used in detergents, and some are useful reagents. Alkyl sulfates consist of a hydrophobic hydrocarbon chain, a polar sulfate group (containing an anion) and either a cation or amine to neutralize the sulfate group. Examples include: sodium lauryl sulfate (also known as sulfuric acid mono dodecyl ester sodium salt) and related potassium and ammonium salts.
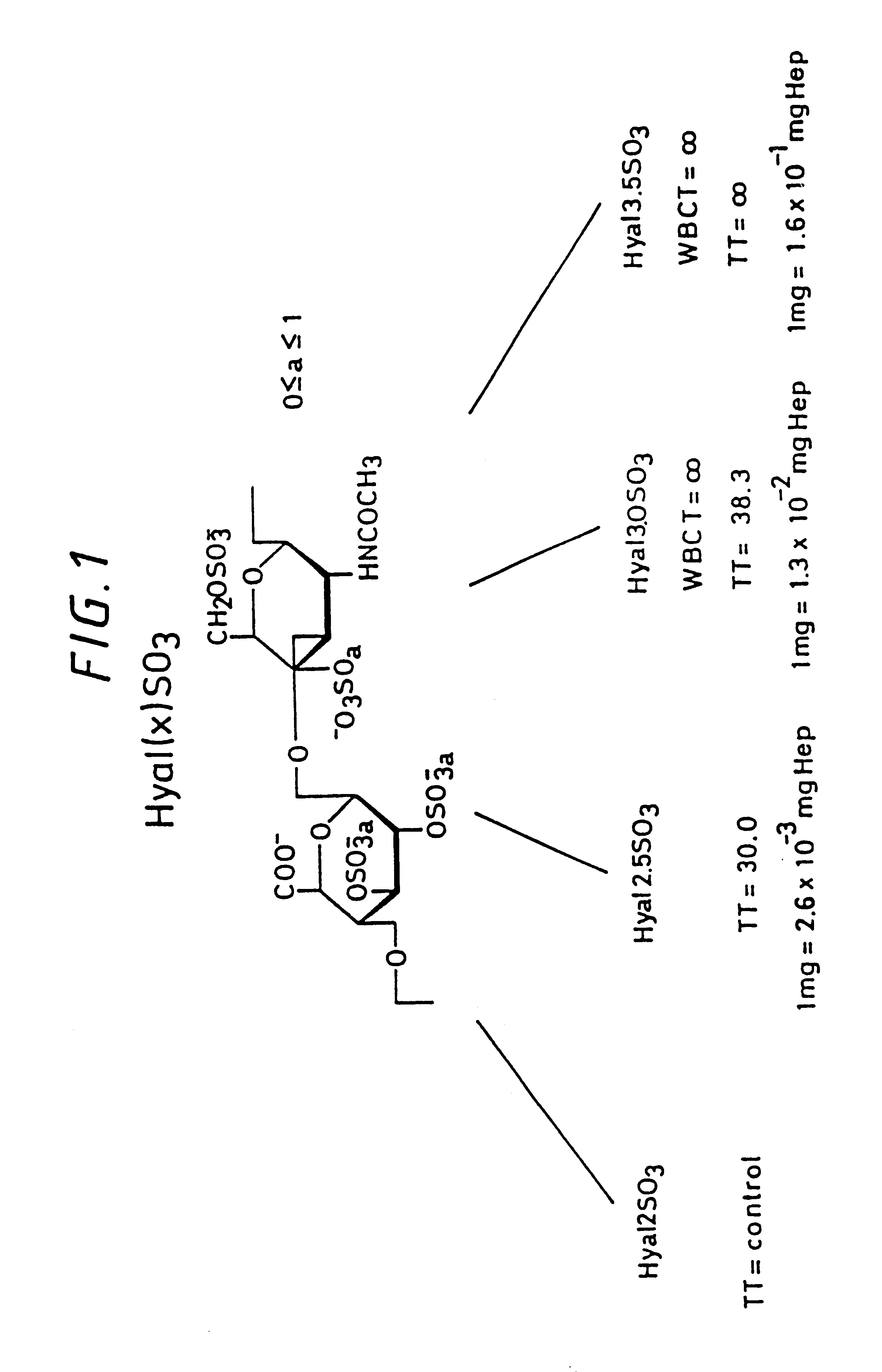
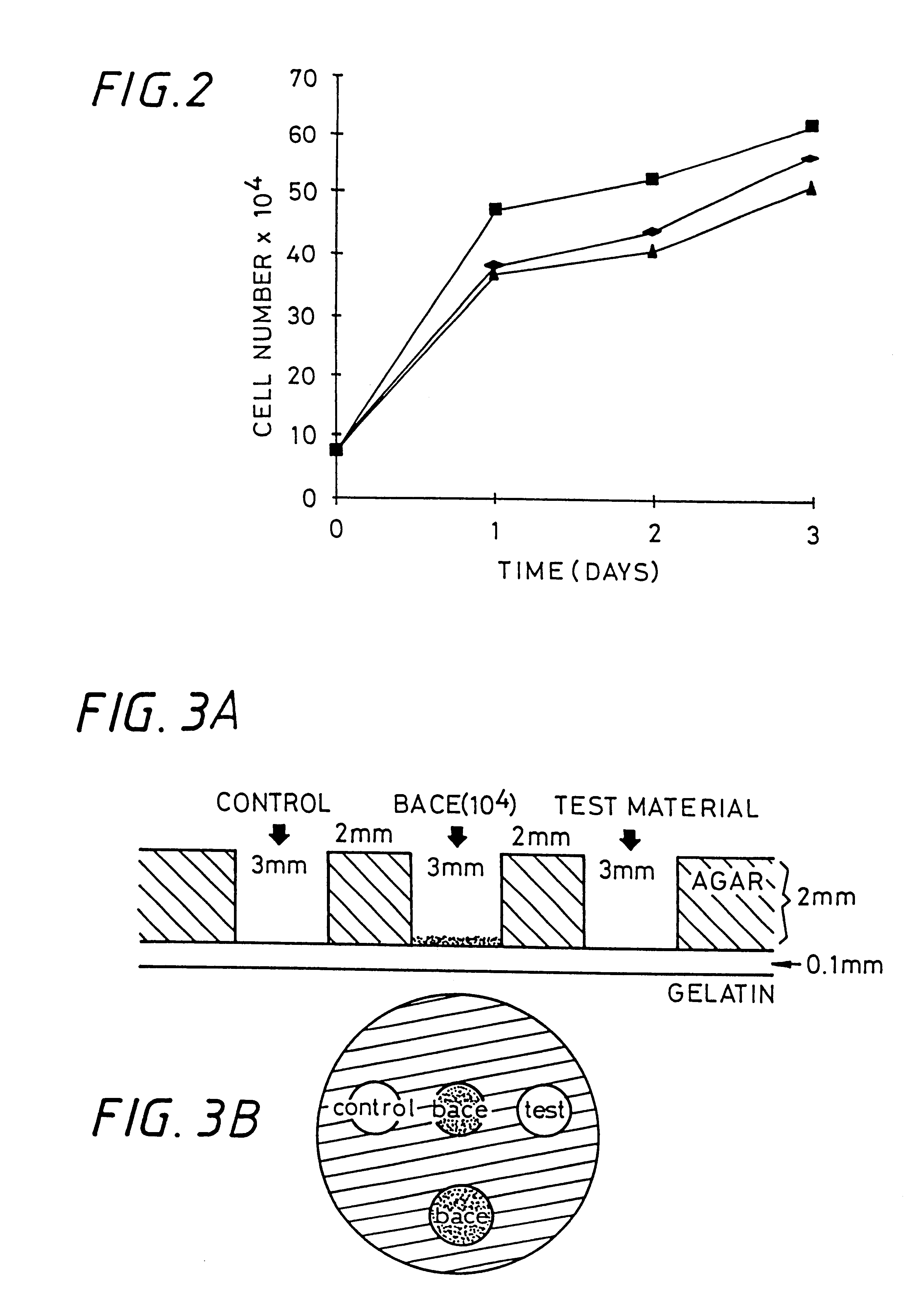
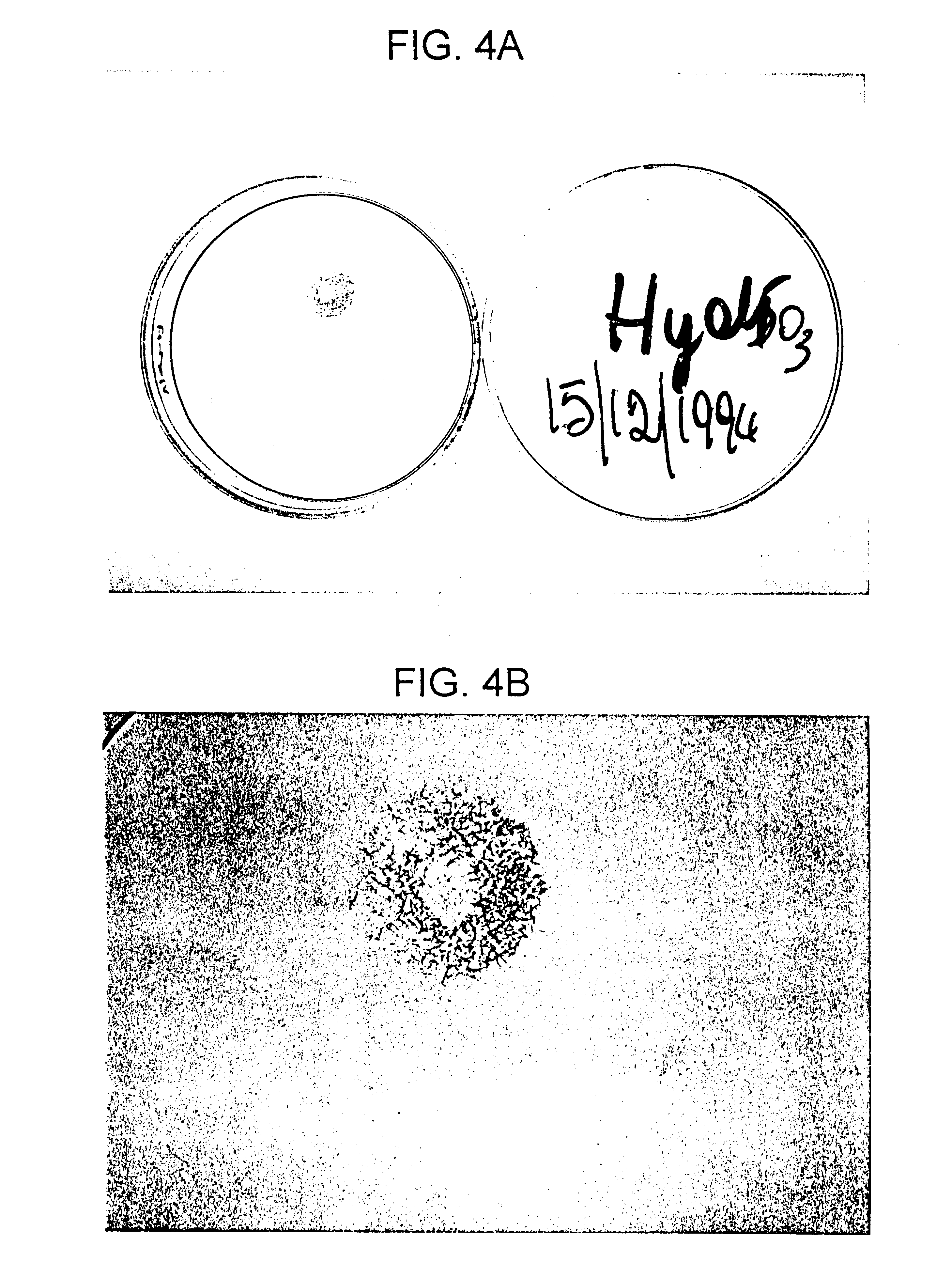
Sulfated hyaluronic acid and esters thereof
Hyaluronic acid, hyaluronate esters and salts thereof are sulfated such that the number of sulfate groups per monomeric unit is in the range of from 0.5 to 3.5. The sulfated derivatives exhibit anticoagulant and cell adhesion reduction properties, and may be used to prepare biomaterials.
Owner:FIDIA ADVANCED BIOPOLYMERS SRL
Cyclic sulfate compound, non-aqueous electrolyte solution containing same, and lithium secondary battery
