Solid-phase preparation method for exenatide crude product
A technique for the preparation of exenatide and solid phase, which is applied to the preparation methods of peptides, chemical instruments and methods, peptides, etc., and can solve problems such as difficult stability, many control items, and complicated steps of exenatide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] In one example of the present invention, the preparation method of solid-phase synthesis of exenatide by the polypeptide of the present invention comprises the following steps:
[0085] The first step, the synthesis of Fmoc-linker:
[0086] Use 1.0-5.0 times the amount of Fmoc-Linker, 1.0-5.0 times the amount of HOBt, and 1.0-5.0 times the amount of DIC reagent, and dissolve it in a DMF solvent of 5ml-10ml / g resin. Add it into a reaction kettle equipped with PLAM or MBHA resin, react for 30-60 minutes, then add 1.0-5.0 times the amount of DIC reagent, and react for 3 hours at room temperature. After the reaction, it was washed three times with DMF / MeOH successively. End-capped with Ac2O / Pyridine / DMF;
[0087] The second step, the synthesis of amino acid residue 39#-31#:
[0088]Treat the solid in the reaction kettle twice with a deprotecting agent, wash with DMF / MeOH for 1-3 times, and then add 1.0-5.0 times the amount of Fmoc-protected amino acids (such as: Fmoc-Ser...
Embodiment 1
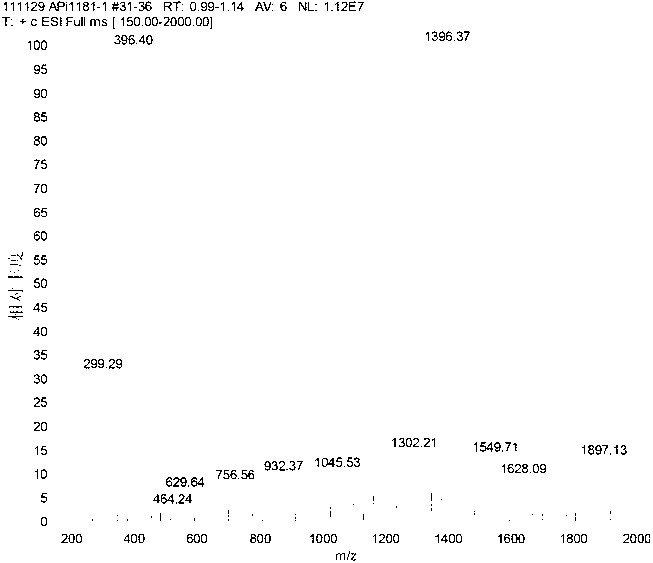
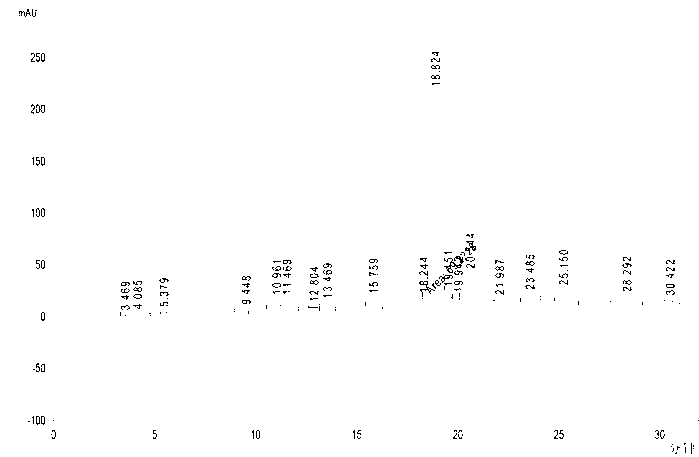
[0117] Synthesis of Crude Exenatide 1
[0118] Synthesis of Fmoc-linker:
[0119] Use 2.0 times the amount of Fmoc-Linker, 2.0 times the amount of HOBt, and 2.0 times the amount of DIC reagent, and dissolve them in a DMF solvent of 8ml / g resin. Add it into a reaction kettle equipped with 5.5 grams of MBHA resin (substitution rate 0.9mmol / g), react for 30 minutes, then add 2.0 times the amount of DIC reagent, and react for 3 hours at room temperature. After the reaction, it was washed three times with DMF / MeOH successively. Capped with Ac2O / Pyridine / DMF.
[0120] Synthesis of amino acid residues 39#-31#:
[0121] Treat the solid in the reaction kettle twice with a deprotecting agent, wash it three times with DMF / MeOH, add 2.5 times the amount of Fmoc protected amino acids, 2.5 times the amount of HOBt, and 2.5 times the amount of DIC reagent, and dissolve them in 8ml / g Mixed solution of resin in DMF solvent. After reacting for 30 minutes, add 2.5 times the amount of DIC re...
Embodiment 2
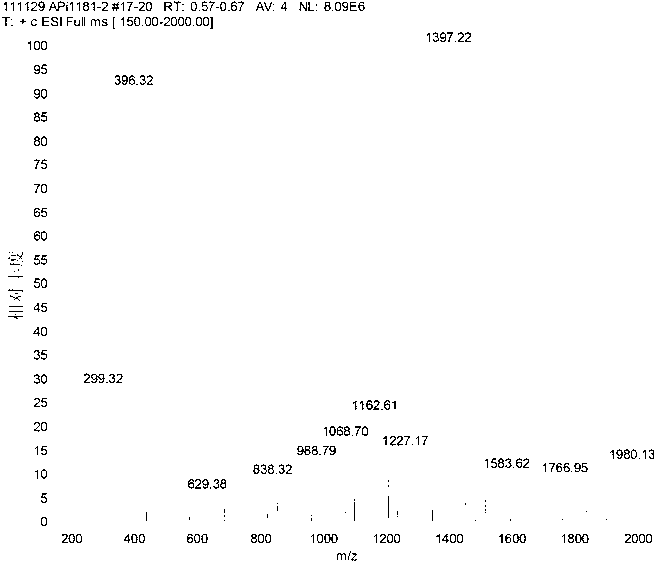
[0137] Synthesis of Crude Exenatide 2
[0138] Synthesis of Fmoc-linker:
[0139] Use 2.0 times the amount of Fmoc-Linker, 2.0 times the amount of HOBt, and 2.0 times the amount of DIC reagent, and dissolve them in a DMF solvent of 8ml / g resin. Add it into a reaction kettle equipped with 2.22 grams of PLAM resin (substitution rate 0.9mmol / g), react for 30 minutes, then add 2.0 times the amount of DIC reagent, and react for 3 hours at room temperature. After the reaction, it was washed three times with DMF / MeOH successively. Capped with Ac2O / Pyridine / DMF.
[0140] Synthesis of amino acid residues 39#-31#:
[0141] Treat the solid in the reaction kettle twice with a deprotecting agent, wash it three times with DMF / MeOH, add 2.5 times the amount of Fmoc protected amino acids, 2.5 times the amount of HOBt, and 2.5 times the amount of DIC reagent, and dissolve them in 8ml / g Mixed solution of resin in DMF solvent. After reacting for 30 minutes, add 2.5 times the amount of DIC r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com