Oat bran phenolic amide alkaloid, preparation method thereof and application of oat bran phenolic amide alkaloid in preparation of antipruritic products
A technology of oat bran amide and gluten amide, which is applied in the application field of oat bran amide alkaloids and its preparation, and preparation of antipruritic products, can solve the problems of limited application and single structure, and achieve the reduction of scratching times, Simple steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
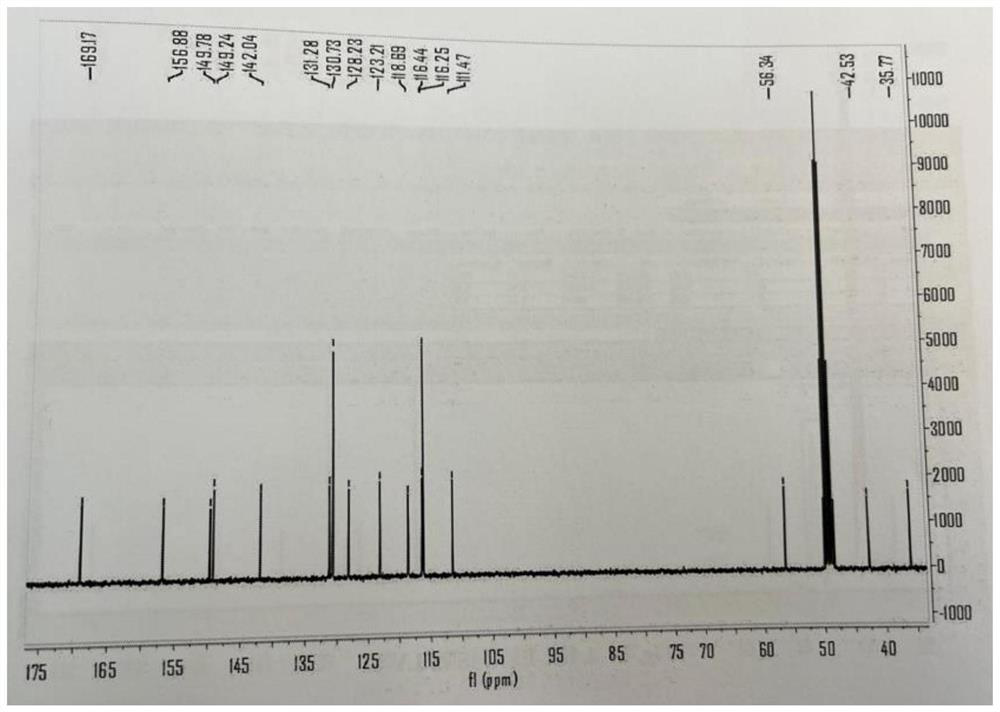
[0044] Preparation of avena gluten amide alkaloid compound-1 (abbreviated as PAA-1) compound
[0045] Weigh (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid (1.0g) into a 100mL clean round-bottomed flask, put it in a magnet, add 40mL anhydrous dichloromethane, and add 4- (2-Aminoethyl)phenol (1.84 g), triethylamine (5.5 mL), and HATU (4.85 g) after 5 minutes. After the addition was completed, the reaction was continued at room temperature for 5 hours, and the disappearance of the reaction raw materials was monitored by TLC. The stirring was stopped, the reaction system was evaporated under reduced pressure to remove the solvent, and the residue was purified by silica gel column chromatography (200-300 mesh) to obtain avena gluten amide alkaloid compound-1. The structure looks like this:
[0046]
Embodiment 2
[0048] Preparation of avena gluten amide alkaloid compound-2 (abbreviated as PAA-2) compound
[0049] Weigh (E)-3-(4-hydroxyphenyl)acrylic acid (1.0g) into a 100mL clean round-bottomed flask, put it in a magnet, add 40mL anhydrous dichloromethane, and add 4-(2-amino) under stirring ethyl)phenol (1.84g), triethylamine (5.5mL), HATU (4.85g) after 5 minutes. After the addition was completed, the reaction was continued at room temperature for 5 hours, and the disappearance of the reaction raw materials was monitored by TLC. The stirring was stopped, the reaction system was evaporated under reduced pressure to remove the solvent, and the residue was purified by silica gel column chromatography (200-300 mesh) to obtain avena glutenamide alkaloid compound-2. The structure looks like this:
[0050]
Embodiment 3
[0052] Preparation of avena gluten phenolamide alkaloid compound-3 (abbreviated as PAA-3) compound
[0053] Weigh (E)-3-(3,4-dihydroxyphenyl)acrylic acid (1.0 g) into a 100 mL clean round-bottomed flask, put it in a magnet, add 40 mL of anhydrous dichloromethane, and add 4-(2 -aminoethyl)phenol (1.84g), triethylamine (5.5mL), after 5 minutes HATU (4.85g) was added. After the addition was completed, the reaction was continued at room temperature for 5 hours, and the disappearance of the reaction raw materials was monitored by TLC. The stirring was stopped, the reaction system was evaporated under reduced pressure to remove the solvent, and the residue was purified by silica gel column chromatography (200-300 mesh) to obtain avena gluten amide alkaloid compound-3. The structure looks like this:
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com