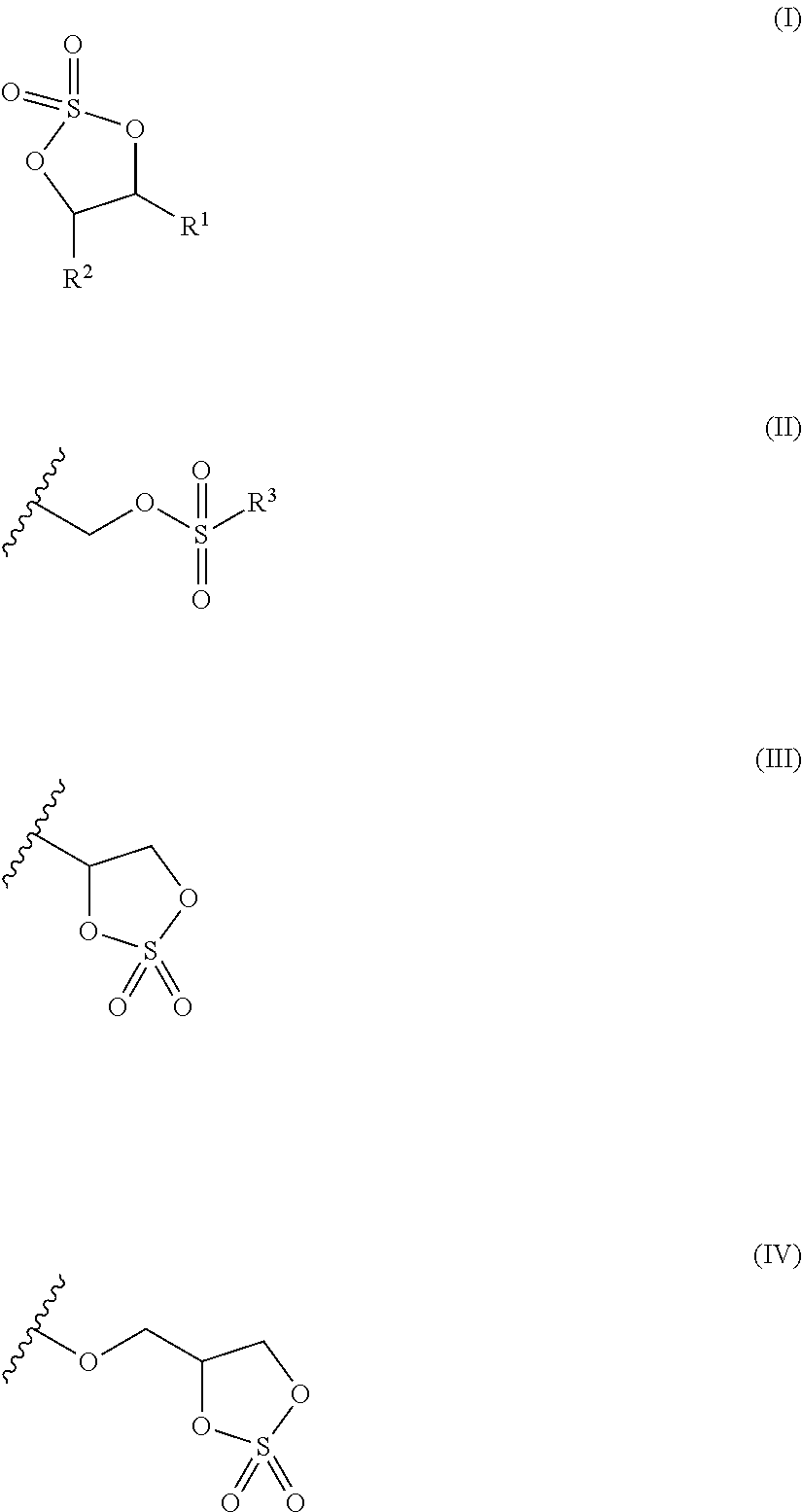
ActiveCN103098290AGood capacity retentionLower open circuit voltageOrganic chemistryFinal product manufactureHydrogen atomHalogen
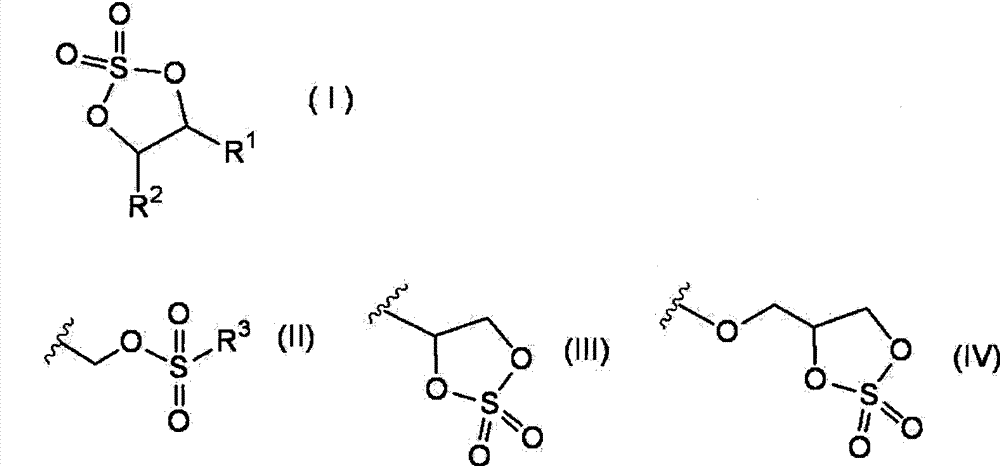
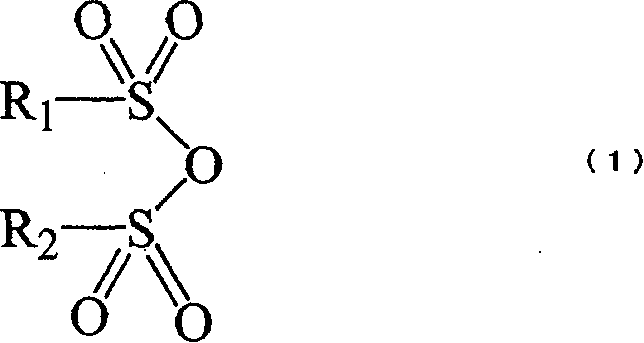
A non-aqueous electrolyte solution containing a cyclic sulfate compound represented by formula (I) is provided, wherein in formula (I), R1 represents a group represented by formula (II) or a group represented by formula (III); R2 represents a hydrogen atom, an alkyl group having from 1 to 6 carbon atoms, a group represented by formula (II), or a group represented by formula (III); and in formula (II), R3 represents a halogen atom, an alkyl group having from 1 to 6 carbon atoms, a halogenated alkyl group having from 1 to 6 carbon atoms, an alkoxy group having from 1 to 6 carbon atoms, or a group represented by formula (IV).
