Method for synthesizing imidazoline intermediate and cationic derivative thereof
A synthesis method and intermediate technology are applied in the synthesis field of imidazoline intermediates and cationic derivatives thereof, and can solve the problems of inclusion of solvents, polyamine residues, influence on corrosion inhibition performance, etc. content, good water film replacement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
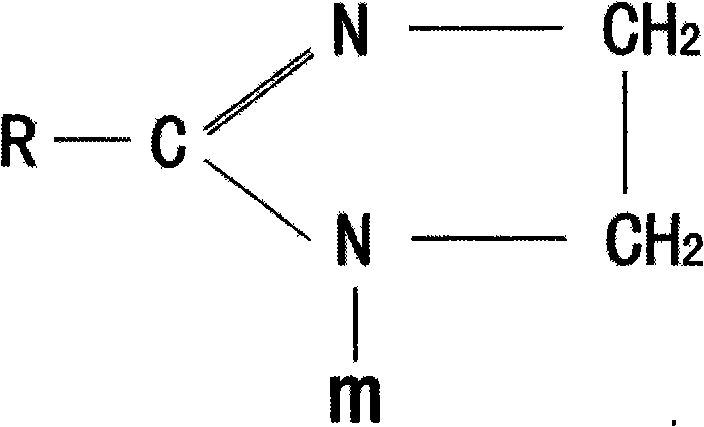
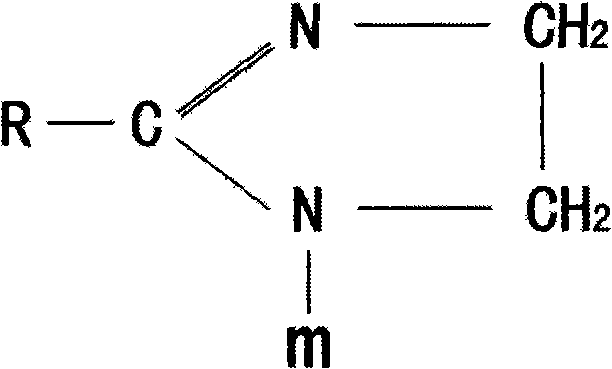
[0030] Step 1: Put 1 mol of oleic acid, 1.2 mol of ethylenediamine, and 4 mol of water into the reaction kettle, stir evenly and gradually heat up. The reaction time for heating up from 100°C to 180°C is 10 hours, and the water and The water generated by the reaction, then turn on the vacuum pump, remove the excess polyamine and the generated water at -0.06MPa, and gradually increase the temperature, and continue the reaction for 4 hours at 180°C to 240°C, thus synthesizing the imidazoline intermediate.
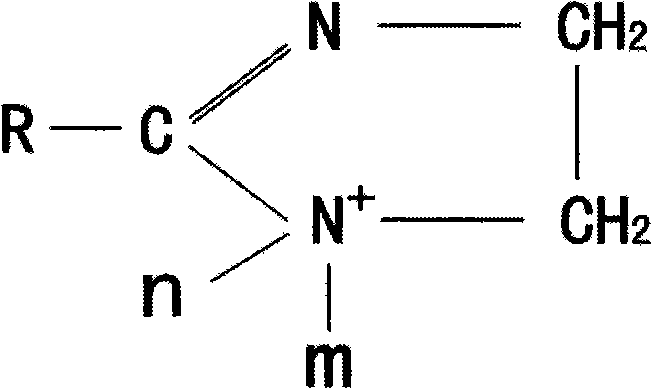
[0031] Step 2: Cool the imidazoline intermediate to 80°C, add isopropanol, the quality of isopropanol is 30% of the imidazoline intermediate, then add 2mol of diethyl sulfate, and keep warm at a temperature of 80°C React for 2 hours, so that cationic imidazoline is synthesized.
Embodiment 2
[0033] Step 1: Put 1 mol of linoleic acid, 1.4 mol of diethylenetriamine, and 3 mol of water into the reactor, stir evenly and gradually heat up. The reaction time for heating up from 100°C to 180°C is 8 hours. Water and the water generated by the reaction, then turn on the vacuum pump, remove the excess diethylenetriamine and the water generated by the reaction at -0.07MPa, and gradually increase the temperature, and continue the reaction for 5 hours at 180 ° C ~ 230 ° C to obtain imidazoline intermediate.
[0034] Step 2: cooling the imidazoline intermediate to 70°C, adding dehydrated ethanol, whose quality is 60% of the imidazoline intermediate, then adding 1.1mol of dimethyl sulfate, at a temperature of 70°C, Heat preservation reaction 3 hours, promptly synthesize cationic imidazoline like this.
Embodiment 3
[0036] step one:
[0037] Heat and melt 1 mol of lauric acid, add 1.6 mol of triethylenetetramine and 2 mol of water, stir evenly and gradually heat up, the reaction time for heating up from 100°C to 180°C is 7 hours, steam the water added in the previous stage and the water generated by the reaction , and then turn on the vacuum pump, remove excess triethylenetetramine and generated water at -0.08MPa, and gradually increase the temperature, and continue the reaction for 6 hours at 180°C to 220°C, thus synthesizing the imidazoline intermediate.
[0038] Step 2: Cool the imidazoline intermediate to 60°C, add 95% ethanol, the quality of 95% ethanol added is 80% of the imidazoline intermediate, then add dimethyl sulfate 1.05mol, at a temperature of 60°C, Heat preservation reaction 4 hours, promptly synthesize cationic imidazoline like this.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com