Method for preparing carfilzomib amorphous crystal
A carfilzomib and amorphous technology, applied in the direction of peptides, etc., can solve the problems of affecting the stability of substances, difficult to remove water, etc., and achieve the effect of easy removal, low moisture content, and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a kind of preparation method of carfilzomib amorphous crystal, comprises the following steps:
[0025] Carfilzomib is dissolved in an organic solvent to obtain a carfilzomib solution;
[0026] Concentrating the carfilzomib solution to obtain amorphous carfilzomib.
[0027] The preparation method of the amorphous crystal provided by the present invention uses an organic solvent without adding water, the organic solvent is easy to remove during the concentration process, no residue will be generated in the product, and the moisture content is low, and no crystal water will be formed, so that the obtained Carfilzomib amorphous crystal has high stability. And the preparation method of carfilzomib amorphous crystal provided by the present invention is after dissolving carfilzomib, directly concentrates and dries the obtained carfilzomib solution, without going through the process of crystal growth and crystallization, the obtained carfilzomib The non...
Embodiment 1
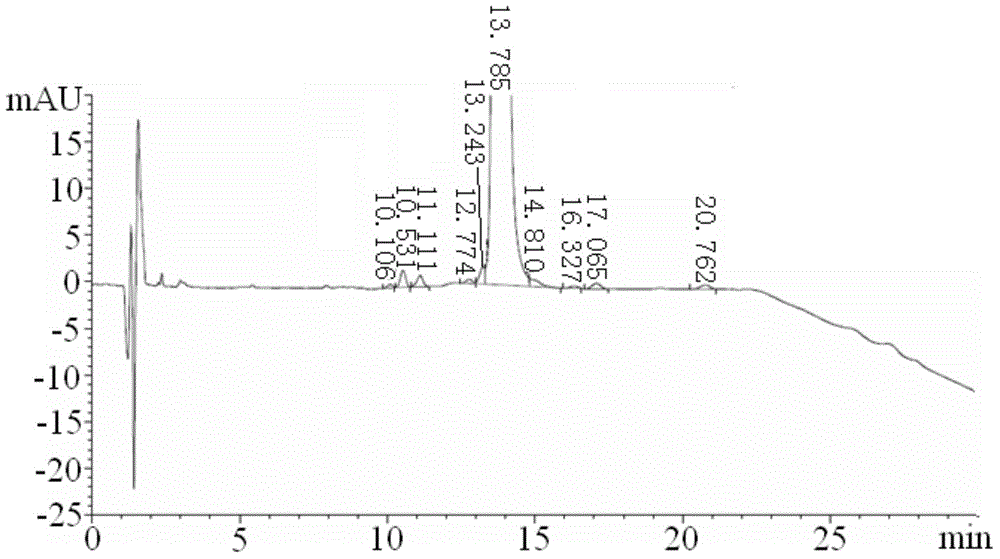
[0064]1g of carfilzomib crude product is dissolved in the mixed solution of ethyl acetate and n-hexane with a volume ratio of 2:1, and the obtained concentration of carfilzomib crude product solution of 0.5g / mL is added to a silica gel column, and the silica gel The particle size of the silica gel filled in the column is 200 mesh to 250 mesh, the control parameter of the wet packing column is 15g carfilzomib / kg silica gel, and the mixed solution of ethyl acetate and n-hexane with a volume ratio of 2:1 is used as the mobile phase. Carry out column chromatography separation, the flow velocity of mobile phase in the column chromatography separation is 5mL / min, track with HPLC, the parameter that HPLC detects is:
[0065] The chromatographic column is Agilent Extend-C18 150mm×4.6mm, 3.5μm; the column temperature is 30°C; the injection volume is 10μL; the flow rate is 1mL / min; the mobile phase includes solution A and solution B, and the molar concentration of solution A is 0.02mol / m...
Embodiment 2
[0079] Under the condition of stirring, 1g of carfilzomib crude product was dissolved in 15mL of methanol, filtered to remove insoluble impurities, the filtrate was concentrated to dryness under reduced pressure at a vacuum of 0.1 and a temperature of 40°C, and then Under the conditions of 0.1 and a temperature of 50° C., dry under reduced pressure for 12 hours to obtain carfilzomib crystals.