Owner:MITSUI CHEM INC
Preparation method of fucosan sulphate oligosaccharide
A process for preparing the oligose of fucosan sulfate includes such steps as preparing the aqueous solution of fucosan sulfate, adding oxidant chosen from hydrogen peroxide, hypochlorous acid, nitrous acid, or their salts, heating, membrane ultrafilter, vacuum concentrating and freeze drying. Its advantages are high medical effect to protect liver and take care of health, and low cost.
Owner:OCEAN UNIV OF CHINA
Glycosidase inhibitors and methods of synthesizing same
The compounds of the present invention relate to chain-extended and chain-modified analogues of salacinol, including embodiments where the sulfate moiety has been substituted with a carboxylate or phosphate moiety. In other embodiments the sulfate moiety has been shifted by one carbon atom in the zwitterionic structure. In another embodiment the polyhydroxylated side chain may be replaced with a lipophilic alkyl chain and a suitable counterion. The invention also encompasses methods for synthesizing the salacinol analogues and using the analogues for enzyme inhibition applications.
Owner:SIMON FRASER UNIVERSITY
Non-aqueous electrolyte secondary battery
ActiveCN1877897AImprove securityImprove charge and discharge cycle characteristicsActive material electrodesLi-accumulatorsSulfonateSulfate
A non-aqueous secondary battery contains a positive electrode, a negative electrode, a separator and a non-aqueous electrolytic solution. The positive electrode contains a layered structure lithium-containing compound oxide, or a spinel lithium-containing compound oxide containing manganese as an active material. The non-aqueous electrolytic solution contains at least one additive selected from a sulfonic acid anhydride, a sulfonate ester derivative, a cyclic sulfate derivative and a cyclic sulfonate ester derivative, and a vinylene carbonate or a derivative of the vinylene carbonate.
Owner:MAXELL HLDG LTD
Preparation for polysaccharide selenite and application thereof
InactiveCN101104644AHigh yieldQuality improvementOrganic active ingredientsAntineoplastic agentsSulfateSugar
Disclosed are a preparation method and the applications of a polysaccharide selenous acid ester, belonging to the polysaccharide selenide technical field. The invention is characterized in that the polysaccharide selenous acid ester is made essentially by reaction between polysaccharide or carbohydrate complex and selenide reagent which is selenium Oxyhalide or the compound contains the selenium Oxyhalide. Feeding molar ratio of the selenide reagent and hydroxyl groups in the polysaccharide is 1:0.1-20. The invention also comprises the applications of the polysaccharide selenous acid ester in preparing anticancer drug and ant-oxidation drug. The polysaccharide selenous acid ester is produced by the reaction between the selenium reagent and the hydroxyl groups in sugar units, but not by selenite radicals substituting sulfate groups of the polysaccharide sulfate. The yield and the quality of the product are both higher than that of the products produced by other methods, and the selenium content is much higher than that of the products produced by the present technique.
Owner:陈艺新
Preparation method of sulfated polysaccharide
The invention relates to a preparation method of sulfated polysaccharide. Firstly, the polysaccharide reacts with organic silicon and then reacts with sulfuric acid esterification reagent such as sulfur trioxide-pyridine complex, chlorosulfonic acid and the like, and finally the sulfated polysaccharide is prepared. The method has the advantages of mild reaction, easy control, high yield and good repeatability. By utilizing the method, sulfated polysaccharide with wider scope of sulfated degree can be prepared, the sulfated substitution degree can be up to 2.95 at most, moreover, the polysaccharide degradation in the reaction process is less, the side effects are less, the product color is light, and the product is especially suitable for being applied as a drug.
Owner:WUHAN UNIV
Synthetic method of cyclosulphate
InactiveCN110386916AHigh reaction conversion rateImpurities increaseOrganic chemistrySecondary cellsCatalytic oxidationOrganic layer
The invention discloses a synthetic method of cyclosulphate. A peroxysulfate solution is pre-synthesized through sulfuric acid and hydrogen peroxide, then under existence of an organic solvent, a metal inorganic compound and a catalyst, the peroxysulfate solution is dropwise added to generate a catalytic oxidation reaction with cyclosulfite, after the reaction is completed, salt residue is filtered out, still standing for layering is conducted, an organic layer is taken to be distilled, concentrated and purified, and a cyclosulphate high-quality product is obtained. According to the syntheticmethod of the cyclosulphate, the cheap sulfuric acid and hydrogen peroxide are used as the raw materials, the peroxysulfate solution is prepared to conduct catalytic oxidation on the cyclosulfite, onthe one hand, the reaction is mild, control is easy, and the reaction conversion rate is high; on the other hand, the evaporation capacity of water is small, energy consumption is low, generated wastewater is less, and a synthetic process is more environmentally friendly; and the prepared cyclosulphate is few in impurity, high in purity and wide in market prospect.
Owner:CHANGSHU CHANGJI CHEM
Fucosylated glycosaminoglycan derivative and preparation method thereof
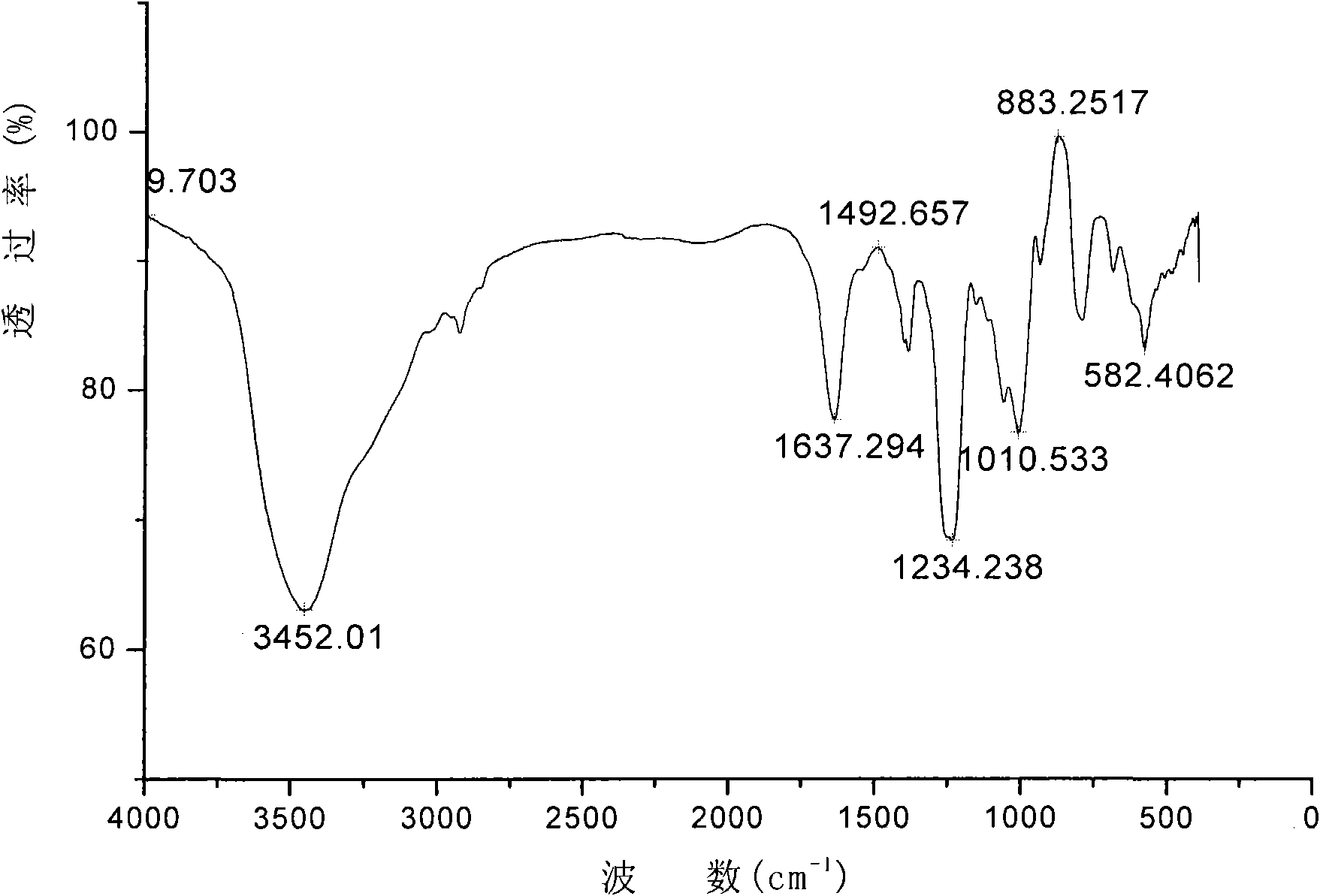
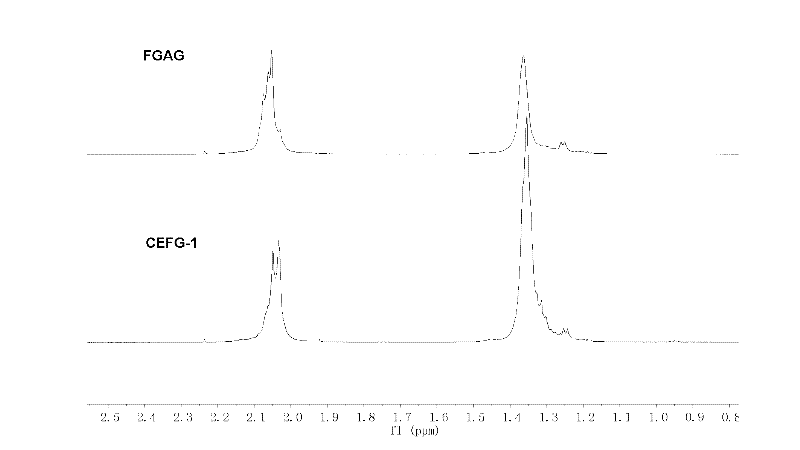
InactiveCN102329397APotent anticoagulant activityOrganic active ingredientsBlood disorderOrganosulfateCarboxylic ester
The invention discloses a carboxylic ester of fucosylated glycosaminoglycan (CEFG) with anticoagulation activity, a pharmaceutically acceptable salt thereof, a preparation method of the CEFG and the pharmaceutically acceptable salt thereof, a pharmaceutical composition containing the CEFG or the salt thereof, and application of the pharmaceutical composition in preparation of anticoagulants. The monosaccharides for preparing the CEFG comprise D-glucuronic acid or D-glucuronate (D-GlcU), D-2-deoxy-2-acetyl galactosamine sulfate (D-GalNAcS) and L-fucose sulfate (L-FucS), wherein the molar ratio of D-GlcU to D-GalNAc to L-Fuc to -OSO3<-> is 1:(1+ / -0.3):(1+ / -0.3):(3.5+ / -0.5); the esterification degree of the D-GlcU is not lower than 20%; and the weight average molecular weight of the CEFG is 3000-20000 Da. The glycosylated chondroitin sulfate esterification derivative has strong anticoagulation activity, and can be applied in preparation of drugs for preventing and / or treating thrombotic diseases.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Cyclic sulfate compound, non-aqueous electrolyte solution containing same, and lithium secondary battery
ActiveUS20130171514A1Reduce voltageGood capacity retentionOrganic chemistryFinal product manufactureHydrogen atomHalogen
A non-aqueous electrolyte solution containing a cyclic sulfate compound represented by formula (I) is provided, wherein in formula (I), R1 represents a group represented by formula (II) or a group represented by formula (III); R2 represents a hydrogen atom, an alkyl group having from 1 to 6 carbon atoms, a group represented by formula (II), or a group represented by formula (III); and in formula (II), R3 represents a halogen atom, an alkyl group having from 1 to 6 carbon atoms, a halogenated alkyl group having from 1 to 6 carbon atoms, an alkoxy group having from 1 to 6 carbon atoms, or a group represented by formula (IV).
Owner:MITSUI CHEM INC
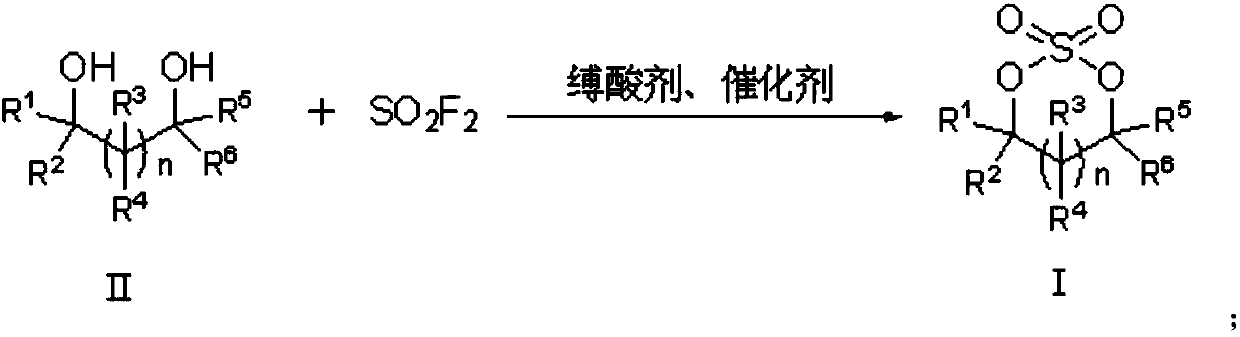
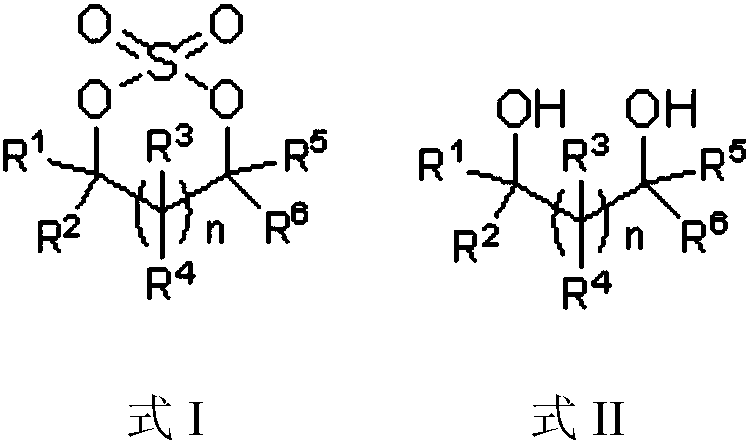
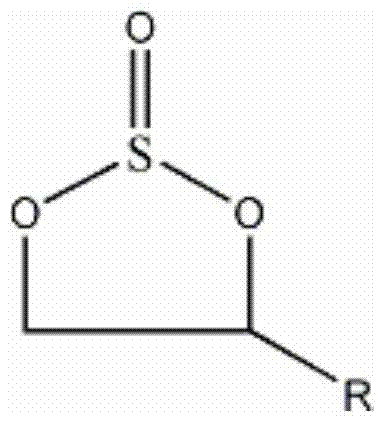
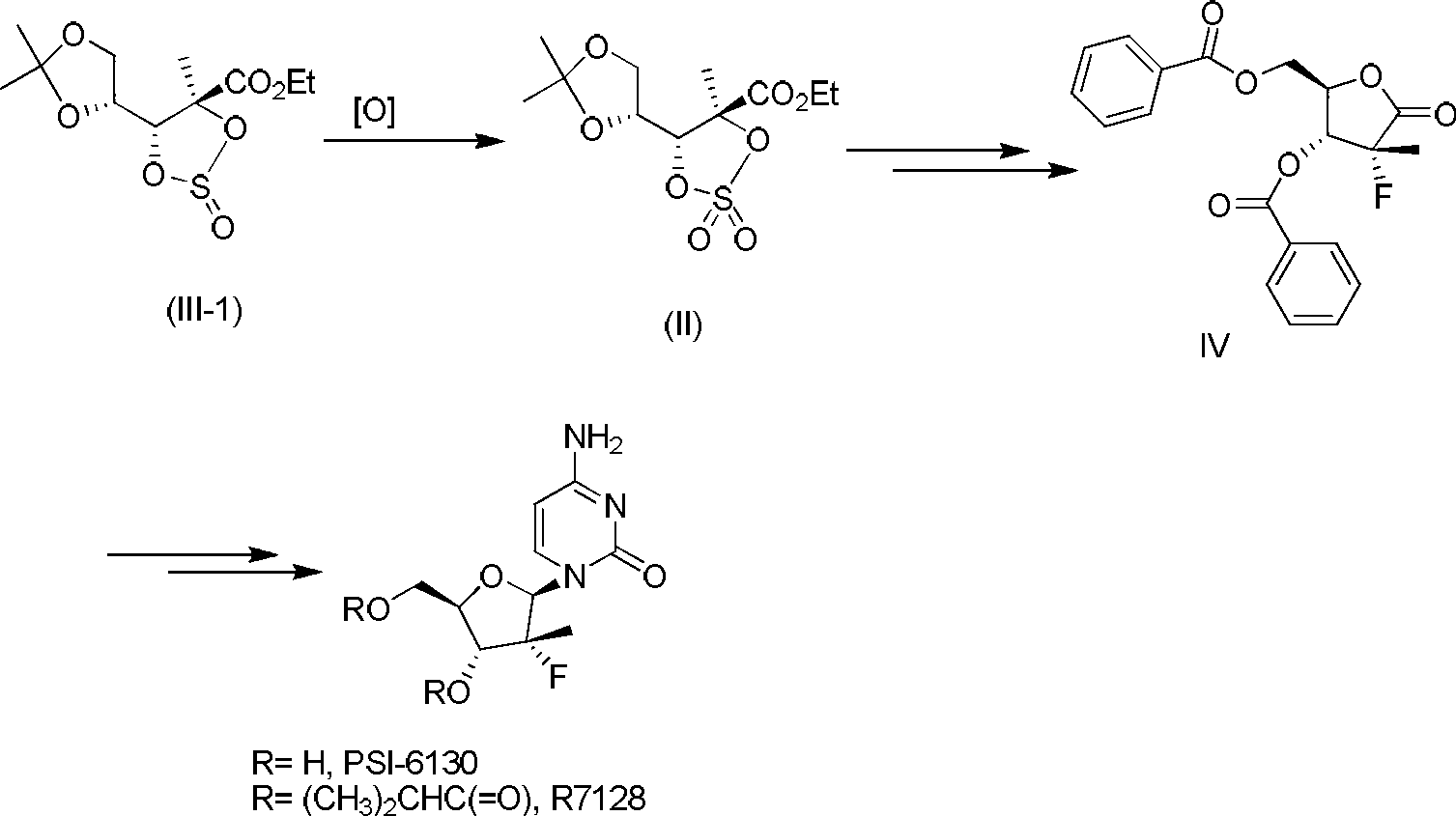
Preparation method of cyclic sulfate
The invention relates to the field of organic synthesis, in particular to a preparation method of cyclic sulfate. The structural formula of cyclic sulfate is shown as formula I. The preparation methodof cyclic sulfate comprises the following step: cyclic sulfate in the formula I is prepared from a compound in formula II and sulfuryl fluoride by reacting in the presence of an acid binding agent and a catalyst. The preparation method of cyclic sulfate adopts short reaction step, cyclic sulfate can be prepared with the one-step reaction, cost of raw and auxiliary materials is low, and expensiveraw materials and oxidizing agent are not used.