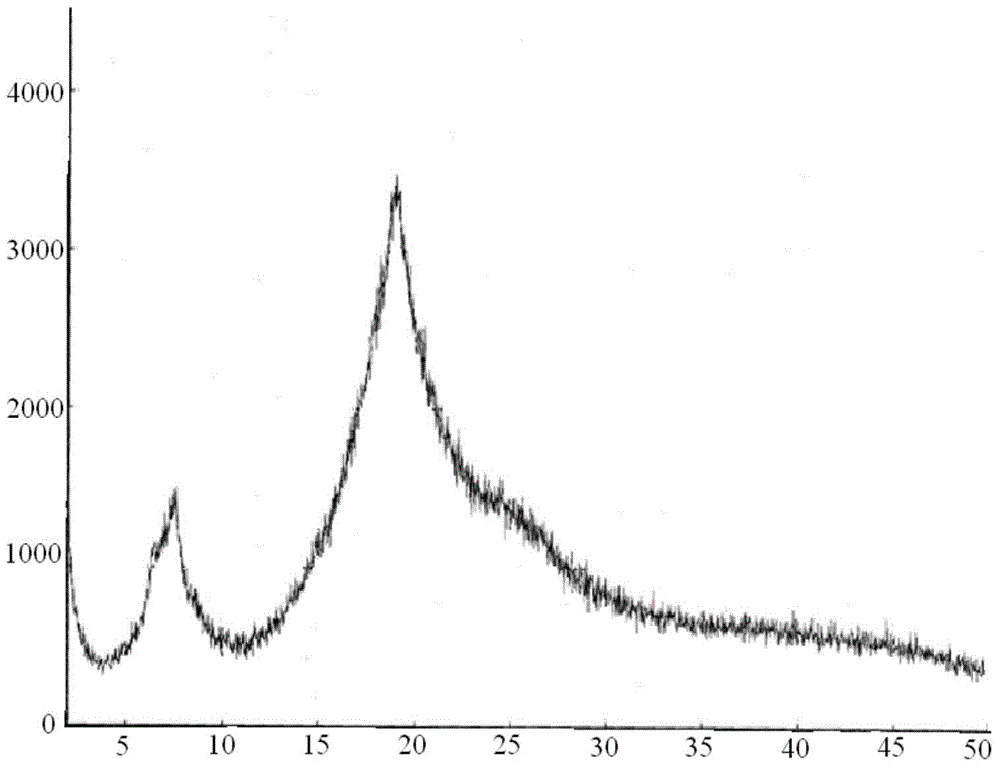
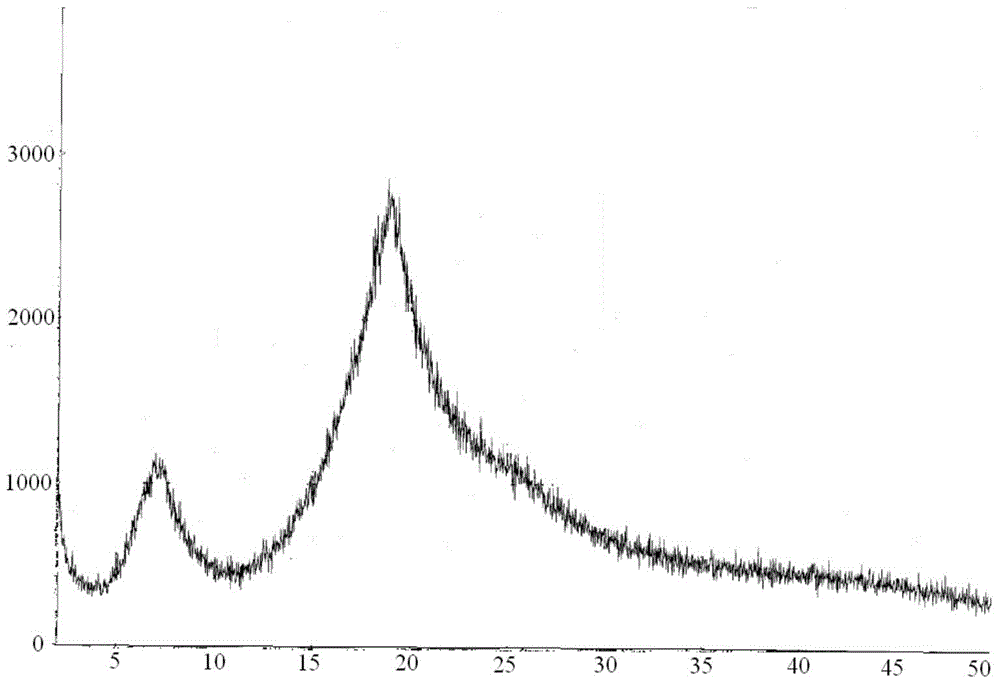
[0080] The present invention adopts X-powder diffraction method to detect the carfilzomib crystal obtained, and the result is as follows: image 3 as shown, image 3 For the XRPD spectrum of the carfilzomib crystals of Example 2 of the present invention, by image 3 It can be seen that the carfilzomib crystals obtained in this example are amorphous carfilzomib crystals.
[0081] The water content of the amorphous carfilzomib crystal obtained by the detection of the present invention, the results show that the water content of the amorphous carfilzomib crystal obtained by the method of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com