Owner:SHANGHAI CHEMSPEC CORP +1
Method for preparing cyclic sulphate
InactiveCN104744427AEasy to transportEasy to storeSilicon organic compoundsDistillationOrganic layer
The invention discloses a method for preparing cyclic sulphate. The method comprises the following steps: in turn, adding cyclic sulphite, a polar solvent and a catalyst into a reaction container, dipping an oxidant water solution to perform catalytic oxidation reaction under a reaction temperature, adjusting the pH value of the reaction system by alkali in the catalytic oxidation reaction process, standing after finishing reaction, separating the water layer, removing the solvent by distilling an organic layer to obtain the cyclic sulphite crude product, and re-crystallizing or performing vacuum refinery distillation on the crude product to obtain the cyclic sulphate. The method has the advantages of mild catalytic oxidation reaction, easy control, easily available raw materials and cheap raw materials, simple process, convenient operation, high purity and yield of the obtained cyclic sulphate, good stability and easy transportation and storage.
Owner:CHANGSHU CHANGJI CHEM
Method for preparing sulfated bagasse xylan
The invention discloses a method for preparing sulfated bagasse xylan. The method comprises the following steps of: dehydrating and cooling pyridine; dripping chlorosulfonic acid into the pyridine, wherein the volume ratio of the pyridine to the chlorosulfonic acid is between 15 and 25; when white solid appears in the reaction flask, adding anhydrous formamide to help dissolving, and continuing stirring for 1 to 2 hours; transferring the obtained solution into a constant-temperature water bath kettle, adding amino-sulfonic acid and bagasse xylan into the water bath kettle, and esterifying for 2.5 to 4 hours, wherein the ratio of the volume of the chlorosulfonic acid to the mass of the xylan is 1.2-2.0:0.7-1.5; adding excessive acetone and standing for 2 hours; pouring out the supernatant and adding the mixed solution of 75 to 90 volume percent methanol and water; depositing and then filtering; collecting the deposit and washing for multiple times; washing the deposit with the aqueous solution of ethanol at least once; washing and dehydrating with acetone; and drying in vacuum at 50 DEG C for 6 hours. The method has the characteristics of high substitution degree, high utilization rate of raw materials, easy control on synthesis process, simple post-treatment process and stable product quality; moreover, the prepared sulfated bagasse xylan has high water solubility.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
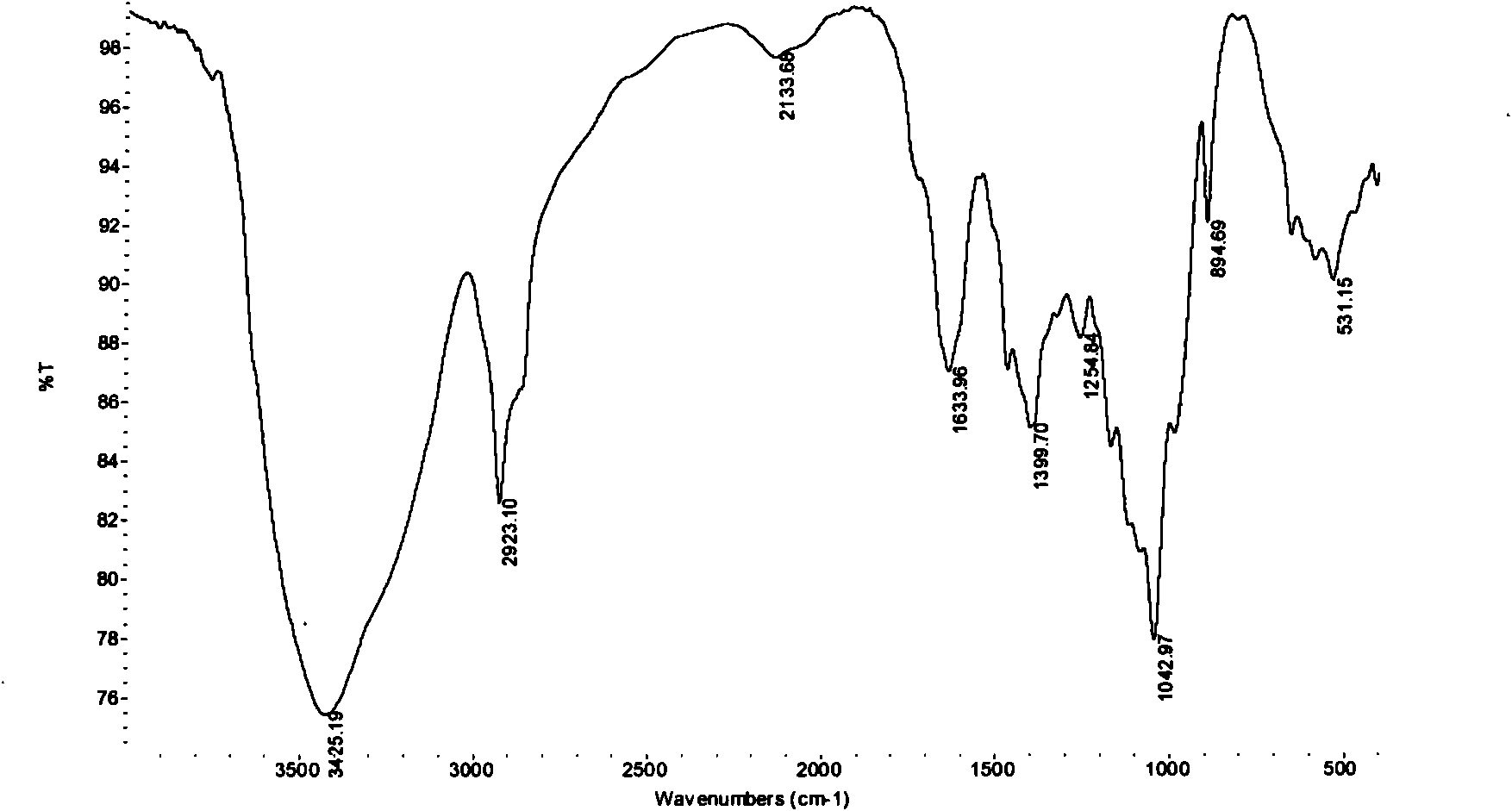
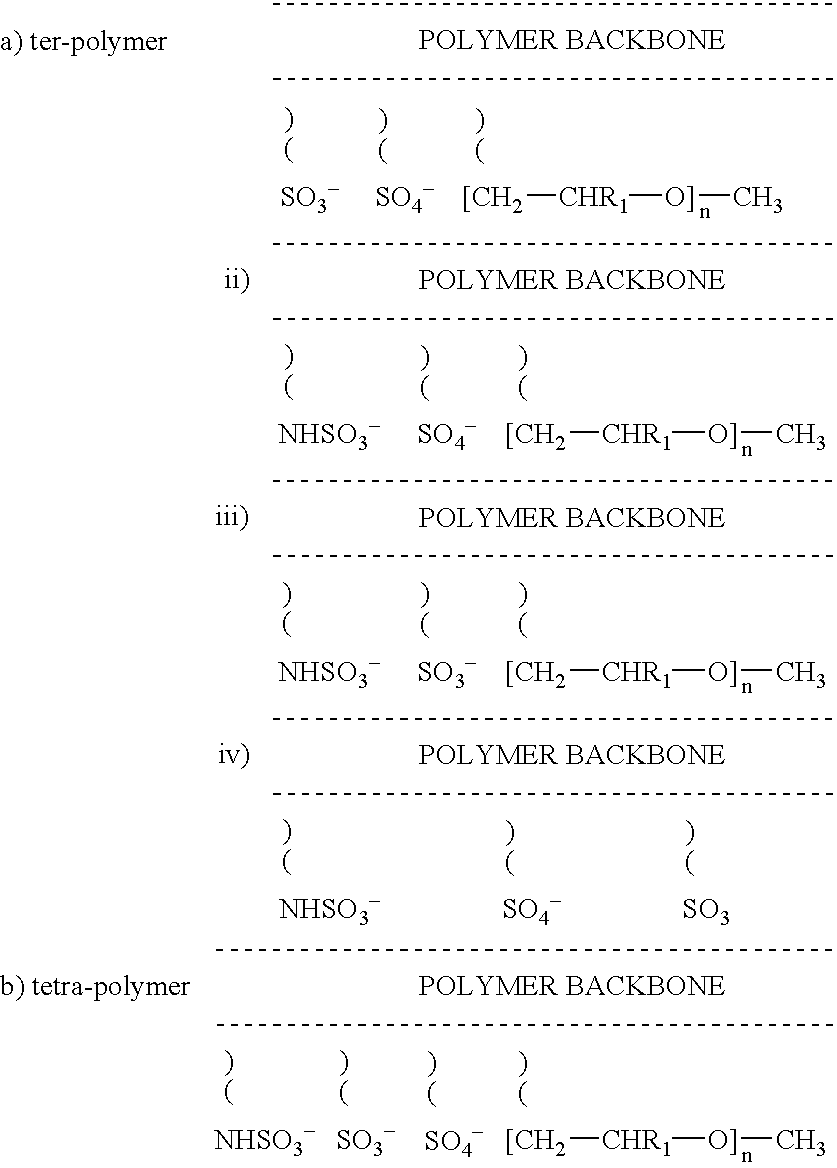
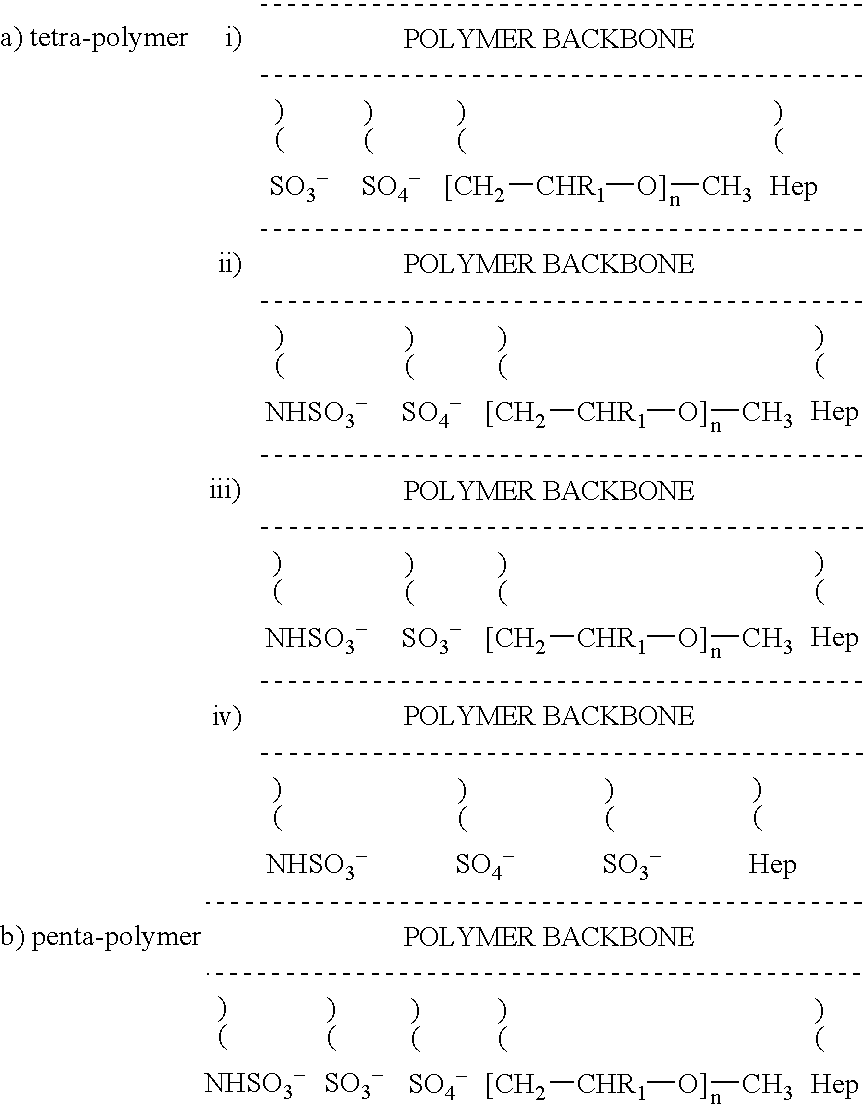
Non-thrombogenic and anti-thrombogenic polymers
InactiveUS7034061B1Improve attachment stabilityPreferential ionic bondingPeptide/protein ingredientsPharmaceutical non-active ingredientsWarfarinPolymer science
Polymers having non-thrombogenic properties can be prepared by copolymerizing monomers of at least three classes selected from (a) monomers having sulphate groups, (b) monomers having sulphonate groups, (c) monomers having sulphamate groups, (d) monomers having polyoxyalkylene ether groups, and (e) monomers having zwitterionic groups. The polymers can additionally be provided with anti-thrombogenic properties by including an additional comonomer having a pendant heparin (or hirudin, warfarin or hyaluronic acid) group. The polymers can be used as coating materials for medical devices, such as tubing or connectors, in order to provide them with non-thrombogenic, and optionally anti-thrombogenic, properties.
Owner:BIOINTERACTIONS
Polysaccharide sulfate selenide compound and its preparation method and application
InactiveCN101104643AHigh yieldQuality improvementOrganic active ingredientsAntineoplastic agentsFuranSulfate radicals
Disclosed are a selenium polysaccharide sulfate compound and a preparation method and applications of the selenium polysaccharide sulfate compound, belonging to the polysaccharide selenide technical field. The selenium polysaccharide sulfate compound is characterized in containing pyran sugar units or / and furan sugar units. The preparation method adopts reaction between polysaccharide sulfate and selenide reagent which is selenous acid and selenite. Molar ratio of the selenide reagent and groups of the polysaccharide sulfate is 1: 0.1-10. The compound of the invention is applied in preparing anticancer drug and ant-oxidation drug. The polysaccharide selenide sulfate of the invention is produced by substitution reaction between selenous acid and sugar unit sulfate radicals. Therefore, the yield and the quality of the product are both higher than that of the product produced by other methods. The invention also has advantage of high selenium content: the highest content of the selenium can reach to 45 percent which is much higher than 12.5 percent reported in literature.
Owner:陈艺新
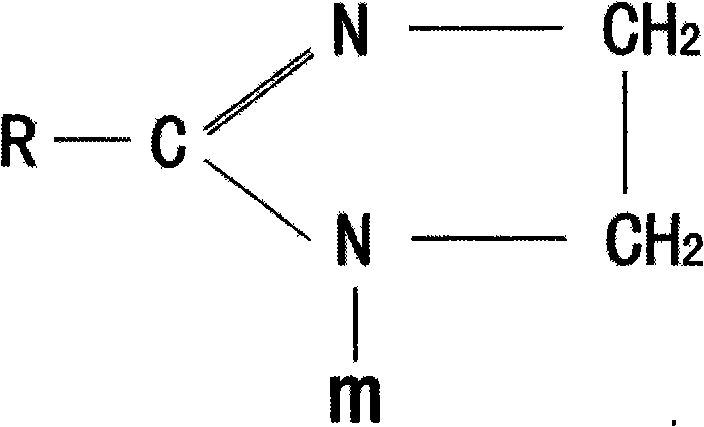
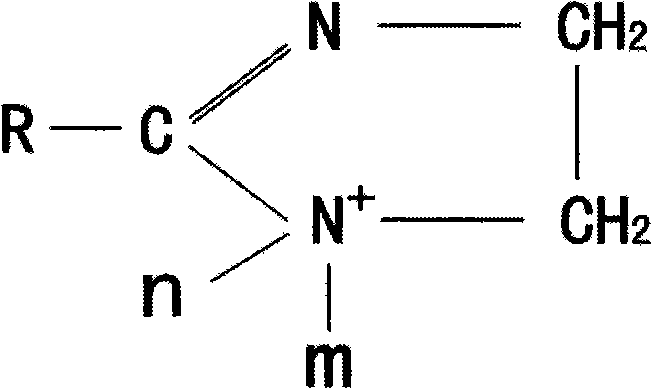
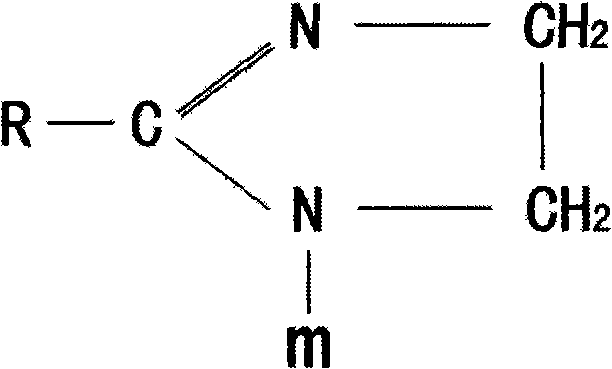
Method for synthesizing imidazoline intermediate and cationic derivative thereof
The invention relates to a method for synthesizing an imidazoline intermediate and a cationic derivative thereof. The method comprises the following process steps of: putting fatty acid, polyamine and water into a reaction kettle in a mole ratio of 1:1.1-2:1-5, heating to the temperature of between 100 and 180 DEG C to distill over the water added before and the water generated in the reaction, removing redundant polyamine and water under a pressure of between -0.04 and -0.1MPa and performing a reaction at a temperature of between 180 and 240 DEG C for 3 to 8 hours to synthesize the imidazoline intermediate; and reducing the temperature of the imidazoline intermediate to be below 80 DEG C, adding a solvent of which the mass is 30 to 100 percent of the imidazoline intermediate into the imidazoline intermediate, then adding 1 to 2mol of sulfate into the mixture and performing heat preservation reaction at the temperature of between 50 and 80 DEG C for 2 to 4 hours to synthesize cationic imidazoline. The fatty acid is oleic acid, linoleic acid, stearic acid, palmitic acid, lauric acid, coconut oil acid, capric acid and octanoic acid; the polyamine is ethylenediamine, diethylenetriamine, triethylene tetramine and tetraethylene pentamine; the solvent is isopropanol, absolute ethyl alcohol or 95 percent ethanol; and the sulfate is dithyl sulfate and dimethyl sulfate.
Owner:苗俊良
Method for preparing k-carrageenan with high gel strength from eucheuma
The invention relates to a method for preparing k-carrageenan with high gel strength from eucheuma. The method comprises the following steps of: (1) adding aqueous solution of ethanol into red algae eucheuma, treating under the ultrasonic condition, and draining water from the algae residue; (2) adding alkali modifier into the algae residue, and heating to remove sulfate groups on the C6 site in carrageenan so as to form 3,6-inner ether-galactose; (3) boiling gel: cleaning the algae residue by using tap water, draining water, adding deionized water, boiling the gel, and filtering to obtain carrageenan gel solution; (4) adding macroporous weak alkaline styrene series anion exchange resin into the carrageenan gel solution, decolorizing, and filtering to obtain liquid for later use; and (5) adding a flocculation accelerator into the liquid obtained by filtering, filtering to obtain white gel, performing vacuum drying, and crushing the product obtained by vacuum drying to obtain the k-carrageenan. By the method, the loss of gel is low, the consumption of alkali is greatly reduced, and the water treatment cost and the environmental pollution are reduced.
Owner:上海国矗生物科技有限公司
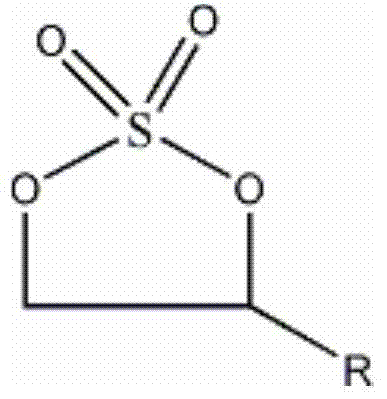
Preparation method of five-membered cyclic sulphate
The invention discloses a preparation method of five-membered cyclic sulphate, which comprises the following steps that: in the presence of an organic solvent and sodium bicarbonate, five-membered cyclic sulphite and OXONE are subjected to oxidation reaction; after reacting, treatment is carried out to obtain the five-membered cyclic sulphate. When the method disclosed by the invention is adopted for synthesizing the five-membered cyclic sulphate, the operation process is safe, simple and easy to control; the process cost is low; use of a chlorine-containing oxidant is avoided; the chlorine content and the metal content in a product are controlled; the yield of the product is high; and the prepared five-membered cyclic sulphate is an important drug synthetic midbody and has important application implications.
Owner:ZHEJIANG APELOA TOSPO PHARMA +1
Preparation method of water-soluble depepsen
InactiveCN101284878ABiologically activeAvoid the severity of direct sulfonationSodium amylosulfateFiltration
The invention discloses a method for making water-soluble sodium amylosulfate. The method comprises the following steps that: (1) concentrated sulfuric acid is added in a reactor and then alcohol is slowly dripped and stirred with the molar ratio between the alcohol and the sulfuric acid ranging from 1:1.6 to 1:2.8; moreover, the concentrated sulfuric acid and the alcohol are evenly stirred to be made into a sulfonating agent; (2) starch is added in the sulfonating agent with the mass ratio between the sulfonating agent and the starch ranging from 18:1 to 60:1; then, the mixture undergoes stirring reaction, keeping stand and sedimentation and filtration so as to obtain starch sulphate after a plurality of times of alcohol flushing and deposition; (3) the starch sulphate is added in water with the pH value adjusted to between 8 and 10 by sodium hydroxide solution, and sodium amylosulfate solution is obtained after filtration; (4) ethanol is added in the sodium amylosulfate solution to carry out deposition, centrifugal separation and drying, thereby obtaining sodium amylosulfate. The making process does not adopt toxic solvent and the made sodium amylosulfate has the characteristics of higher degree of substitution, less molecular degradation of starch and greater molecular weight and so on.
Owner:ZHEJIANG UNIV
Urushi polysaccharide sulfation and its preparation method and uses
InactiveCN1544477AHigh molecular weightHigh degree of substitutionOrganic active ingredientsBlood disorderLacquerDrug biological activity
The invention relates to a sulfation raw lacquer polysaccharide with biological activity prepared through the steps of, (2) dissolving or suspending the raw lacquer polysaccharide in 50-100 times weight ratio of drying solvent, stiring 10-30 min at 40-100 deg. C, charging trioxide pyridine compound of 1-4 times molar weight of raw lacquer polysaccharide residue or 1:2-4:1 volumetric ratio of sulfuric chlorohydrin pyridine mixture, reacting 0.5-4 hrs, cooling down the reaction mixture through ice bath, charging ice water with 1 / 3-1 / 2 times of solvent volume, neutralizing with 5-20wt% of NaOH, dialyzing in distilled water, subjecting the dialysate to vacuum distillation concentration at 30-70 deg. C, settling with anhydrous alcohol, and vacuum drying with P2O5.
Owner:WUHAN UNIV
Sulfate preparation method
The invention relates to the field of organic synthesis, and especially relates to a sulfate preparation method. The invention provides a sulfate preparation method. According to the preparation method, a compound represented by a formula (II) reacts with sulfuryl fluoride in the presence of a reaction solvent to prepare a compound represented by a formula (I). During the sulfate preparation process, the introduction of water and chlorine ions is avoided effectively, the situation that the product is degraded and the chlorine ion content is high is avoided therefore; moreover, the steps of thepreparation method are simple and short, the raw material are common chemical products on the market, the kinds of raw materials and the side reactions are few, and the yield is high. The manufacturing cost is low, only recyclable organic solvents are used, the reaction byproduct is a single inorganic salt solid and can be easily recovered, no wastewater is generated, the environment is better protected, and the sulfate preparation method is green and environmentally friendly and is suitable for industrial large scale production.
Owner:SHANGHAI CHEMSPEC CORP +1
Starch sulphate and its application in concrete
The present invention relates to starch sulfate and its preparation and application in concrete. Starch sulfate is prepared through one semi-dry process with chrlorosulfonic acid and starch in the molar ratio of 0.5-1.5 to 1 and organic solvent in 1-2 times volume of chrlorosulfonic acid, and through reaction for 1-4 hr. It has degree of substitution in 0.04-0.12, and molecular weight of (40-55)x10<6>. The starch sulfate is used as water reducing agent for concrete, and the added amount is 0.3-2 wt%. The starch sulfate itself is one kind of surfactant and possesses excellent application as concrete water reducing agent.
Owner:TIANJIN UNIV
Method for converting dimethyl sulfate waste residue into dimethyl sulfate product
InactiveCN102249953AZero emissionEliminate pollutionSulfur compoundsSulfonic acid esters preparationDistillationSulfate
The invention discloses a method for converting dimethyl sulfate waste residue into a dimethyl sulfate product, belongs to a method for preparing sulfate, and in particular relates to a method for recycling waste residue generated by a dimethyl sulfate device in the production running process. The method is characterized by comprising the following steps of: hydrolyzing and etherifying dimethyl sulfate in the dimethyl sulfate waste residue to convert the dimethyl sulfate into dimethyl ether; and performing esterification on the generated dimethyl ether and sulfur trioxide to obtain the dimethyl sulfate product. The method comprises the following process steps of: hydrolysis, etherification, separation of distillates, esterification, and distillation. The invention provides a method for converting the dimethyl sulfate waste residue into the dimethyl sulfate product; and the dimethyl ether is recycled from the dimethyl sulfate waste residue and is further converted into the dimethyl sulfate product. The waste residue generated by the dimethyl sulfate device is recycled, and the environmental pollution of the dimethyl sulfate waste residue is eliminated. The purity of the recycled dimethyl ether gas is 99.0 to 99.75 percent; the dimethyl sulfate product has the dimethyl sulfate content of more than or equal to 99.5 percent and the acidity of less than or equal to 0.6 percent; and the content of a byproduct of sulfuric acid is 50 to 70 percent.
Owner:SHANDONG XINGHUI CHEM
Preparation method of cyclic sulfates
The invention discloses a preparation method of cyclic sulfates. The cyclic sulfates comprise ethylene sulfate, butylene sulfate, propylene sulfate. The preparation method comprises the following steps: (1) adding an organic solvent into a reaction kettle, then adding a cyclic sulfite and hydrogen peroxide with a mass concentration of 30% into a reaction kettle, adding a catalyst, and controllingthe reaction temperature to be 30-40 DEG C, wherein the cyclic sulfite comprise ethylene sulfite, butylene sulfite or propylene sulfite, and the catalyst is a TS-1 (titanium-silicon molecular sieve);(2) separating the reaction liquid after the reaction is finished, taking a primary organic layer, washing the primary organic layer by water, and then carrying out layering by standing to obtain a secondary organic layer; and (3) evaporating out the organic solvent from the secondary organic layer for crystallization, and carrying out drying to obtain a finished product. The preparation method has the advantages that the reaction process is mild, heat removal is not required, the reaction is easy to control, by-products are few, the reaction is thorough, and the conversion rate is high.
Owner:ZHANGJIAGANG GUOTAI HUARONG NEW CHEM MATERIALS CO LTD
Technique for extracting sulfate polysaccharide from Laminaria digitata
The invention relates to a technique for extracting sulfate polysaccharide from Laminaria digitata, which comprises the following steps: cleaning Laminaria digitata, drying, pulverizing, adding a certain volume of water, carrying out ultrasonic assisted extraction for some time to obtain an extracting solution, centrifugating, adding proteinase into the supernate to decompose protein into amino acid, concentrating with a nanofiltration membrane to 1 / 3-1 / 5 of the original volume to obtain a crude polysaccharide concentrated solution, adding ethanol into the crude polysaccharide concentrated solution until the volume percent is 20-40%, standing, centrifugating, concentrating the centrifugate with a nanofiltration membrane to 1 / 2-1 / 3 of the original volume, adding ethanol until the volume percent is 70-85%, and carrying out centrifugal separation to obtain the sulfate polysaccharide. The technique is simple, and adopts the membrane to filter out water and amino acid, thereby saving the ethanol amount for the next purification step; and meanwhile, compared with the traditional heating concentration, the membrane filter concentration technique effectively keeps the structure and stability of the polysaccharide, and enhances the extraction rate of the sulfate polysaccharide.
Owner:ZHEJIANG YUXIANG BIOLOGICAL SCI & TECH
Fucoidan-galactosan sulfate, extracting, separating, and purifying method thereof, and application thereof
ActiveCN102234336AGood curative effectIncrease valueOrganic active ingredientsMetabolism disorderSulfated polysaccharidesSulfate
The invention discloses a fucoidan-galactosan sulfate with a novel structure. Fucoidan-galactosan sulfate provided by the present invention is a sulfated polysaccharide composed of fucose and galactose. According to the chemical constitution of the sugar chain, a fundamental chain is formed by -4-alpha-L-Fuc-(1,3)-alpha-L-Fuc-(1,3)-alpha-L-Fuc-(1,3)-alpha-L-Fuc-(1-residue. Every 4 sugar residues comprise a branched chain, which is composed of alpha-L-Fuc(1- or beta-D-Gla-(1,6)-beta-D-Gla-(1-. Sulfated group is connected to the 2 or 4 site of fucose, or on the 3 or 4 site of galactose. Meanwhile, the invention discloses a preparation method and an application of fucoidan-galactosan sulfate. Fucoidan-galactosan sulfate provided by the present invention can be exploited into medicines for treating kidney diseases.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Method for preparing 4-aminophenyl-beta-hydroxyethyl sulfone sulfate
InactiveCN101255128AReduce productionEasy to handleOrganic chemistryOrganic compound preparationChlorosulfuric acidNitrobenzene
The invention relates to a preparation method for 4-(beta-sulfatoethyl sulfone sulfonyl) aniline, and relates to a preparation method for a sulfuric ester. The invention solves the problems that chlorosulfonic acid is greatly overused and environmental protection cost is high in the preparation process of para-ester at present. The preparation method of the invention comprises: condensing nitro halogeno benzene and beta-mercaptoethanol to produce 4-(beta-sulfatoethyl sulfone sulfide) nitrobenzene, adding hydrogen peroxide in aqueous medium after separating out dissolvent, water and salts to produce 4-nitrobenz-beta-hydroxyethyl sulfone by catalytic oxidation, deoxidizing nitro of 4-nitrobenz-beta-hydroxyethyl sulfone to be amino by catalytic hydrogenation reaction, and homogeneously esterifying with sulfuric acid to obtain the product. The method does not use chlorosulfonic acid, generation amount of waste waters is little and easy to treat, by-product is few and easy to separate. The purity of the product is high, the amino number and ester number are higher than 97. 0and 95. 0respectively, the difference between the two number is less than 2, and the product has light color and pure tint.
Owner:HEILONGJIANG INST OF PETROCHEM
Method for preparing rubber tackifier p-tert-octylphenol formaldehyde resin
ActiveCN101190961ASolve the problem of high import costsAvoid difficultiesP-tert-octylphenolPolystyrene
The invention provides a preparation method of p-tert-octyl phenol formaldehyde resins. Mixture of C4 fraction by additive reaction after being treated with a sulfated-degassing process is adopted as raw material; isobutene dimmer with targeted distillate content more than 98 percent after respective evaporation and cutting at the temperature of 75 DEG C-105 DEG C is adopted as alkylating agent; Na-sulfonated polystyrene-polyvinylidene copolymer strong-base anion exchange resin and H-sulfonated polystyrene-polyvinylidene copolymer strong-acid cation exchange resin are adopted as alkylation catalyst so as to obtain the p-tert-octyl phenol alkylate mixture by alkylation reaction under the temperature of 90-100 DEG C; the mixture is then treated with respective evaporation and cutting under the temperature of 220-270 DEG C to prepare the p-tert-octyl phenol with the melting point of 87.5-89.5 DEG C; finally the p-tert-octyl phenol and formaldehyde solution are treated with condensation reaction catalyzed by acid in toluol medium to obtain the p-tert-octyl phenol formaldehyde resins.
Owner:SINO LEGEND CHINA CHEM +1
Synthesis method of 6-O-carboxymethyl chitosan sulfuric sulfation product
The invention discloses a synthesis method of a 6-O-carboxymethyl chitosan sulfation product. The method comprises the following steps: (1) chitin alkaline treatment of chitin; (2) carboxymethylation of C6-O site of chitin; (3) deacetylation reaction of 6-O-carboxymethyl chitin; and (4) sulfation of 6-O-carboxymethyl chitosan. The synthesis method disclosed by the invention is a bran-new method for selectively replacing and controlling the replacement rate by using chitin. The synthesis product of the method provides N-site -SO3H with main anticoagulant activity and introduces -COOH, so that a lot of -COOH and SO3H which have negative electricity in the molecular structure are regularly distributed, and an anticoagulant effect of heparinoid is generated by the synergistic effect. High molecular polysaccharide is a heparinoid drug which is selectively modified by chitin via a safe reagent, so that reagent pollution of bulk drugs is reduced, the virus contamination risk of heparin biological extraction is avoided, the safety performance of the drug in the clinical experiment is more excellent in theory as compared with heparin sodium, so the 6-O-carboxymethyl chitosan sulfation product provided by the invention is expected to serve as a cheap direct thrombin inhibitor to replace heparin sodium anti coagulation drugs.
Owner:SHENZHEN BRIGHT WAY NOVEL BIO MATERIALS TECH CO LTD
Sulfated chitosan quaternary ammonium salt as well as preparation and application thereof
InactiveCN103665185AImproves antioxidant activityImprove biological activityCosmetic preparationsToilet preparationsWater bathsDrug biological activity
The invention relates to the fields of daily use chemicals and pharmaceuticals industry and in particular relates to a sulfated chitosan quaternary ammonium salt as well as a preparation and an application thereof. The sulfated chitosan quaternary ammonium salt is shown as a formula (1). The preparation method specifically comprises the following steps: uniformly mixing chitosan quaternary ammonium salt and an N,N-dimethyl formamide (DMF) solution, slowly dropwise adding a sulfonated reagent under the stirring condition after uniformly mixing, reacting in a water bath at the temperature of 30-60 DEG C for 1.5-3 hours, cooling at room temperature, performing absolute ethyl alcohol precipitation, filtering, washing by using absolute ethyl alcohol, and drying to obtain the sulfated chitosan quaternary ammonium salt. The obtained sulfated chitosan quaternary ammonium salt is easily dissolved in water; due to introduction of sulfonate, the biological activity of the chitosan quaternary ammonium salt is enhanced, and a synergistic effect is achieved, so that the sulfated chitosan quaternary ammonium salt has higher biological activity and can be widely applied to the fields of medicines and daily use chemicals.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com